Patents
Literature
77results about How to "Method route short" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
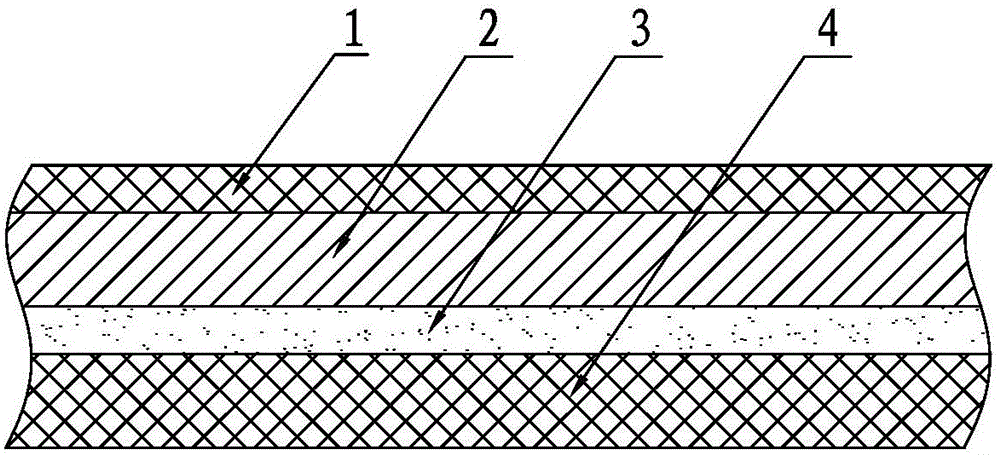
Environment-friendly breathable TPU (Thermoplastic polyurethanes) synthetic leather and preparation method thereof
InactiveCN105966027AReduce pollutionMethod route shortLamination ancillary operationsSynthetic resin layered productsPolyurethane adhesiveAdditive ingredient
The invention discloses an environment-friendly breathable TPU synthetic leather and a preparation method thereof. The environment-friendly breathable TPU synthetic leather comprises a decorative surface layer, a TPU surface layer, an adhesive layer and a base cloth layer. The decorative surface layer is coated on The treatment agent on the upper surface of the TPU surface layer is generated, and the TPU surface layer is combined with the base cloth layer through the adhesive layer. The components and parts by weight of the TPU surface layer are: 30 to 95 parts by weight of TPU particles; 3 to 95 parts by weight of the flame retardant 25 parts by weight; 2-15 parts by weight of pigment; 0-5 parts by weight of light stabilizer; 0-5 parts by weight of anti-scratch agent; 0-20 parts by weight of filler. The adhesive is a water-based polyurethane adhesive; the surface treatment agent is a water-based polyurethane surface treatment agent. Adopting the preparation method of extrusion casting, shaping first, then compounding, and negative pressure embossing, the products are environmentally friendly, non-toxic, low-odor, low-VOC, clear in structure, soft in hand, scratch-resistant, wear-resistant, hydrolysis-resistant, genuine leather Strong features.
Owner:常州三聚塑胶科技有限公司
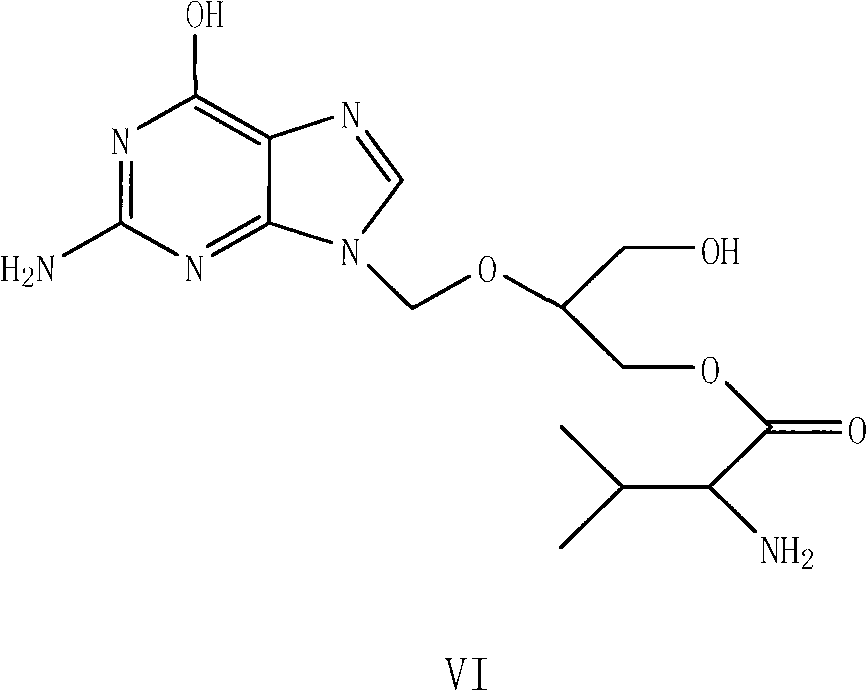
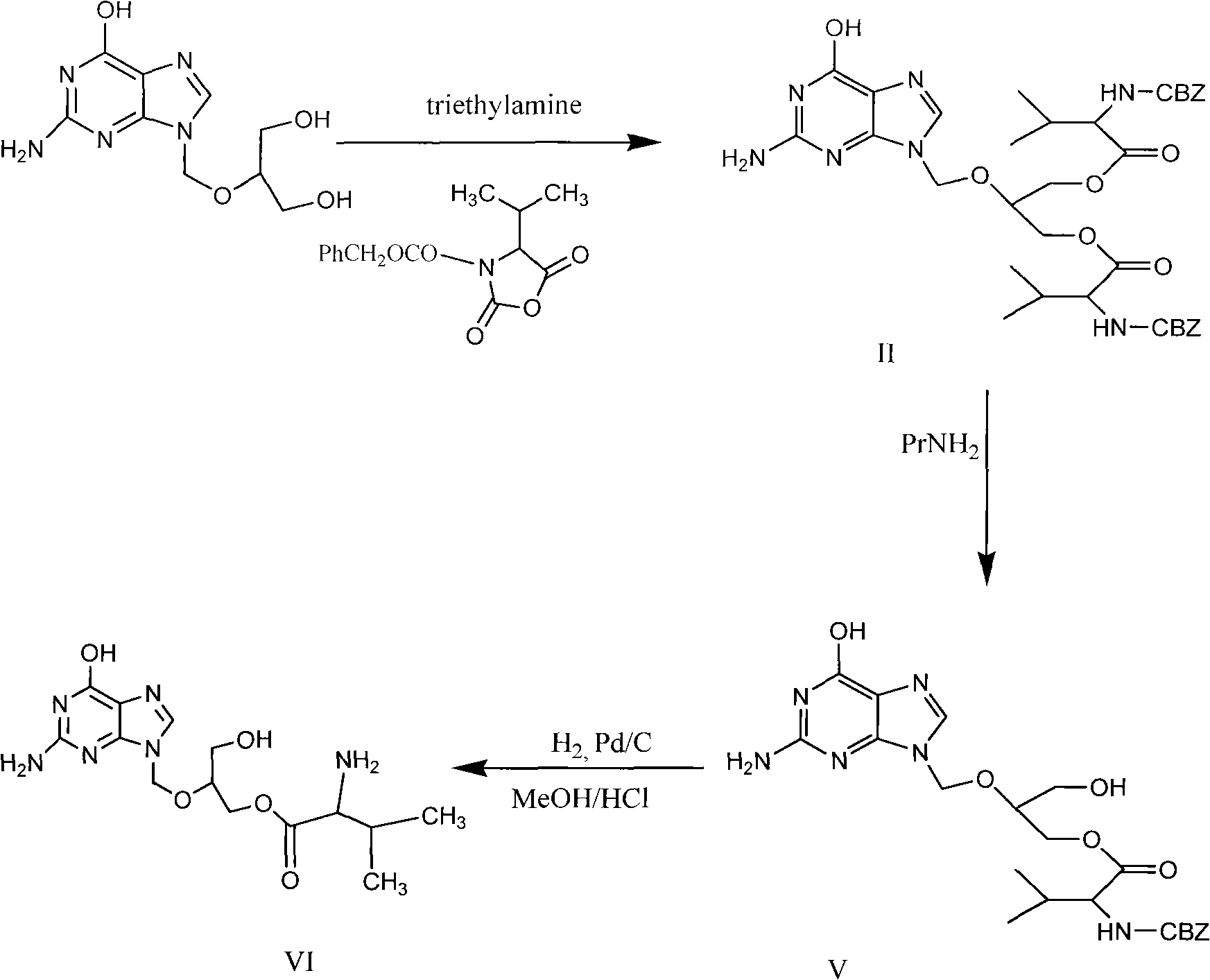
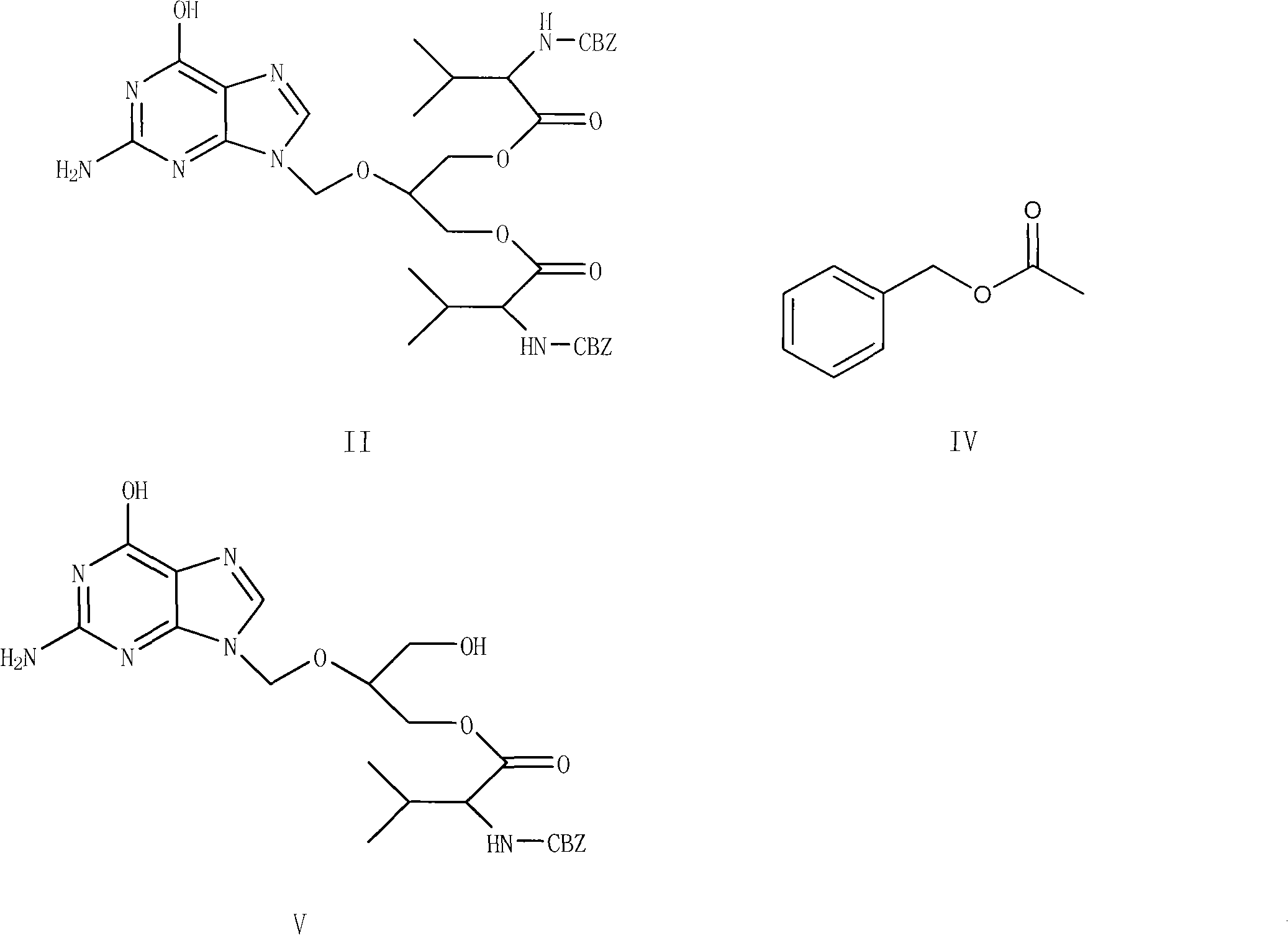
Preparation method for ganciclovir valine ester derivative
The invention discloses a preparation method for a ganciclovir valine ester derivative. The method comprises the following steps of: dissolving ganciclovir-CBZ-L-dual-valine ester shown as a formula II in a reaction solvent B at the temperature of 0 to 60 DEG C; adding a basic catalyst; keeping the temperature and reacting; tracking and monitoring the reaction solution until the reaction is finished; and obtaining a dissolving ganciclovir-CBZ-L-single-valine ester pure product by operations such as extracting, leaching, purifying and the like. The method has the advantages that: the route is simple and short; used auxiliary materials have no pollution to the environment basically; high-purity ganciclovir-CBZ-L-single-valine ester can be obtained; the molar yield of a refined product can reach over 50 percent and the content is easy to reach over 99.0 percent; complicated means such as column or column chromatography and the like are not needed; therefore, the method is very beneficialfor industrial production.
