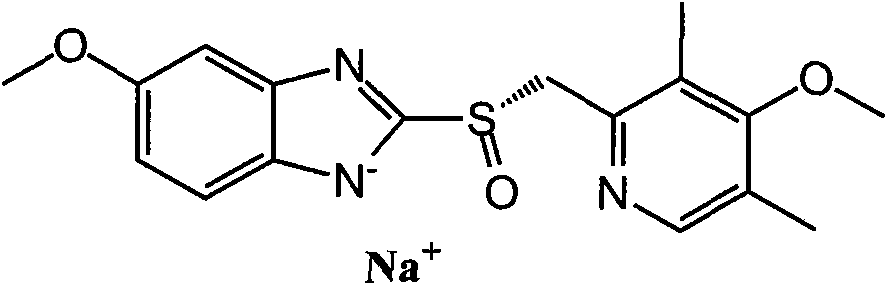
Esomeprazole sodium enteric-coated tablet and preparation method thereof
A technology of esomeprazole sodium and enteric-coated tablets, which is applied in pill delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as increasing gastrointestinal irritation and increasing operation complexity, and achieve increased drug use Safety, improved drug stability, improved release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Tablet core prescription
[0027]
[0028] Barrier Formulation
[0029]
[0030] Enteric layer formula
[0031]
[0032] Preparation Process
[0033] Mix esomeprazole sodium with lactose, sodium carboxymethyl starch, and microcrystalline cellulose evenly, add aqueous sodium hydroxide solution, granulate, dry, add magnesium stearate, mix evenly, and compress to obtain the product.
[0034] The compressed tablet is coated with an isolation coat with a weight gain of 5%, and then with an enteric coating with a weight gain of 15%.
Embodiment 2
[0036] Tablet core prescription
[0037]
[0038] Barrier Formulation
[0039]
[0040] Enteric layer formula
[0041]
[0042] Preparation Process
[0043] Mix esomeprazole sodium, sodium carboxymethyl starch and microcrystalline cellulose evenly, add sodium carbonate aqueous solution, granulate, dry, add magnesium stearate, mix evenly, and tablet to obtain.
[0044] The compressed tablet was coated with an isolation coat with a weight gain of 6%, and then with an enteric coating with a weight gain of 18%.
Embodiment 3
[0046] Tablet core prescription
[0047]
[0048] Barrier Formulation
[0049]
[0050]
[0051] Enteric layer formula
[0052]
[0053] Preparation Process
[0054] Mix esomeprazole sodium with lactose and crospovidone evenly, add sodium hydroxide aqueous solution, granulate, dry, add magnesium stearate, mix evenly, and compress into tablets to obtain the product.
[0055] The compressed tablet is coated with an isolation coat with a weight gain of 5%, and then with an enteric coating with a weight gain of 15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com