Therapy for human cancers using cisplatin and other drugs or genes encapsulated into liposomes
a technology of cisplatin and other drugs, applied in the direction of drug compositions, genetic material ingredients, peptide/protein ingredients, etc., can solve the problems of limited therapeutic efficacy, limited therapeutic efficiency, and no synthesized compound showed significant cytotoxic activity against three, so as to improve the entrance across the cell membrane, high therapeutic efficacy, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
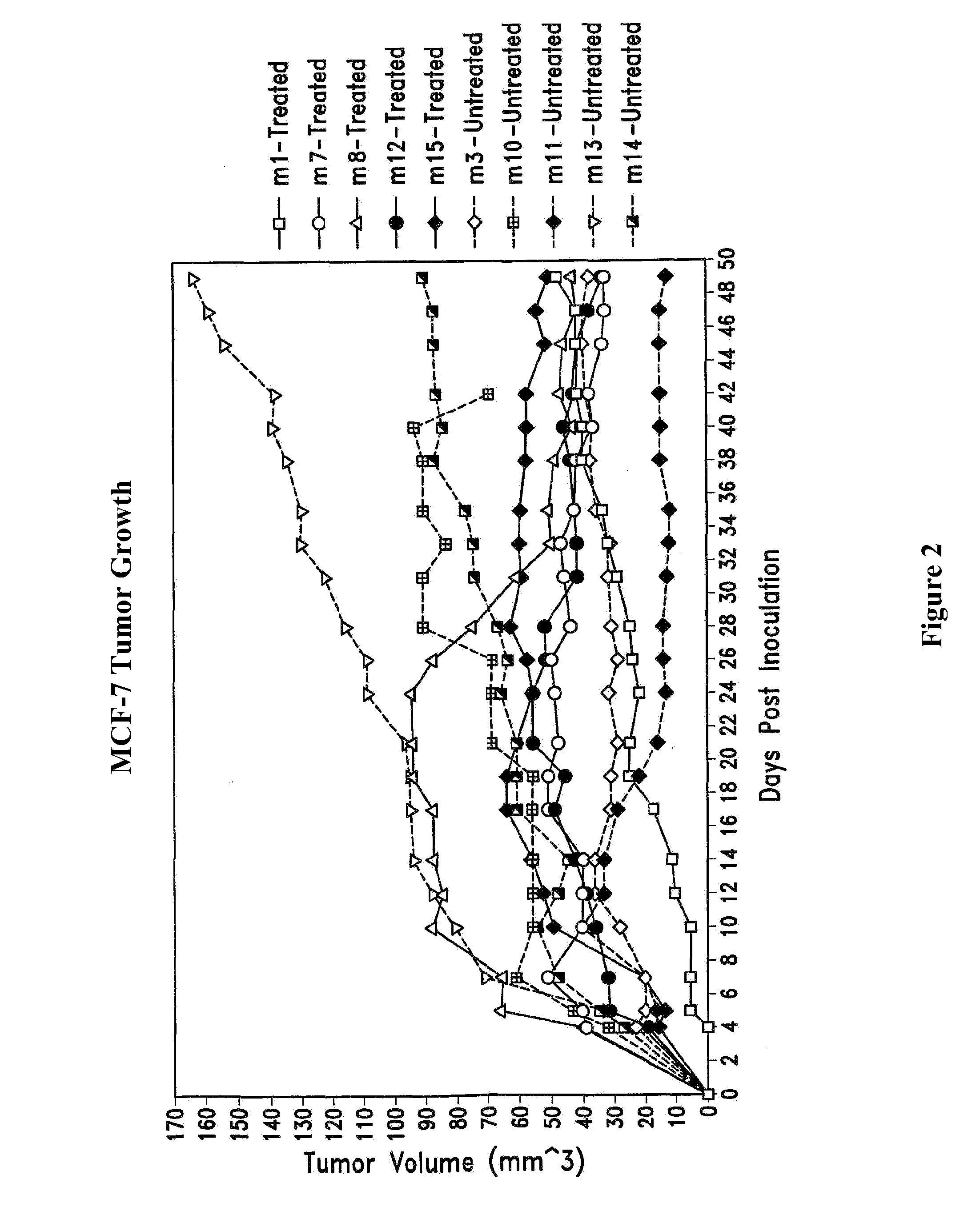
Examples
examples
