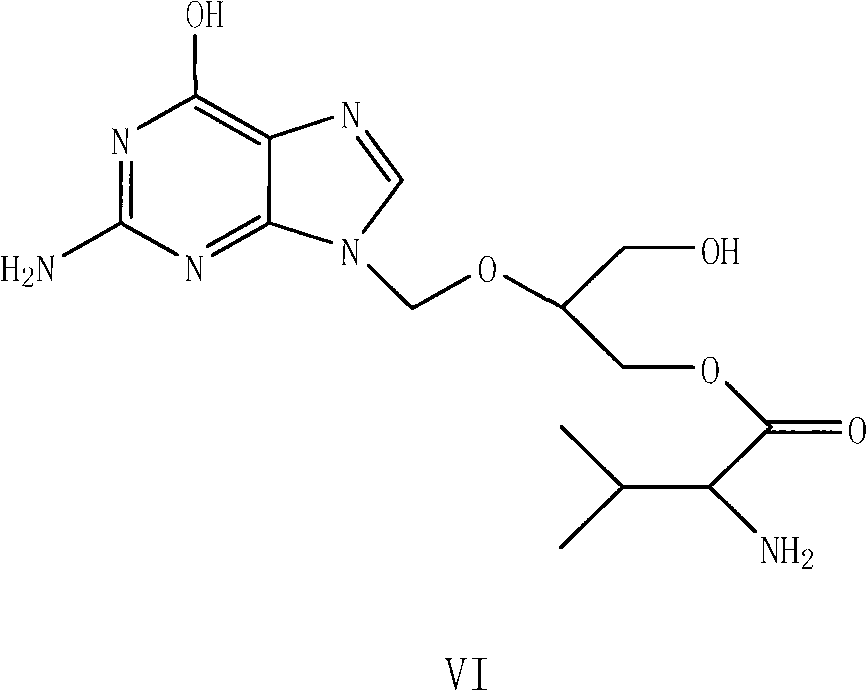
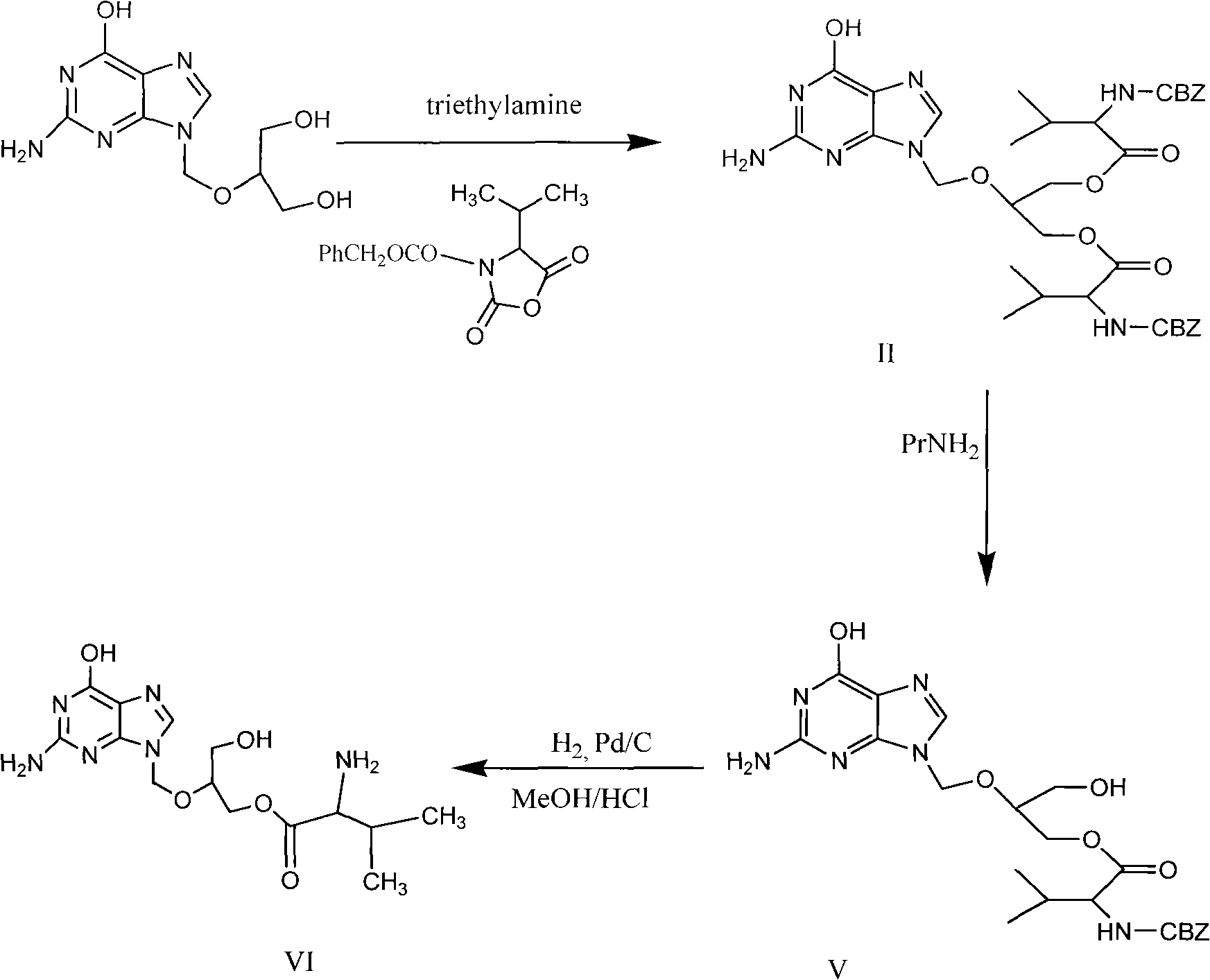
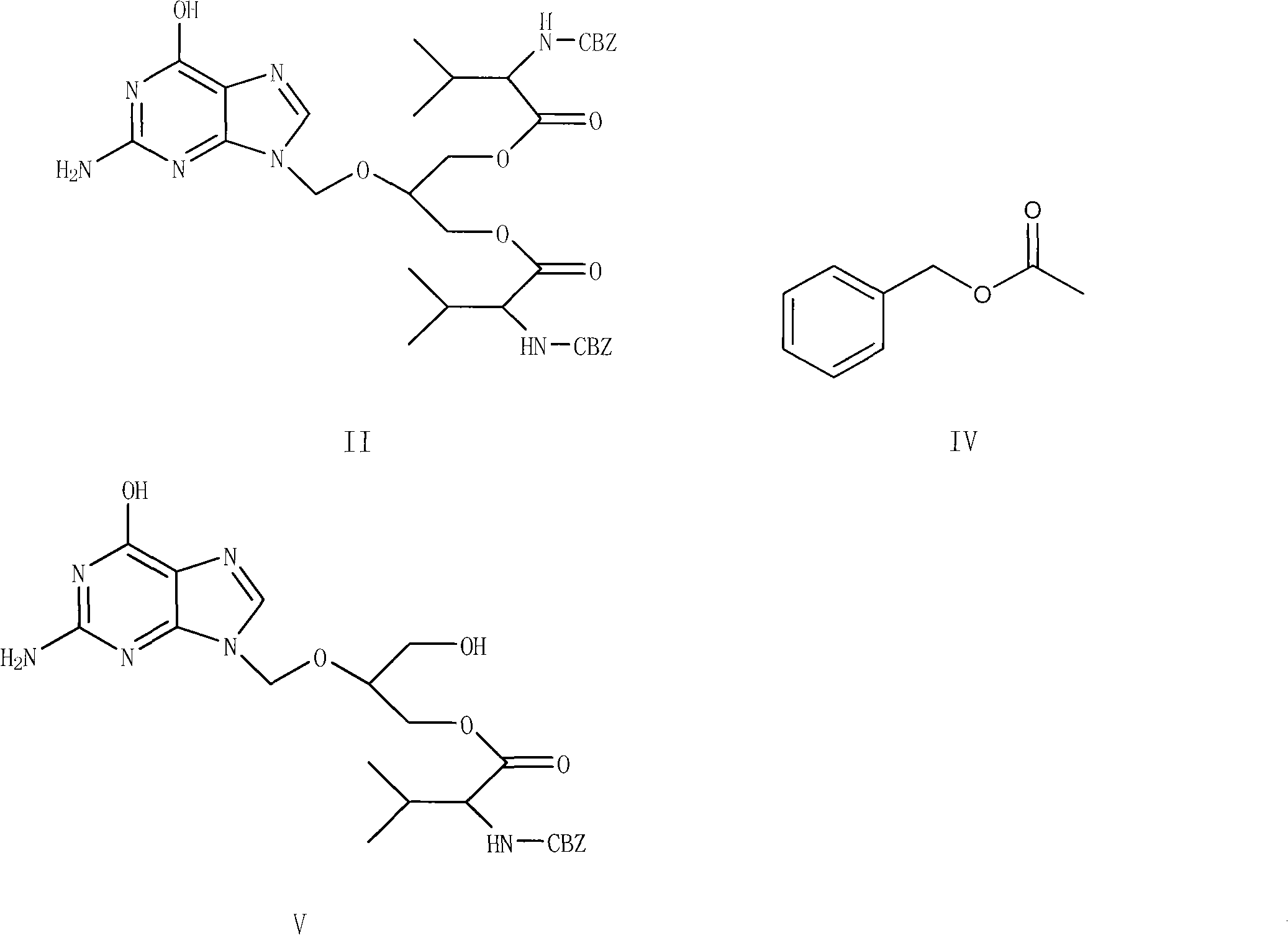
Preparation method for ganciclovir valine ester derivative
A technology of lovir valine ester and ganciclovir, which is applied in the field of preparation of ganciclovir valine ester derivatives, can solve the problems of high irritation, complicated extraction process, influence on yield and final product quality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Put 10 g of ganciclovir, 30 g of CBZ-L-valine, 1.92 g of DMAP, and 100 ml of DMF into the reaction pot, and stir for 0.5 hours. Add 25 g of DCC dissolved in 20 ml of DMF dropwise at room temperature to the above solution system, and the dropwise addition is completed within 2 hours, then stir the reaction at room temperature, and monitor the progress of the reaction with TLC. The mixed solution of methane and methanol is developed as a