Ganciclovir for injection and preparation method thereof
A technology for ganciclovir and injection, which is applied in the field of ganciclovir freeze-dried powder injection for injection and its preparation, can solve the problem of powder injection skeleton and freeze-dried microstructure collapse, skeleton freeze-dried microstructure Structural collapse, large safety risks and other problems, to achieve the effect of loose and uniform appearance, reduce production costs, and improve product uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 ganciclovir freeze-dried powder injection
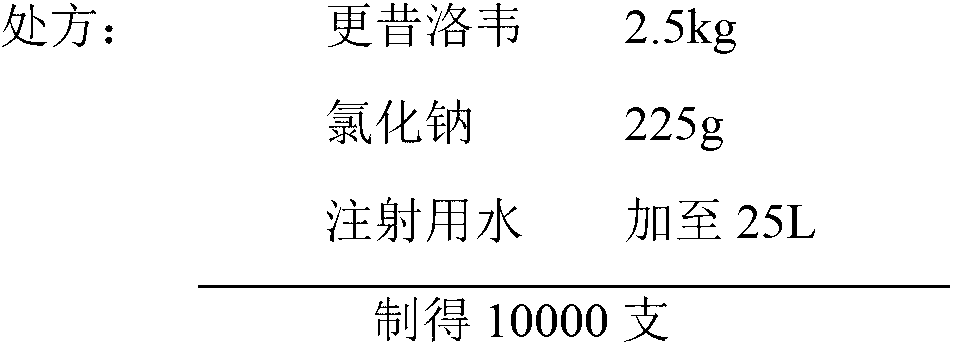
[0028]
[0029] Preparation: Weigh 2.5kg of ganciclovir and 225g of sodium chloride, add 80-85% of the prescription amount to water for injection cooled to room temperature, stir to dissolve completely, adjust the pH of the liquid to 10.5-11.0 with sodium hydroxide, add water for injection to 25L, stir evenly. Then go through double-stage sterilization filtration with 0.22μm sterile filter membrane, take a sample of the filtrate to detect the content of the semi-finished product, fill a clean vial with a drug solution of about 2.5ml / bottle according to the content, add a rubber stopper halfway, and put it into a freeze-dry Freeze-dried in the machine. The freeze-drying process is as follows, after the freeze-drying is completed, the vacuum plug is pressed, the aluminum cover is rolled out, visually inspected, and packaged.
[0030] Freeze drying process:
[0031] (1) repeated freezing
[...
Embodiment 2
[0037] The preparation of embodiment 2 ganciclovir freeze-dried powder injection
[0038]
[0039]
[0040] The preparation process is the same as in Example 1.
Embodiment 3
[0041] The preparation of embodiment 3 ganciclovir freeze-dried powder injection
[0042]
[0043] The preparation process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com