Owner:ZHEJIANG CHARIOTEER PHARMA
Medicinal composition for treating hepatitis and diabetes
InactiveCN1686141AHigh purityQuality is easy to controlOrganic active ingredientsMetabolism disorderActive componentHepatitis
A composite medicine for treating hepatitis and diabetes is prepared from twospot swertia herb through extracting its active components (oleanolic acid, etc).
Owner:昆明生物谷医药研究院有限公司
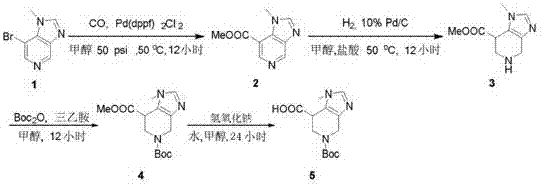
Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate
ActiveCN111620869AReasonable reaction process designMethod route shortOrganic chemistryBulk chemical productionPalladium on carbonNonane
The invention relates to a synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate, and mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following seven steps: 1, reacting a compound 1 with ethyl malonate added into a solvent ethanol to obtain a compound 2; 2, reacting the compound 2 with lithium borohydridein tetrahydrofuran to obtain a compound 3; 3, reacting the compound 3 with p-toluenesulfonyl chloride in dichloromethane to obtain a compound 4; 4, adding cesium carbonate into the compound 4 in acetonitrile serving as a solvent for cyclization to obtain a compound 5; 5, adding magnesium chips into the compound 5 in methanol serving as a solvent for reduction to obtain a compound 6, 6, reacting the compound 6 with Boc anhydride in dichloromethane to obtain a compound 7, and 7, reacting the compound 7 with palladium on carbon in methanol to obtain a final compound 8.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate
InactiveCN107383038AReasonable reaction process designShort routeOrganic chemistryChemistryTert-Butyl formate
The invention relates to a synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate, and mainly solves the technical problem that no industrial method suitable for industrial synthesis exists in the prior art. The synthesis method comprises three steps that 1, a compound 1, a compound 2 and TMEDA react in a solvent of tetrahydrofuran to obtain a compound 3; 2, the compound 3 is subjected to intramolecularly ring closing to obtain a compound 4 under the gathering effects of chlorine tosylate by using n-butyllithium as the alkali; 3, the compound 4 and ozone react to obtain a final compound 5. A reaction formula is shown in the specification.
