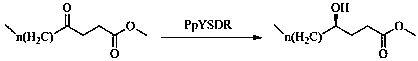Patents
Literature
121 results about "Chiral selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Beta-cyclodextrin derivative, preparation thereof and use as chiral selector
InactiveCN101372516AWide variety of sourcesMild reaction conditionsOther chemical processesHydrogenChiral selectivity
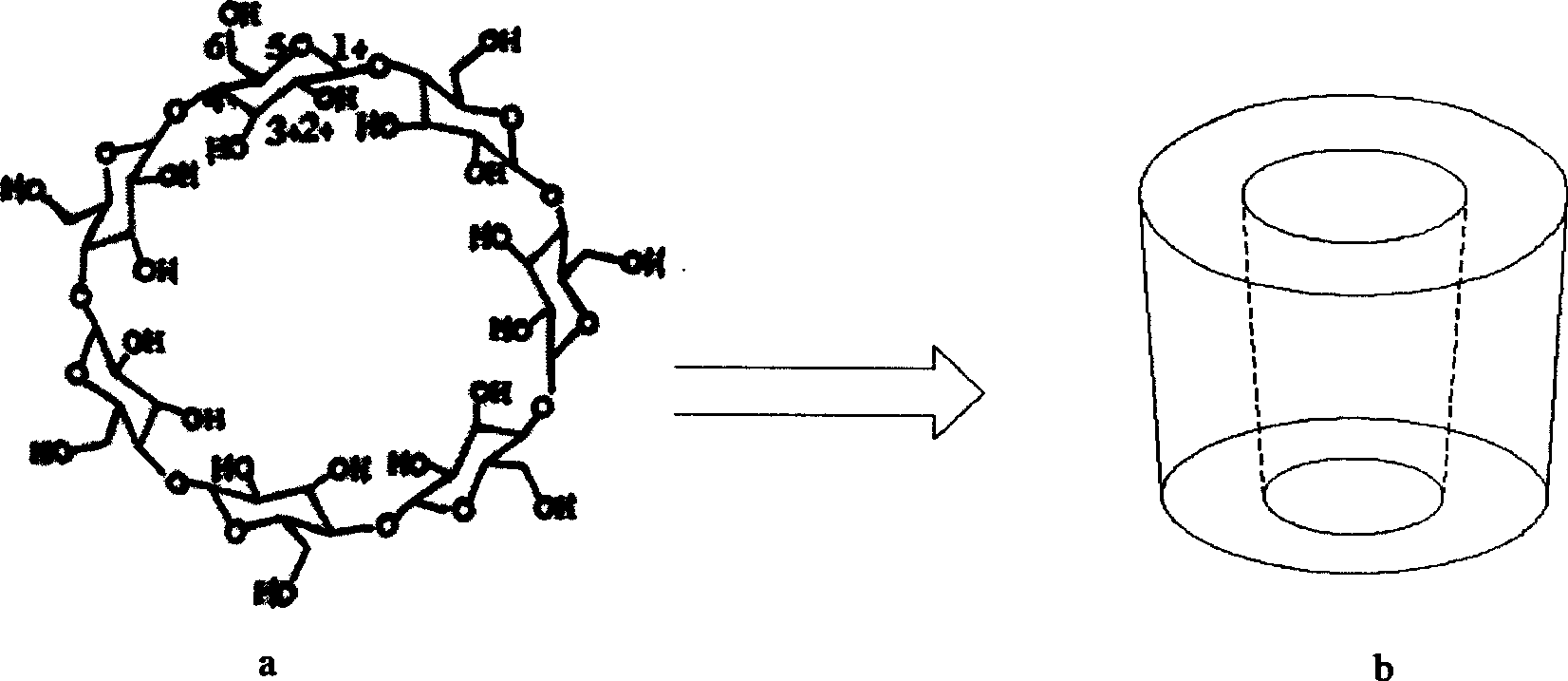
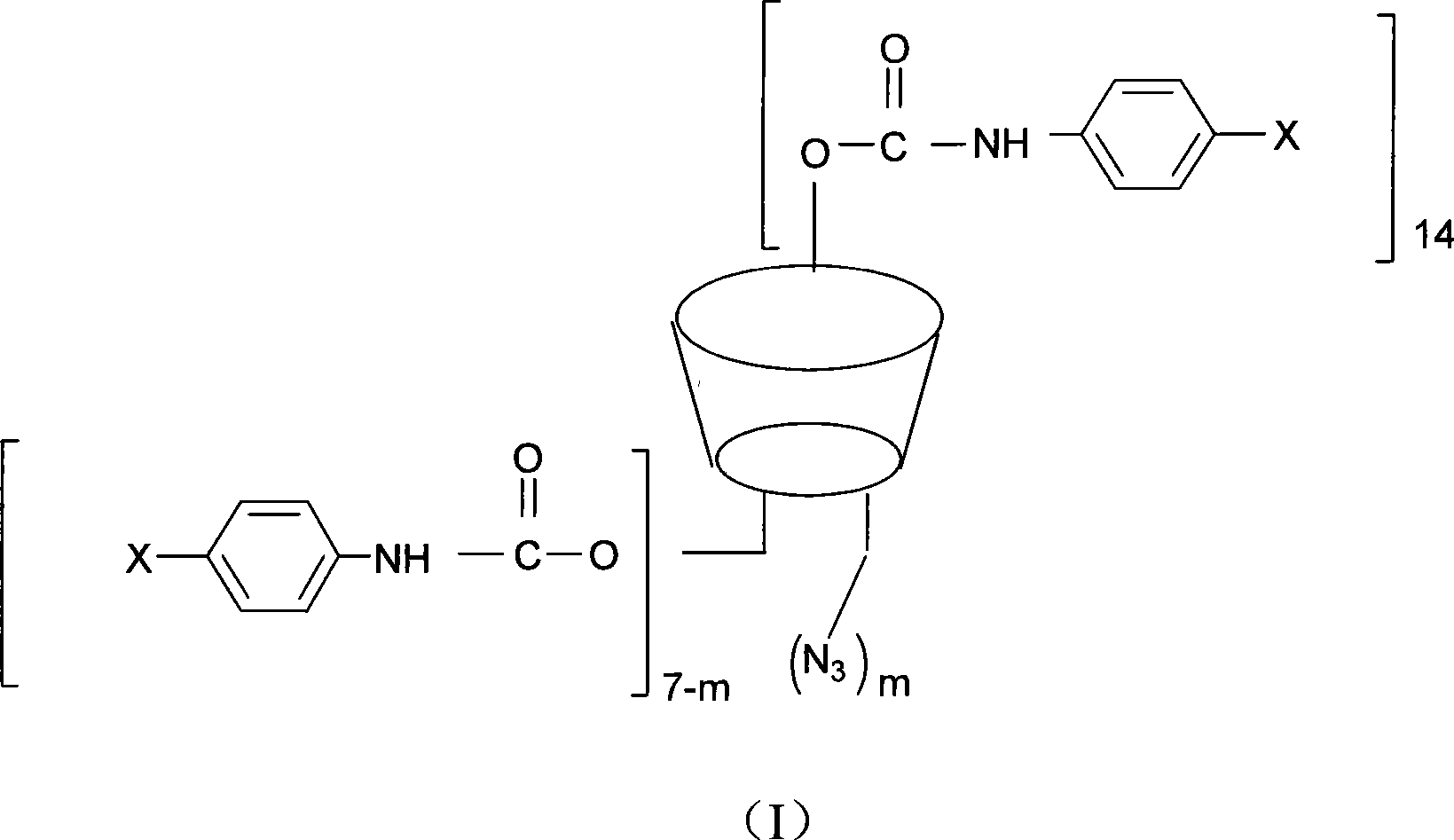
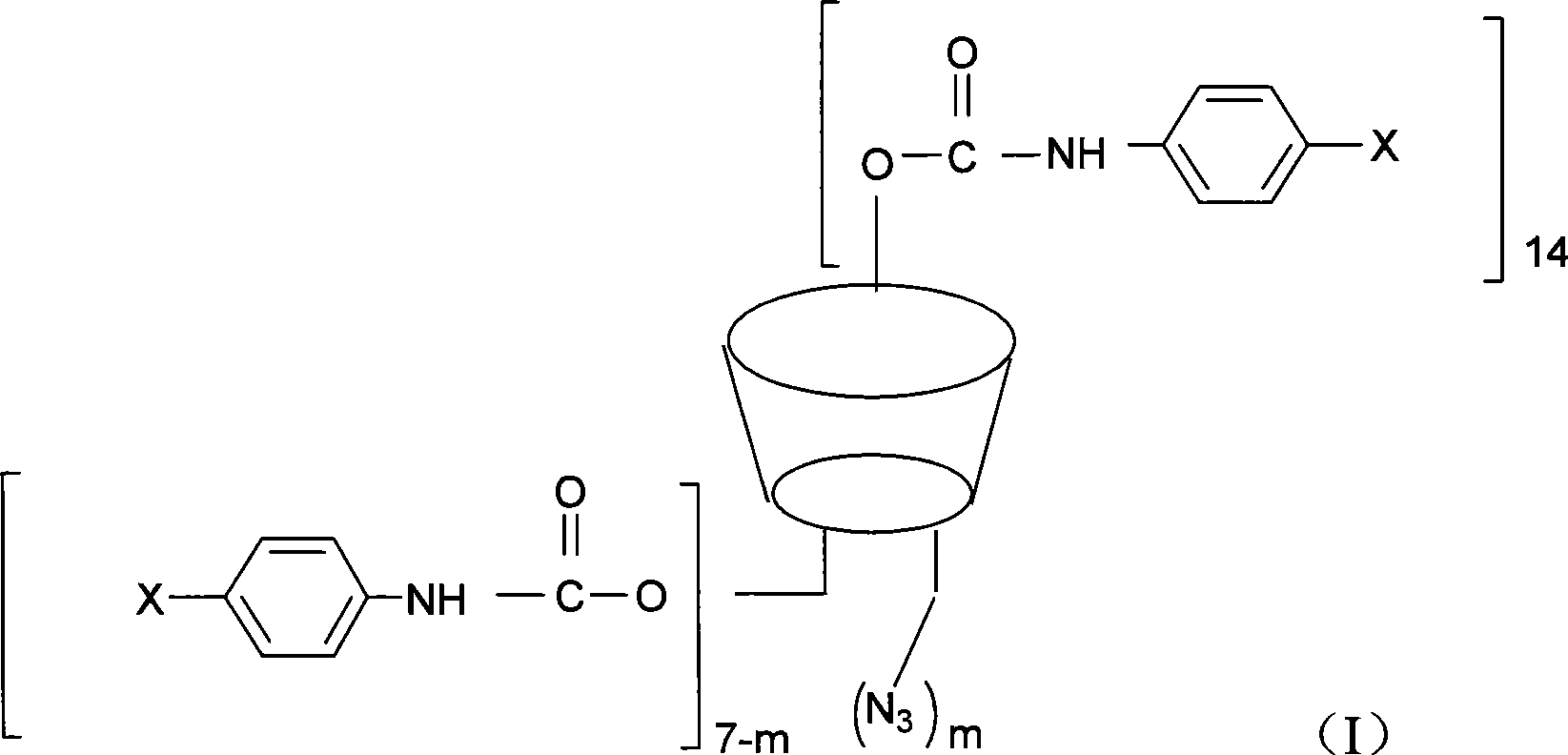
The invention discloses a beta-cyclodextrin derivative, a preparation method and the application that the beta-cyclodextrin derivative is taken as chiral selective agent; the derivative is formed on the basis that hydrogen on beta-cyclodextrin hydroxide radical is respectively replaced by XC6H5NHCO- and -N3, and has the general molecular formula of ((XC6H5NHCO) n (C42H49O34) (N3) m); the structural formula is shown as formula (I). The beta-cyclodextrin derivative has wide material source, mild reaction conditions and low cost; when beta-cyclodextrin derivative is taken as chiral selective agent, the chiral compound separated by the derivative has board application scope and good effect; furthermore, when the chiral selective agent is linked with matrix, the fastness level, density and chiral selectivity can be adjusted and controlled by manpower.
