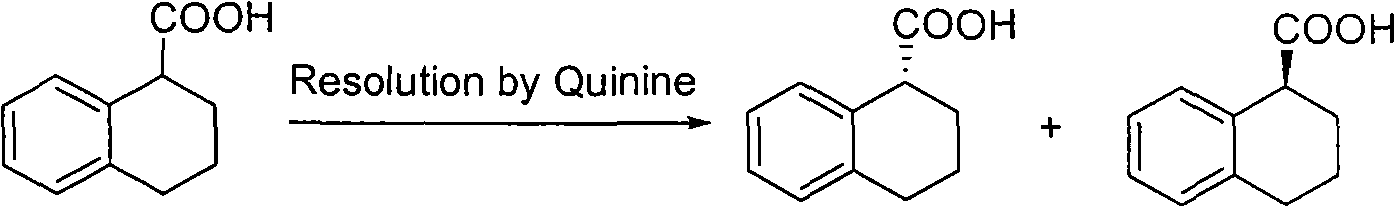
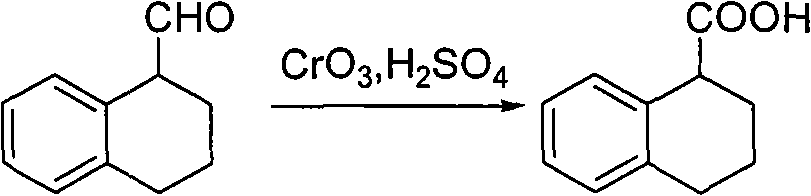Method for preparing chiral 1, 2, 3, 4-tetrahydro-1-naphthoic acid
A technology of naphthoic acid and chirality, which is applied in the field of preparing chirality, can solve problems such as serious environmental pollution of chromium trioxide oxidant, and achieve the effects of easy separation and purification, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of (S)-1,2,3,4-tetrahydro-1-naphthoic acid
[0027] Put 5.00g (S)-1,2,3,4-tetrahydro-1-naphthalenemethanol into 100ml acetone, cool to -78°C in an ice-water bath, add 20ml Jones reagent dropwise under stirring (dissolved by 5.34g chromium trioxide in 4.6ml of concentrated sulfuric acid and diluted to 20ml with water to obtain), about 1 hour to complete the dropwise reaction, heat preservation reaction for 2 hours, the reaction solution was poured into about 30ml of ice water, after fully stirring, extracted with dichloromethane (20ml×3), combined The organic layer was washed with water (20ml×3), then the organic layer was extracted with 5% aqueous sodium hydroxide solution (20ml), the sodium hydroxide layer was washed with ethyl acetate (20ml), and the pH was adjusted to 2-3 by adding concentrated HCl, and then Extracted with dichloromethane (15ml×3), combined the organic layers, dried with anhydrous magnesium sulfate, and recovered the solvent under reduced...
Embodiment 2
[0031] Preparation of (R)-1,2,3,4-tetrahydro-1-naphthoic acid
[0032] Put 5.00g (S)-1,2,3,4-tetrahydro-1-naphthalenemethanol into 50ml dichloromethane, cool to -78°C in an ice-water bath, add 20ml Jones reagent dropwise under stirring (from 5.34g trioxide Chromium was dissolved in 4.6ml of concentrated sulfuric acid and diluted with water to 20ml), and the dropwise addition was completed in about 1 hour, and the reaction was kept for 2 hours. Extracted with methane (15ml×2), combined the organic layers and washed with water (20ml×3), then extracted the organic layer with 5% aqueous sodium hydroxide solution (20ml), washed the sodium hydroxide layer with ethyl acetate (20ml) and concentrated Adjust the pH to 2-3 with HCl, then extract with dichloromethane (15ml×3), combine the organic layers, dry over anhydrous magnesium sulfate, and recover the solvent under reduced pressure to obtain 4.07g of a light yellow solid, which was recrystallized in n-hexane 3.80g of white crystals...
Embodiment 3
[0036] Preparation of (S)-1,2,3,4-tetrahydro-1-naphthoic acid
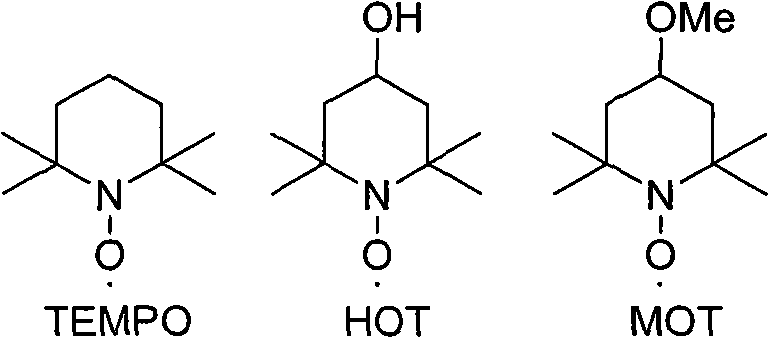
[0037] Put 4.00g (S)-1,2,3,4-tetrahydro-1-naphthalenemethanol into 200ml acetone, 60ml carbonate buffer solution, cool to -20℃ in an ice-water bath, add 0.49g sodium bromide, 0.08 g 2,2,6,6-tetramethyl nitroxide free radical piperidine (TEMPO), add 3.50g trichloroisocyanuric acid (TCCA) under stirring, finish adding in about 30 minutes, keep warm for 1.5 hours, add 12ml Isopropanol, filter, evaporate about 1 / 2 of the solvent under reduced pressure, add 60ml of saturated aqueous sodium carbonate solution, then extract with ethyl acetate (50ml), separate the water layer and add concentrated hydrochloric acid to adjust the pH value to 2~3, and use ethyl acetate Extract the ester (50ml×2), combine the organic phases and extract with 5% NaOH aqueous solution, add concentrated hydrochloric acid to the aqueous layer to adjust the pH value to 2~3, extract with dichloromethane (15ml×2), combine the organic phases, anhydrou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com