Owner:SHANGHAI STA PHARMA R&D CO LTD
Recovery method of fiber reinforced composite material
ActiveCN111333905ARealize full resource recyclingLow reaction temperatureSulfur compoundsCarbon preparation/purificationEconomic benefitsFiber-reinforced composite
The invention relates to a recovery method of a fiber reinforced composite material. The method comprises: (1), mixing a fiber reinforced composite material and acid, performing heating, and carryingout solid-liquid separation to obtain a modified fiber and a filtrate; and (2), carbonizing the filtrate obtained in the step (1) to obtain a carbon material. Resin of the fiber composite material isdissolved through microwave-enhanced acid and the fiber surface is subjected to chemical oxidation treatment, so that the resin material is dissolved in a sulfuric acid solution, and thus the fiber and the resin material are separated to obtain the fiber material. According to the method, full resource recycling of the fibers and the resin material is achieved through microwaves under the action of biomass or the catalyst, and the recycling energy consumption is greatly reduced through the low reaction temperature; and the method is short in route, easy to operate, low in energy consumption and wide in application range and has great economic benefits and environmental benefits.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester
InactiveCN105294442AMethod route shortHigh yieldPreparation from carboxylic acid halidesMeth-Combinatorial chemistry
The invention relates to a preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester, and mainly solves the technical problem that a method suitable for industrial synthesis does not exist at present. The preparation method comprises the following six steps: firstly, reacting a compound (1) and bromoform under an alkaline condition to obtain a compound (2); secondly, reacting the compound (2) and lithium methide to obtain a compound (3); thirdly, illuminating the compound (3) and diacetyl by a high-pressure mercury lamp to obtain a compound (4); fourthly, treating with sodium hypochlorite to obtain a compound (5); fifthly, reacting the compound (5) and thionyl chloride to obtain a compound (6); and finally, treating the compound (6) under the effect of methanol to obtain a compound (7) which is a final product. A reaction formula is as shown in the specification, and the acquired bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester is a useful midbody or product for synthesis of many drugs.
Owner:SHANGHAI STA PHARMA R&D CO LTD +3
Synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid
ActiveCN105418620AReduce pollutionMethod route shortOrganic chemistryFuranTert-Butyloxycarbonyl protecting group
The present incention relates to a synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid, and mainly solves the technical problem of no synthesis method suitable for industrialization at present. The synthesis method comprises six steps: first esterifying a compound 1 to obtain a compound 2; then performing iodination to produce a compound 3; acetylating the compound 3 to obtain a compound 4; then performing coupling to obtain a compound 5; performing cyclization to obtain a compound 6; performing hydrogenation to obtain a compound 7, then reacting with Boc anhydride to obtain a compound 8; and performing hydrogenation to obtain a final compound 9. The reaction formula is shown in the description.
Owner:WUXI APPTEC (TIANJIN) CO LTD
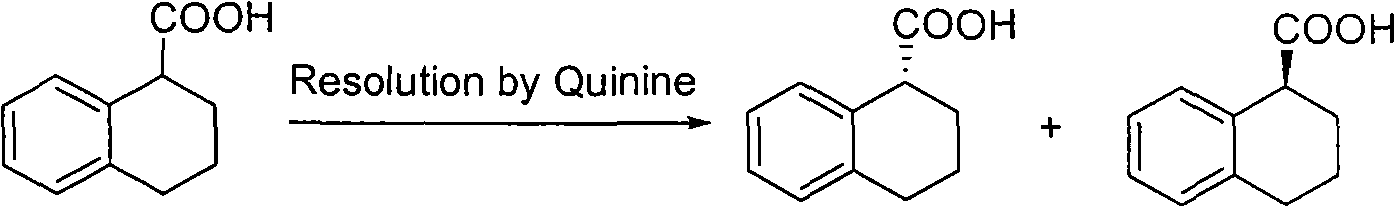
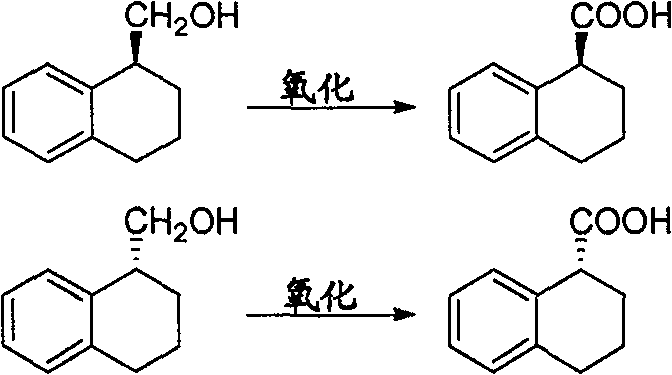
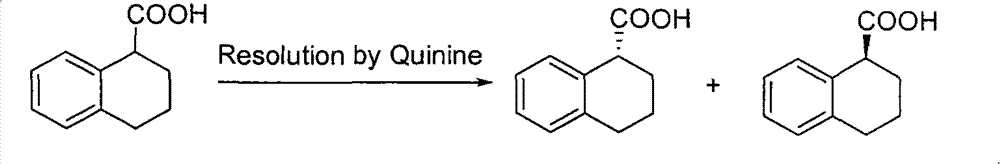
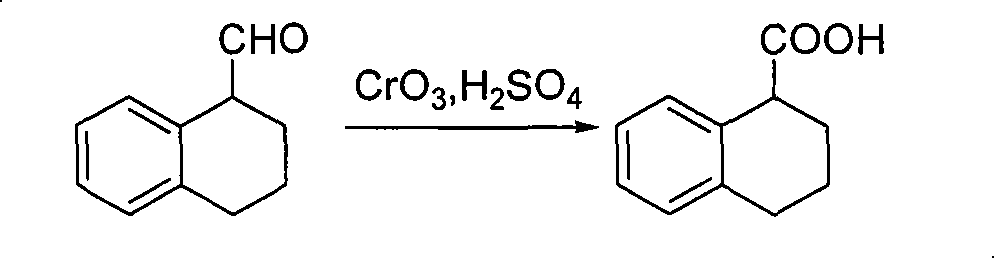
Method for preparing chiral 1, 2, 3, 4-tetrahydro-1-naphthoic acid
ActiveCN101591241ALow costMild method conditionsCarboxylic preparation by oxidationCarboxylic acidMedicinal chemistry
The invention provides a method for preparing chiral 1, 2, 3, 4-tetrahydro-1-naphthoic acid. The method takes the chiral 1, 2, 3, 4-tetrahydro-1-napthyl methanol as the raw material, and the raw material is oxidized under the existence of oxidant to obtain the carboxylic acid with a corresponding configuration. The method is simple in circuit, simple and convenient to operate, high in yield, and suitable for industrial production.
Owner:国药集团工业有限公司 +1
Synthesis method of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester
ActiveCN107383026AReasonable reaction process designEasy to getOrganic chemistryTriethylphosphiteSynthesis methods
The invention relates to a synthesis method of a compound of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester, and mainly solves the technical problem that no industrial method suitable for industrial synthesis exists in the prior art. A compound 1 and triethyl-phosphite are used as raw materials for synthesizing and obtaining the final compound through eight steps. A reaction formula is shown in the specification.
Owner:成都药明康德新药开发有限公司
Synthesis method of ethyl-3-oxo-1-oxa-4-azaspiro[5.5]undecane-9-carboxylate
PendingCN111662245AReasonable reaction process designShort routeOrganic chemistryDichloromethaneCarboxylate
The invention relates to a synthesis method of ethyl-3-oxo-1-oxa-4-azaspiro[5.5]undecane-9-carboxylate. The synthesis method mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following three steps: 1, adding triethylamine and chloroacetyl chloride into a solvent dichloromethane of a compound 1, and reacting to obtain a compound 2; 2, reacting the compound 2 with sodium iodide in acetone to obtain a compound 3; and 3, reacting the compound 3 with potassium tert-butoxide in tert-butyl alcohol and tetrahydrofuran to obtaina final compound 4. The reaction formula is shown in the specification.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester
InactiveCN107383033AReasonable reaction process designMethod route shortOrganic chemistry methodsFuranDiisopropyl azodicarboxylate
The invention relates to a synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester. The method mainly solves the technical problem that no proper industrial synthesis method exists in the prior art. The method comprises five steps that: 1, a compound 1 uses sodium borohydride as a reducing agent to react in an ethanol solvent to obtain a compound 2; 2, the compound 2 uses tetrahydrofuran as a solvent to obtain a compound 3 under the effects of triphenylphosphine and diisopropyl azodicarboxylate; 3, the compound 3 and the N-methoxymethyl-N-(trimethylsilyl) benzylamine use dichloromethane as a solvent to obtain a compound 4 through room temperature reduction under the action of trifluoroacetic acid; 4, the compound 4 and thionyl chloride react under the methanol backflow condition to obtain a compound 5; 5, the compound 5 uses palladium hydroxide catalysts and Boc anhydride auxiliary agents to obtain a final compound 6 through catalytic hydrogenation reaction.