Owner:广州研创生物技术发展有限公司
Magnetic nano particle enzyme immobilization as well as preparation method and uses thereof
InactiveCN101177678AEasy to manufactureEasy to operateFermentationOn/in inorganic carrierChiral selectivityMagnetite Nanoparticles
The invention relates to a functionalized magnetic nano particle immobilized enzyme and a preparation method thereof. Bisphenols with active functional groups are used to coordinate with defect sites on the surface of magnetic nanoparticles, thereby introducing active functional groups on the surface of magnetic nanoparticles, and forming covalent bonds with enzymes to achieve the purpose of immobilizing enzymes. This type of immobilized enzyme is easy to prepare, easy to operate, and exhibits high reactivity and chiral selectivity in chiral resolution and chiral synthesis. This type of immobilized enzyme is also very stable, and can still maintain high reactivity and chiral selectivity after repeated use. The method is universal and applicable to the immobilization of other biomacromolecules.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
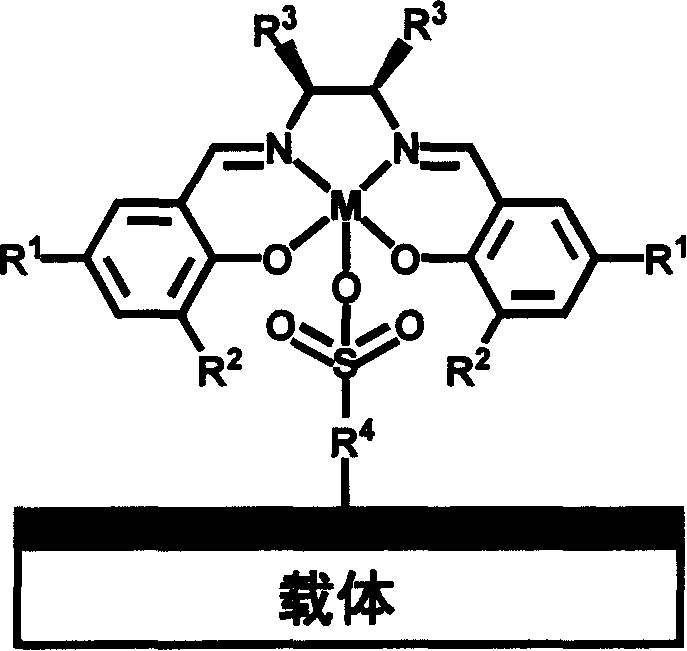
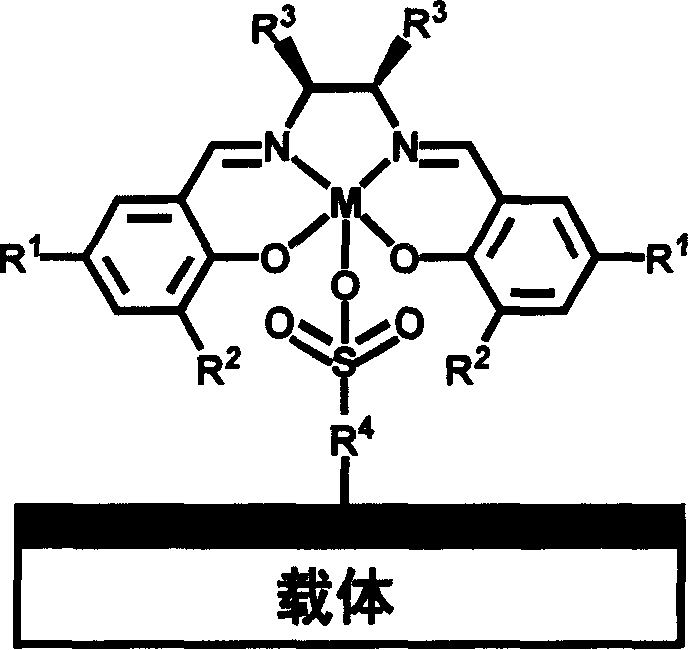
Chiral schiff base-metal heterogeneous epoxidation catalyst and its prepn. method