Preparation of Micelles and Lipid-Encapsulated Cisplatin
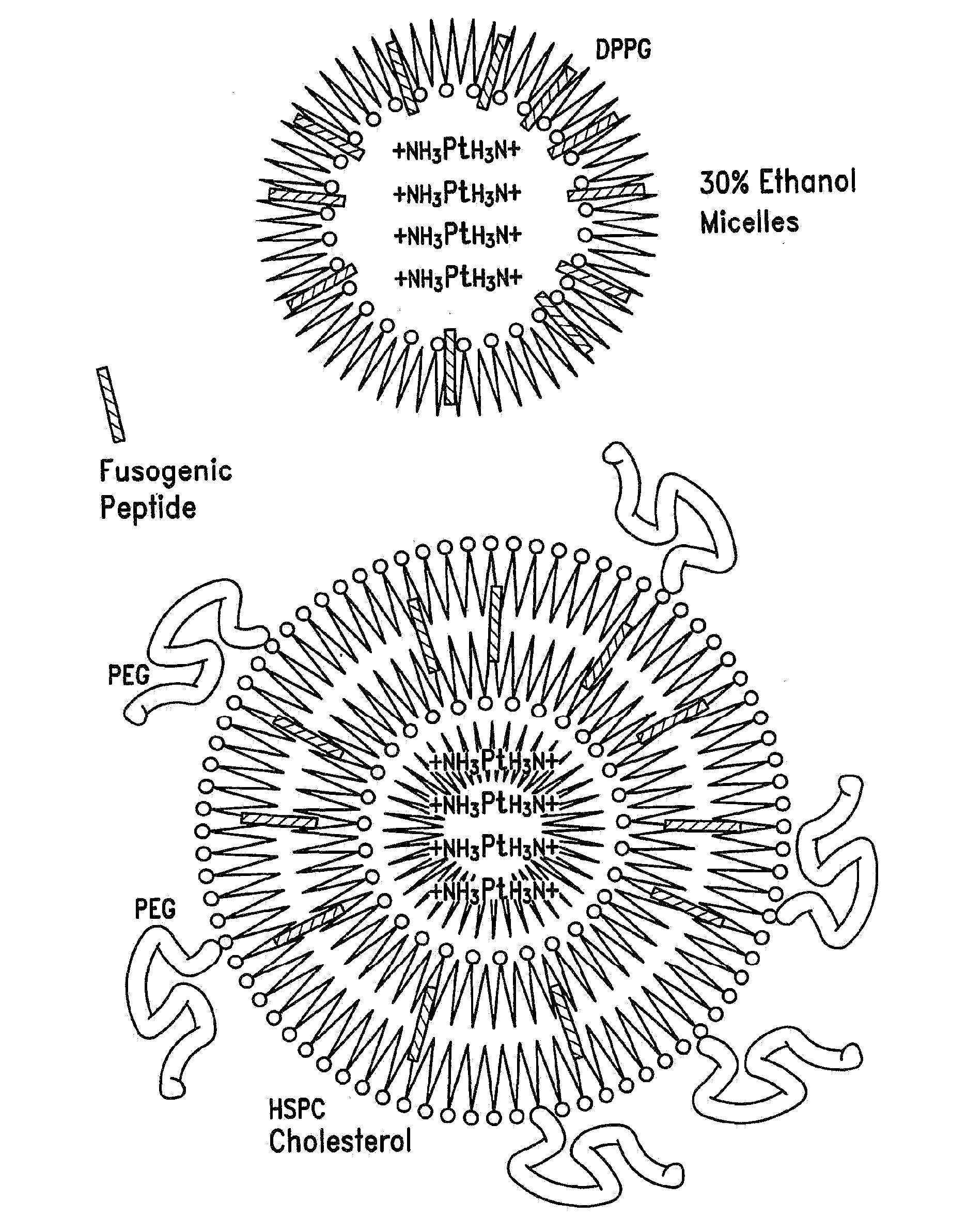
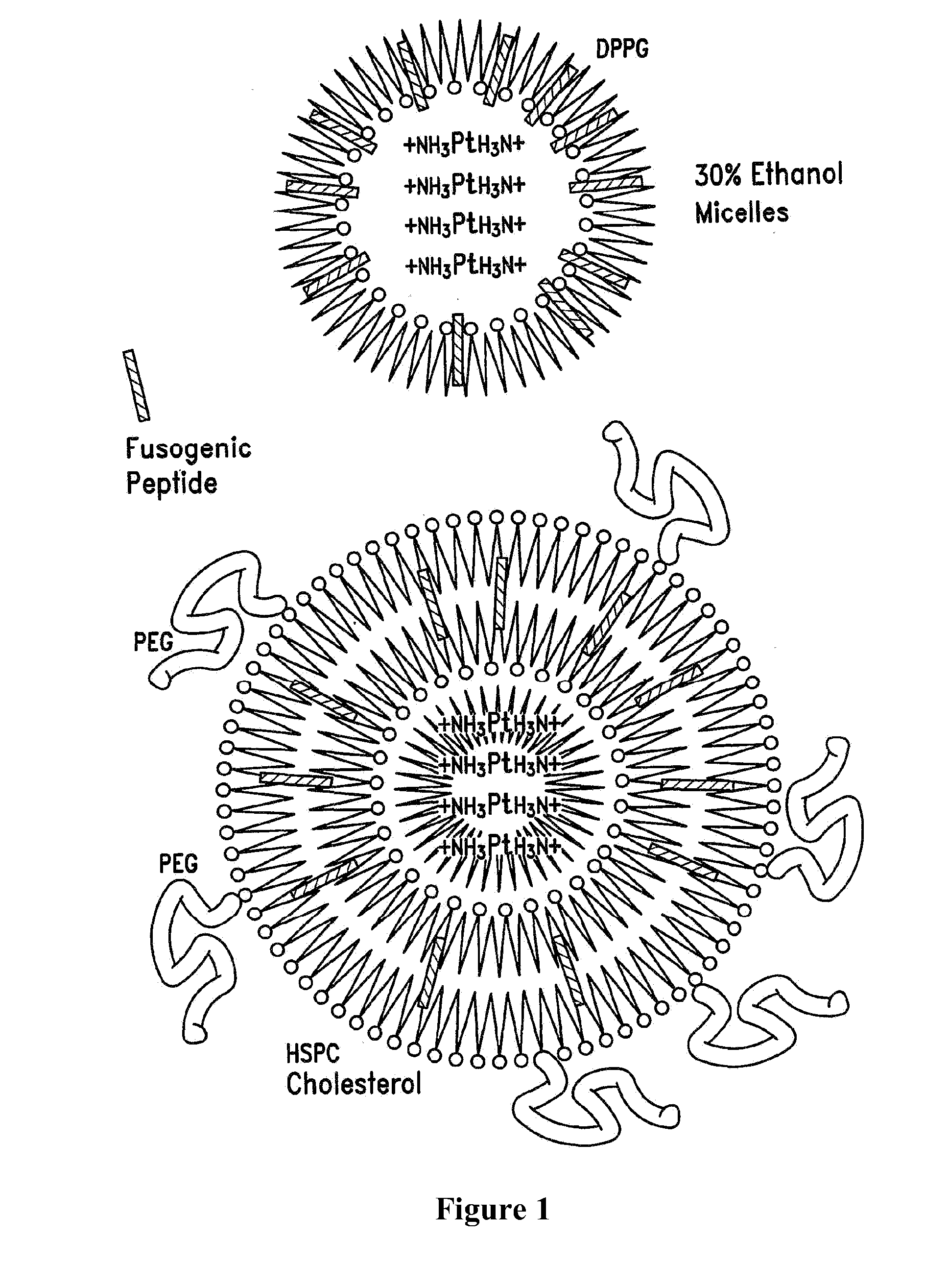
[0100]One formula for encapsulation includes the steps of: (A) mixing cisplatin (in powder or other form) with DPPG (dipalmitoyl phosphatidyl glycerol) or other negatively-charged lipid molecules at a 1:1 to 1:2 molar ratio in at least a 30% ethanol, 0.1 M Tris HCl, pH 7.5 to achieve about 5 mg / ml final cisplatin concentration. Variations in the molar ratio between cisplatin and DPPG are also of therapeutic value targeting different tissues. (B) Heating at 50 .degree. C.
[0101]During steps A and B the initial powder suspension, which tends to give a precipitate of the yellow cisplatin powder, is converted into a gel (colloidal) form; during steps A and B there is conversion of cisplatin to its aqua form (by hydrolysis of the chloride atoms and their replacement by water molecules bound to the platin) which is positively-charged and is the active form of cisplatin endowed with the antineoplastic activity; the aqua cisplatin is si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean size | aaaaa | aaaaa |
| depth | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com