Owner:上海药明康德新药开发有限公司 +4
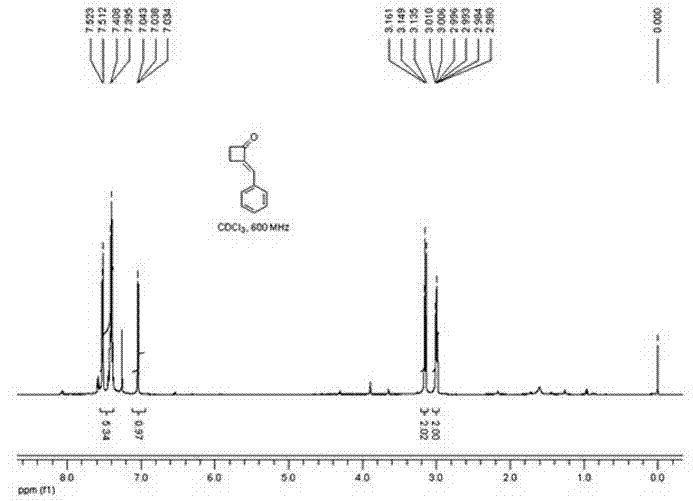
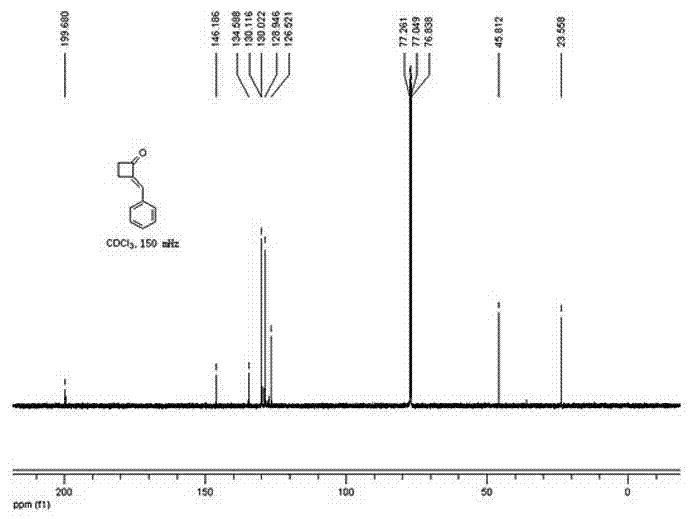
Method for preparing 2-alkylene cyclobutanone
InactiveCN102503784AShort routeImprove efficiencyOrganic compound preparationCarbonyl compound preparationMethylene radicalKetone
Provided is a method for preparing 2-alkylene cyclobutanone. The method relates to the technical field of synthesis methods of synthesized building block alkylene cyclobutanone and includes that cyclobutanone and aldehyde ketone serve as raw materials, are catalyzed by alkali and performed with aldol condensation, and directly synthesize the complex 2-alkylene cyclobutanone in one step. Compared with the traditional synthesis methods, the method for preparing 2-alkylene cyclobutanone is simple, short in line and mild in reaction conditions, the raw materials are easy to obtain, and reaction is easy to operate. By using the method for preparing 2-alkylene cyclobutanone, synthesizing cost is greatly reduced, synthesizing work load is reduced, and efficiency is improved.
Owner:YANGZHOU UNIV
4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method
InactiveCN105669687AMethod route shortHigh yieldOrganic chemistryFuranTert-Butyloxycarbonyl protecting group
The present invention relates to a 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method and mainly solves a technical problem that currently no suitable industrial synthetic method exists. The synthetic method comprises the following four steps: firstly, a compound 1 is subjected to an alkylation reaction with bromo chloroethane to obtain a compound 2; then the compound 2 is subjected to an intra-molecular cyclization reaction to obtain a compound 3; the compound 3 is subjected to a double bond hydrogenation reduction to obtain a compound 4; and the compound 4 is hydrolyzed to obtain a compound 5. The response equations are described as follows.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
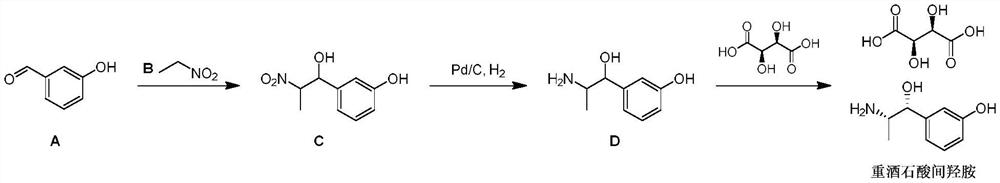
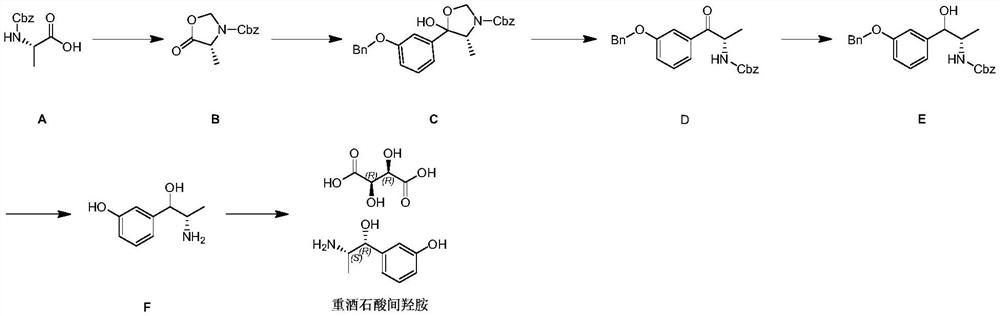
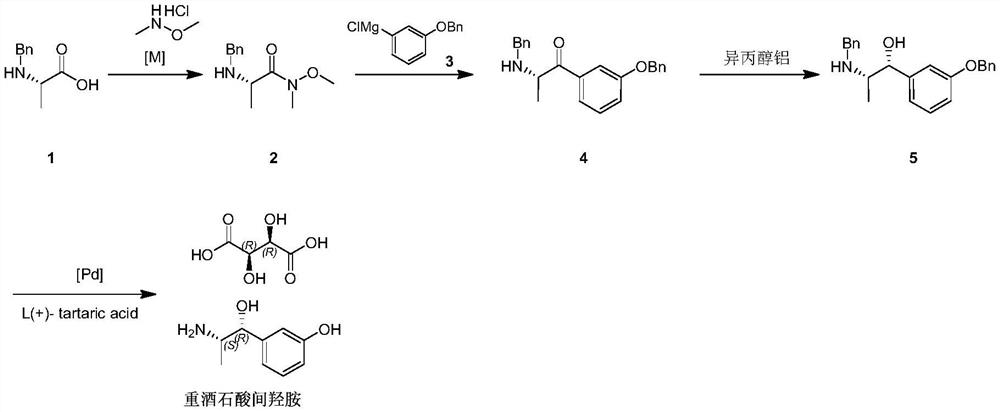
Preparation method of metaraminol bitartrate
PendingCN114478273AShort routeGood chiral selectivityOrganic compound preparationCarboxylic acid salt preparationAluminium isopropoxideMETARAMINOL BITARTRATE
The invention discloses a preparation method of metaraminol bitartrate, which sequentially comprises the following steps: reacting a compound 1 with N, O-dimethyl hydroxylamine hydrochloride to obtain a compound 2, and adding alkali in the reaction process; reacting the compound 2 obtained in the previous step with a compound 3 to obtain a compound 4; reacting the compound 4 obtained in the previous step with aluminum isopropoxide to obtain a compound 5; and adding the compound 5 obtained in the previous step into a solvent, adding a metal catalyst, introducing hydrogen for debenzylation to obtain m-hydroxylamine, then adding tartaric acid, and stirring and filtering to obtain metaraminol bitartrate. The method is short in route, aluminum isopropoxide is used for asymmetric reduction, chiral selectivity is good, isomer resolution is avoided, and cost is saved.