developer, and basically no ganciclovir spots can be seen as the reaction end point. After the reaction was finished, filter and wash the filter cake with 20ml DMF. Combine the washing liquid and filtrate, concentrate in vacuo at no higher than 100°C until almost no liquid drops, cool to room temperature, add 120ml CH 2 Cl 2 Stir to dissolve, then fully wash with 120ml of 5wt% sodium bicarbonate aqueous solution, and then wash the organic phase with 120ml×2 water (that is, wash twice with water, each time with a volume of 120ml). The s...
Embodiment 2
[0072] Other operations are the same as in Example 1, except that the solvent in step a is changed to 100 ml of acetone, the DMF of 5 times of amount in step g is changed to 8 times of acetonitrile, the temperature of water is changed to 30 ° C, other reaction conditions and The operation was the same as in Example 1, and 11.8 g of the crude product of Ganciclovir-CBZ-L-monovaline ester was obtained, the mass yield was 118%, and the content was 96.1%, and 9.6 g of the pure product was obtained after purification, and the content was 99.3%. The molar yield is 50.2%, and the mass yield is 96.0%.
Embodiment 3
[0074]Other operations are the same as in Example 1, the difference is that the solid sodium hydroxide in step a is changed to 3.3g, and the aqueous phase C in step d is adjusted to 7 with saturated sodium carbonate solution at 0°C, and 20 times in step g The amount of methyl alcohol was changed to 20 times the amount of acetone, and other reaction conditions and operations were the same as in Example 1 to obtain 12.9 g of Ganciclovir-CBZ-L-monovaline ester crude product, with a mass yield of 129% and a content of 95.8%. , 10.3g of pure product was obtained after purification, with a content of 99.25%, a molar yield of 53.8%, and a mass yield of 103.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com