Owner:汉瑞药业(荆门)有限公司
Method for preparing chiral 1, 2, 3, 4-tetrahydro-1-naphthoic acid
ActiveCN101591241BLow costMild method conditionsCarboxylic preparation by oxidationHydrogenCarboxylic acid
The invention provides a method for preparing chiral 1, 2, 3, 4-tetrahydro-1-naphthoic acid. The method takes the chiral 1, 2, 3, 4-tetrahydro-1-napthyl methanol as the raw material, and the raw material is oxidized under the existence of oxidant to obtain the carboxylic acid with a corresponding configuration. The method is simple in circuit, simple and convenient to operate, high in yield, and suitable for industrial production.
Owner:国药集团工业有限公司 +1
6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method
ActiveCN105601639AMethod route shortHigh yieldOrganic chemistryTert-Butyloxycarbonyl protecting groupCarboxylic acid
The present invention relates to a 6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method. In the prior art, the suitable industrial synthesis method does not exist. A purpose of the present invention is to mainly solve the technical problem in the prior art. The synthesis method comprises six steps and specifically comprises that a compound 1 and 1-bromo-3-chloro-propane are subjected to an alkylation reaction to obtain a compound 2, an intramolecular cyclization reaction is performed to generate a compound 3, the double bond of the compound 3 is subjected to hydrogenation reduction to obtain a compound 4, and the compound 4 is subjected to hydrolysis to obtain a compound. The reaction formula is defined in the specification.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
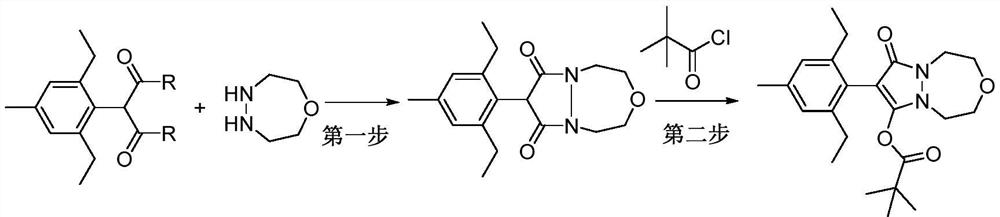
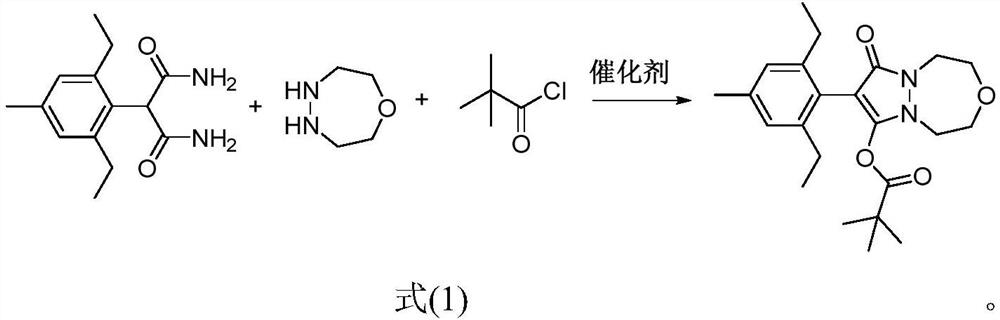
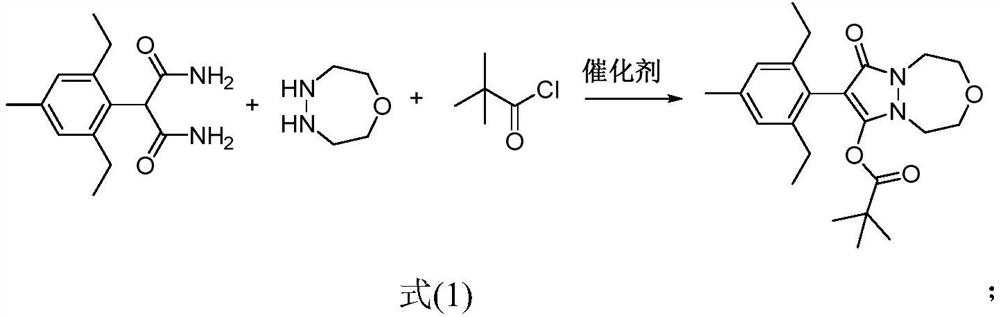
A method for preparing pinoxaden by "one pot method"
ActiveCN112028906BImprove conversion rateMethod route shortOrganic chemistryPtru catalystProcess engineering
The invention discloses a method for preparing pinoxaden by "one-pot cooking method". The method uses 2-(2,6-diethyl-4-methylphenyl) malonamide, 1-oxygen-4,5 ‑Diazepane and pivaloyl chloride are used as raw materials, and under the action of a catalyst, pinoxaden is prepared in one step through the "one-pot method". The general reaction formula is as shown in formula (1). The method of the invention has short route, simple operation and high conversion rate, can effectively reduce the amount of three wastes and online reaction time, and is very suitable for industrial production.
Owner:SOUTHEAST UNIV
Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate
The invention relates to a synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate, and mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following four steps: 1, reacting a compound 1 with ethyl bromoacetate to obtain a compound 2; 2, adding raney nickel into the compound 2 so as to obtain a compound3 through hydrogenation reduction; 3, cyclizing the compound 3 in ethanol by using sodium ethoxide to obtain a compound 4; and 4, reducing amide of the compound 4 in tetrahydrofuran by using a boranedimethyl sulfide complex to obtain a compound 5, with the reaction formula shown in the descriptions in the invention.
Owner:成都药明康德新药开发有限公司
Synthesis method of (3aS, 9bR)-tert-butyl-3,3 a, 4,5-tetrahydro-pyrrolo [3,4-c] quinolin-2 (9bH) ester
InactiveCN103073547AMethod route shortHigh yieldOrganic chemistryAddition reactionTrans esterification
The invention relates to a synthesis method of a (3aS, 9bR)-tert-butyl-3,3 a, 4,5-tetrahydro-pyrrolo [3,4-c] quinolin-2 (9bH) ester, and mainly solves the technical problem that an appropriate industrial synthesis method does not exist at present. The method comprises six steps that: a compound 1 and methyl acrylate are subjected to a Michael addition reaction to obtain a compound 2, and then a compound 3 is obtained by a 3 +2 reaction; the nitro group of the compound 3 is subjected to hydrogenation reduction, and intramolecular ammonia is subjected to transesterification to obtain a compound 4; and the amide of the compound 4 is reduced with lithium aluminum hydride to obtain a compound 5, and the compound 5 is hydrogenated to remove a benzyl group, and is reacted with Boc anhydride to obtain a final compound. The reaction formula is as follows.
Owner:上海药明康德新药开发有限公司 +2
Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid
InactiveCN110183442AReasonable reaction process designMethod route shortOrganic chemistryN dimethylformamideCarboxylic acid
The invention relates to a synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid, and mainly solves the technical problem that a current method is not suitable for industrial synthesis. The method comprises the following three steps: step 1, firstly performing a reaction on a compound 1 and di-tert-butyl dicarbonate in solvent tetrahydrofuran under the action of 4-dimethylaminopyridine to obtain a compound 2; step2, performing a reaction on the compound 2 and ethyl cyanoacetate in solvent N,N-dimethylformamide under the action of cesium carbonate to obtain a compound 3;and step 3, performing a reaction on the compound 3 under the action of hydrochloric acid-ethyl acetate to obtain the 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid. The reaction formula isshown in the description.
Owner:WUXI APPTEC
5-tert-butyloxycarbonyl octahydrofuro[3,2-c]pyridine-7-carboxylic acid synthesis method
InactiveCN105503890AMethod route shortHigh yieldOrganic chemistryFuranTert-Butyloxycarbonyl protecting group
The present invention relates to a 5-tert-butyloxycarbonyl octahydrofuro[3,2-c]pyridine-7-carboxylic acid synthesis method. In the prior art, the suitable industrial synthesis method does not exist. A purpose of the present invention is mainly to solve the technical problem in the prior art. The synthesis method comprises six steps, and specifically comprises that a compound 1 and 1-bromo-2-chloroethane are subjected to an alkylation reaction to obtain a compound 2, an intramolecular ring closure reaction is performed to generate a compound 3, the double bond of the compound 3 is subjected to hydrogenation reducing to obtain a compound 4, and the compound 4 is subjected to hydrolysis to obtain a compound 5. The reaction formula is defined in the specification.
Owner:WUXI APPTEC (TIANJIN) CO LTD
Synthetic method of 5-(boc t-butoxycarbonyl)-1-methyl-imidazopyridine-7-carboxylic acid
InactiveCN107188891AReasonable reaction process designMethod route shortOrganic chemistryChemical reactionCarboxylic acid
The invention relates to a synthetic method of 5-(boc t-butoxycarbonyl)-1-methyl-4,5,6,7-tetrahydro-1H-imidazo-[4,5-c]pyridine-7-carboxylic acid, and mainly aims to solve the technical problem that a method suitable for industrial synthesis is unavailable at present. The synthetic method comprises the four steps: I, performing a carbonyl inserting reaction under the catalysis of palladium to obtain a compound 2; II, hydrogenating the compound 2 under the catalysis of strong hydrochloric acid to obtain a compound 3; III, reacting the compound 3 with BOC acid anhydride under a triethylamine alkaline condition to obtain a compound 4; IV, hydrolyzing the compound 4 with sodium hydroxide to obtain a compound 5. The chemical reaction formula is shown in the description.
Owner:WUXI APPTEC (TIANJIN) CO LTD
Resourceful treatment method of organic medical wastes
ActiveCN108910858ARealize recycling of resourcesEmission reductionCarbon preparation/purificationAmmonia compoundsHigh concentrationChemicals poison
The invention discloses a resourceful treatment method of organic medical wastes, belonging to the field of resourceful treatment of hazardous wastes. The method comprises the following steps: (1) mixing the organic medical wastes with sulfuric acid and a catalyst, and then heating for carrying out a reaction so as to generate a mixture; (2) cooling the mixture obtained in the step (1) to the roomtemperature, adding a solvent into the mixture, stirring uniformly, washing and drying to obtain a carbon material and dilute acid. The resourceful treatment method uses the organic medical wastes and the high-concentration organic waste sulfuric acid as raw materials, and an acid-heating method is adopted to destroy the life structures of the residual pathogenic microorganisms and chemical poisons in the medical wastes so as to enable the residual pathogenic microorganisms and the chemical poisons lose activity and toxicity, so that the organic matters in the organic medical wastes and the acid in the organic waste acid are recycled; not only is carbon emission significantly reduced, but the obtained carbon material and dilute acid also have a great application value. The method providedby the invention is short in route, simple to operate, low in energy consumption and wide in application range, and meets green chemical requirements.
Owner:BEIJING ZFRK ENVIRONMENT &TECH CO LTD
Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate
InactiveCN110183448AReasonable reaction process designShort routeOrganic chemistrySolventCarboxylate
The invention relates to a synthesis method of tert-butoxycarbonyl-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate, and mainly solve the technical problem that no synthesis method suitablefor industrialization exists at present. The method is divided into three steps: the first step, a compound 1 and benzylamine are reacted to obtain a compound 2 in the solvent toluene under the action of potassium carbonate; the second step, the compound 2 and allyl magnesium bromide are reacted to obtain a compound 3 in toluene; and the third step, the compound 3 and iodine are reacted to obtaina final compound 4 in the solvent acetonitrile under the action of sodium bicarbonate. The reaction formula is as shown in the specification.
Owner:WUXI APPTEC (TIANJIN) CO LTD
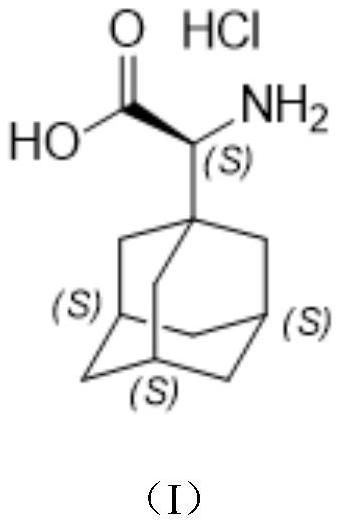
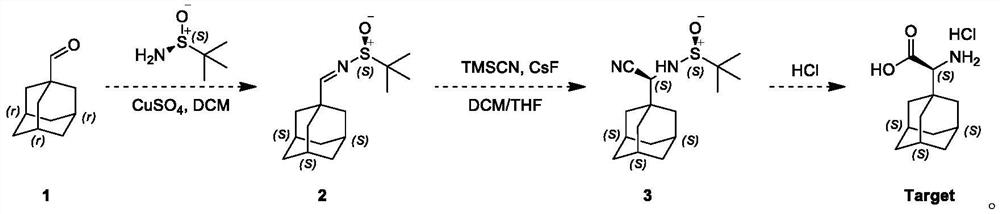
A kind of preparation method of (s)-2-(adamantan-1-yl)-2-aminoacetic acid hydrochloride
ActiveCN112679369BMethod route shortEasy to operateOrganic compound preparationAmino-carboxyl compound preparationCyanide compoundTert butyl
The invention discloses a preparation method of (S)-2-(adamantane-1-yl)-2-aminoacetic acid hydrochloride, wherein, comprising the following steps: firstly, 1-adamantanecarboxaldehyde and (S) ‑(‑)‑tert-butyl sulfinamide was dissolved in methylene chloride, then slowly adding dehydrating agent and pyridine p-toluenesulfonate, and reacted for 16 hours at a temperature of 20 ° C to obtain tert-butyl sulfinyl imide ; tert-butyl sulfenimide is dissolved in dichloromethane and tetrahydrofuran, trimethylnitrile silane and cesium fluoride are added, and the reaction is carried out at a temperature of 20 ° C for 16 hours to obtain cyanide; use a hydrolysis reagent to hydrolyze cyanide, First react for 6 hours at a temperature of 15°C, and then react at a temperature of 110°C for 16 hours to obtain the final compound, namely (S)-2-(adamantan-1-yl)-2-aminoacetic acid hydrochloride Salt, mainly to solve the technical problem that there is no suitable industrial synthesis method at present.
Owner:南通药明康德医药科技有限公司
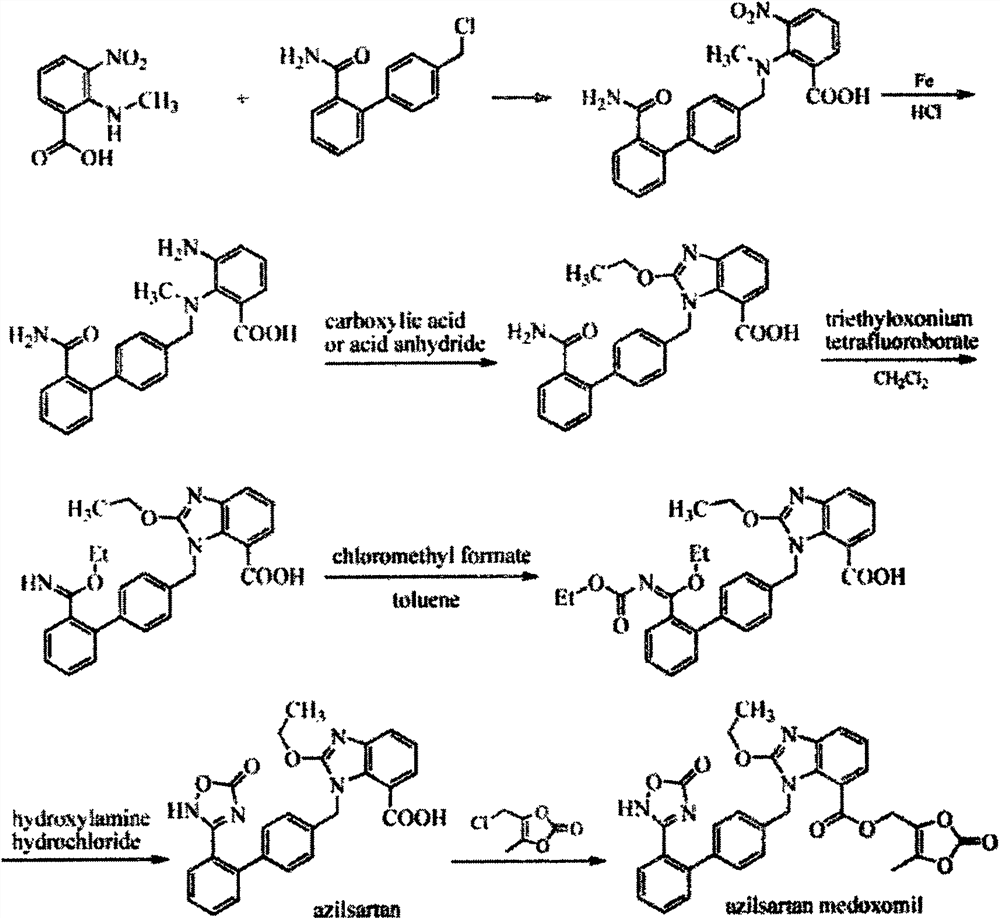
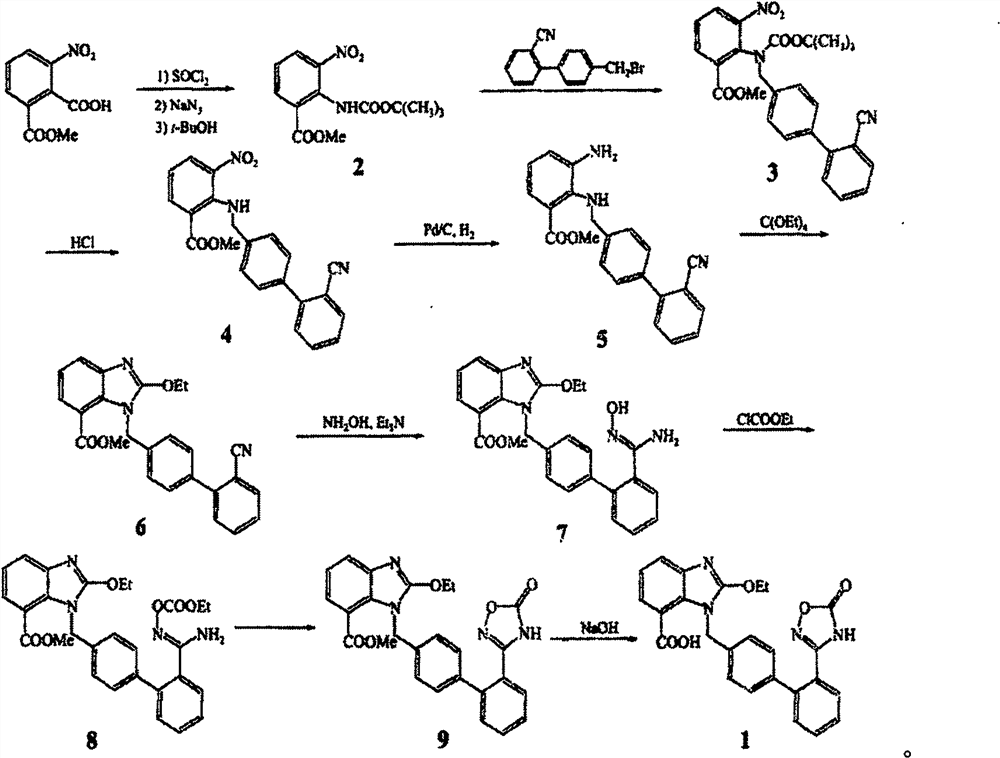
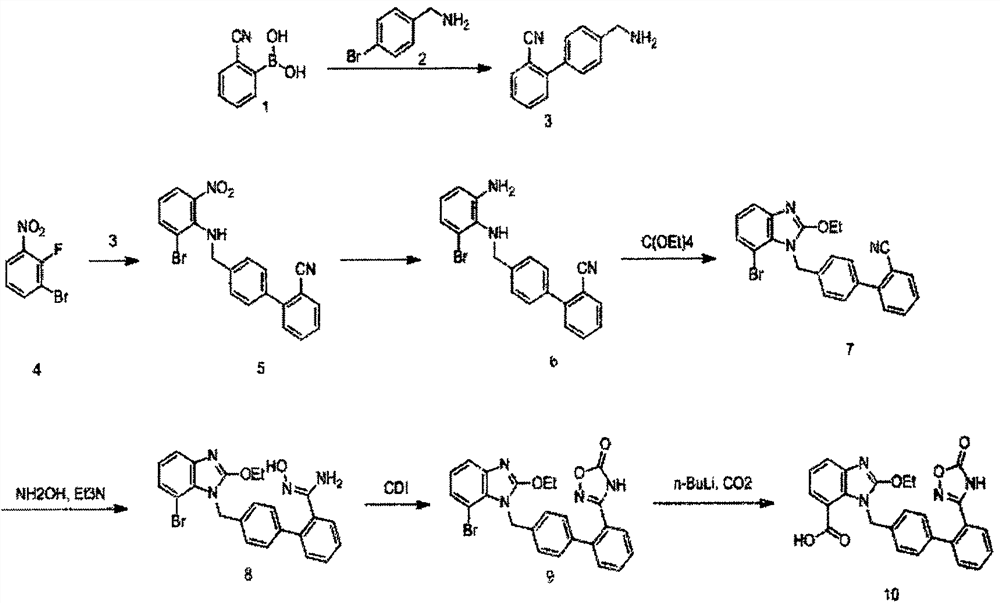
A new preparation process of azilsartan
ActiveCN107325092BRaw materials are easy to getSimple processOrganic chemistryOrthocarbonic acidHydroxylamine
The invention relates to a preparation method for azilsartan. The method is characterized by comprising the following steps: enabling 2-fluorine-3-bromine nitrobenzene to react with a midbody 3 prepared from suzuki reaction, replacing 2 fluorines and then reducing by nitro group; reacting with tetraethyl orthocarbonate, closing the ring and forming a benzimidazole ring; reacting with hydroxylamine hydrochloride and compounding a phenyl substituted oxadiazole ring under the effect of CDI; forming formic acid with carbon dioxide under the catalysis of n-butyllithium, thereby acquiring the product. The preparation method has the advantages of easily acquired raw materials, simple process, high overall yield, few side products, simplicity in post-processing and suitability for industrial production.
Owner:山东鲁宁药业有限公司
Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester
The invention relates to a preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester and mainly solves the technical problem that no appropriate industrial synthesis method exists at present. The method comprises two steps as follows: firstly, a compound 1 and a compound 2 produce a compound 3 under the action of zinc powder and ammonium chloride, then the compound 3 reacts with iodine and sodium bicarbonate in acetonitrile, a compound 4 is obtained, and the equation is shown in the specification. The compound obtained with the method is a useful intermediate for synthesis of numerous drugs or product.
Owner:上海药明康德新药开发有限公司 +2
Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane
ActiveCN106831774AReasonable reaction process designShort routeOrganic chemistry methodsTrifluoromethylPalladium on carbon
The invention relates to a synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane, aiming at mainly solving the technical problem that a suitable industrial synthesis method does not exist at present. The synthesis method is divided into six steps as follows: firstly, taking a compound 1 and oxalyl chloride to react to obtain a compound 2; then, hydrogenating by palladium on carbon to generate a compound 3; taking the compound 3 to react with BOC (Butyloxycarbonyl) acid anhydride under an alkaline condition to obtain a compound 4; taking the compound 4 and acrylonitrile to be subjected to alkylation reaction under the action of sodium ethoxide to obtain a compound 5; carrying out reduction and ring closure on the compound 5 under the action of Raney nickel to obtain a compound 6; reducing the compound 6 with borane-dimethylsulfide to obtain a final compound 7, wherein a reaction formula is shown in the description.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +2
Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid
The invention relates to a synthesis method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid, aiming at mainly solving the technical problem that a suitable industrial synthesis method does not exist at present. The synthesis method is divided into four steps as follows: firstly, taking a compound 1 to react with n-butyl lithium and benzyl chloroformate in tetrahydrofuran to obtain a compound 2; then, taking the compound 2 to react with diphenyl chlorophosphate and lithium hexamethyldisilazide to obtain a compound 3; inserting carbonyl into the compound 3 in the presence of CO under the action of palladium acetate and triphenylphosphine to obtain a compound 4; under the action of hydrogen and palladium on carbon, carrying out hydrogenation reduction again to obtain a target compound 5. A formula is shown I in the description. The compound prepared by the synthesis method is a useful immediate or product synthesized by a plurality of medicines.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka.patsnap.com/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/204216DEST_PATH_IMAGE004.png)
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka.patsnap.com/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/349392DEST_PATH_IMAGE002.png)
![Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/c2bc7891-b076-45ae-af8c-a87decde9903/388939DEST_PATH_IMAGE002.png)
![Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/c2bc7891-b076-45ae-af8c-a87decde9903/97897DEST_PATH_IMAGE002.png)
![Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester](https://images-eureka.patsnap.com/patent_img/96ca2619-778e-460a-a141-f4fa7a99aceb/2014102411696100002DEST_PATH_IMAGE001.PNG)
![Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester](https://images-eureka.patsnap.com/patent_img/96ca2619-778e-460a-a141-f4fa7a99aceb/DEST_PATH_IMAGE001.PNG)
![Synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid Synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/c3c86bdd-fb12-4204-ac96-1283f3eeaca8/DEST_PATH_IMAGE002.PNG)
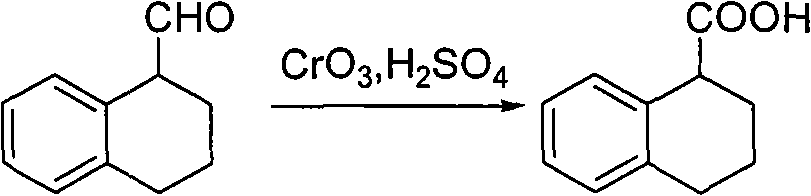
![Synthesis method of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester Synthesis method of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester](https://images-eureka.patsnap.com/patent_img/9922076c-4753-44af-97a5-80cae6f1cda4/DEST_PATH_IMAGE003.png)
![Synthesis method of ethyl-3-oxo-1-oxa-4-azaspiro[5.5]undecane-9-carboxylate Synthesis method of ethyl-3-oxo-1-oxa-4-azaspiro[5.5]undecane-9-carboxylate](https://images-eureka.patsnap.com/patent_img/660c0577-5044-4d3e-9609-85f47b5a3428/78621DEST_PATH_IMAGE004.png)
![Synthesis method of ethyl-3-oxo-1-oxa-4-azaspiro[5.5]undecane-9-carboxylate Synthesis method of ethyl-3-oxo-1-oxa-4-azaspiro[5.5]undecane-9-carboxylate](https://images-eureka.patsnap.com/patent_img/660c0577-5044-4d3e-9609-85f47b5a3428/161480DEST_PATH_IMAGE002.png)
![Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester](https://images-eureka.patsnap.com/patent_img/1b155c81-c2c1-47f6-ba38-9872cf0937bd/240257DEST_PATH_IMAGE002.png)
![Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester](https://images-eureka.patsnap.com/patent_img/1b155c81-c2c1-47f6-ba38-9872cf0937bd/577916DEST_PATH_IMAGE002.png)
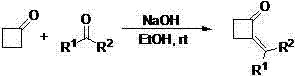
![4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/2399ecb6-2eb4-4916-b39d-5e924440f224/2014106610345100002DEST_PATH_IMAGE002.PNG)
![4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/2399ecb6-2eb4-4916-b39d-5e924440f224/2014106610345100002DEST_PATH_IMAGE004.PNG)
![4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/2399ecb6-2eb4-4916-b39d-5e924440f224/DEST_PATH_IMAGE002.PNG)
![6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method 6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method](https://images-eureka.patsnap.com/patent_img/da2a17e5-6bdd-4ee7-b53b-d70a621ade57/116401DEST_PATH_IMAGE001.PNG)
![6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method 6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method](https://images-eureka.patsnap.com/patent_img/da2a17e5-6bdd-4ee7-b53b-d70a621ade57/383175DEST_PATH_IMAGE002.PNG)
![Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate](https://images-eureka.patsnap.com/patent_img/5ba9af85-40cf-40f8-94e7-4ff1101bb4c1/243538DEST_PATH_IMAGE004.png)
![Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate Synthesis method of tert-butyl-8-oxa-3, 11-diazaspiro [5.6] dodecane-3-formate](https://images-eureka.patsnap.com/patent_img/5ba9af85-40cf-40f8-94e7-4ff1101bb4c1/700298DEST_PATH_IMAGE002.png)
![Synthesis method of (3aS, 9bR)-tert-butyl-3,3 a, 4,5-tetrahydro-pyrrolo [3,4-c] quinolin-2 (9bH) ester Synthesis method of (3aS, 9bR)-tert-butyl-3,3 a, 4,5-tetrahydro-pyrrolo [3,4-c] quinolin-2 (9bH) ester](https://images-eureka.patsnap.com/patent_img/81df6581-0f50-4852-9278-7c4667fb85f1/2013100476634100002DEST_PATH_IMAGE003.PNG)
![Synthesis method of (3aS, 9bR)-tert-butyl-3,3 a, 4,5-tetrahydro-pyrrolo [3,4-c] quinolin-2 (9bH) ester Synthesis method of (3aS, 9bR)-tert-butyl-3,3 a, 4,5-tetrahydro-pyrrolo [3,4-c] quinolin-2 (9bH) ester](https://images-eureka.patsnap.com/patent_img/81df6581-0f50-4852-9278-7c4667fb85f1/339150DEST_PATH_IMAGE002.PNG)
![Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/90380351-ece5-42d0-8697-5638b1785685/32356DEST_PATH_IMAGE003.png)
![Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/90380351-ece5-42d0-8697-5638b1785685/551434DEST_PATH_IMAGE002.png)
![5-tert-butyloxycarbonyl octahydrofuro[3,2-c]pyridine-7-carboxylic acid synthesis method 5-tert-butyloxycarbonyl octahydrofuro[3,2-c]pyridine-7-carboxylic acid synthesis method](https://images-eureka.patsnap.com/patent_img/ca26806c-c786-42e7-a71b-e0bd7998f30f/70584DEST_PATH_IMAGE002.png)
![5-tert-butyloxycarbonyl octahydrofuro[3,2-c]pyridine-7-carboxylic acid synthesis method 5-tert-butyloxycarbonyl octahydrofuro[3,2-c]pyridine-7-carboxylic acid synthesis method](https://images-eureka.patsnap.com/patent_img/ca26806c-c786-42e7-a71b-e0bd7998f30f/196026DEST_PATH_IMAGE002.png)
![Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate](https://images-eureka.patsnap.com/patent_img/0229c71d-9623-4421-9b48-ec93bcf74223/DEST_PATH_IMAGE002.png)
![Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate](https://images-eureka.patsnap.com/patent_img/0229c71d-9623-4421-9b48-ec93bcf74223/DEST_PATH_IMAGE004.png)
![Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester](https://images-eureka.patsnap.com/patent_img/10474158-e881-428a-9b6c-b41d7a7dd716/2015110068842100002DEST_PATH_IMAGE001.PNG)
![Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester](https://images-eureka.patsnap.com/patent_img/10474158-e881-428a-9b6c-b41d7a7dd716/DEST_PATH_IMAGE002.PNG)
![Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane](https://images-eureka.patsnap.com/patent_img/1b74b72f-deec-4a20-bc0c-0c6d6b6c9213/BSA0000140068570000011.png)
![Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane](https://images-eureka.patsnap.com/patent_img/1b74b72f-deec-4a20-bc0c-0c6d6b6c9213/BSA0000140068570000021.png)
![Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane](https://images-eureka.patsnap.com/patent_img/1b74b72f-deec-4a20-bc0c-0c6d6b6c9213/FSA0000140068560000011.png)
![Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid](https://images-eureka.patsnap.com/patent_img/1cb2b678-4d12-49d1-8b23-6bfc618132d5/BSA0000140068260000011.png)
![Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid](https://images-eureka.patsnap.com/patent_img/1cb2b678-4d12-49d1-8b23-6bfc618132d5/BSA0000140068260000021.png)
![Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid](https://images-eureka.patsnap.com/patent_img/1cb2b678-4d12-49d1-8b23-6bfc618132d5/FSA0000140068250000011.png)