InactiveCN1868595AEasy to manufactureEasy to purifyOrganic-compounds/hydrides/coordination-complexes catalystsChemical industryChiral selectivityHigh activity
A multi-phase epoxidizing catalyst with high activity, chiral selectivity and stability, and short reaction time is prepared through using sulfonyl group to axially fix the complex of the chiral Schiff base and metal to inorganic carrier and / or polymer carrier.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Chiral inorganic-organic composite porous material and method for preparing the same
InactiveUS20090018334A1Improve physiological activityHigh selectivitySolid sorbent liquid separationGlass/slag layered productsChiral selectivityIon exchange
The present invention provides a chiral inorganic-organic composite porous material in which cationic chiral organic molecules are present as charge-balancing cations in a porous material containing charge-balancing cations, as well as a method for preparing the same by an ion exchange process. The chiral inorganic-organic composite porous material according to the present invention is excellent in stability, selectivity and durability, and thus, will be useful as a chiral-selective catalyst or a material of separating an isomeric mixture.
Owner:CHIROLITE INC
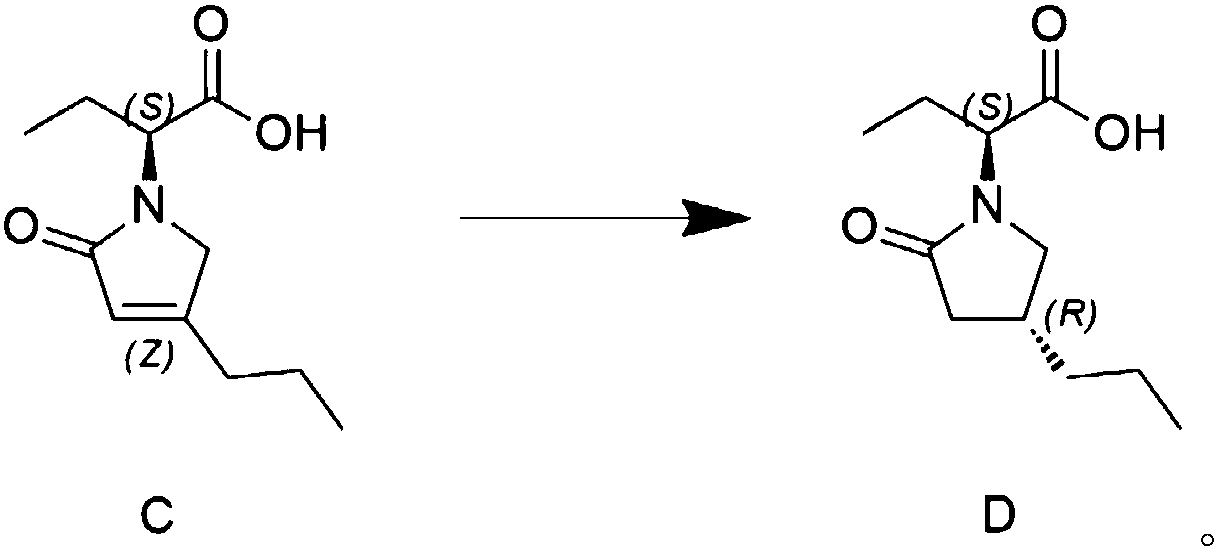
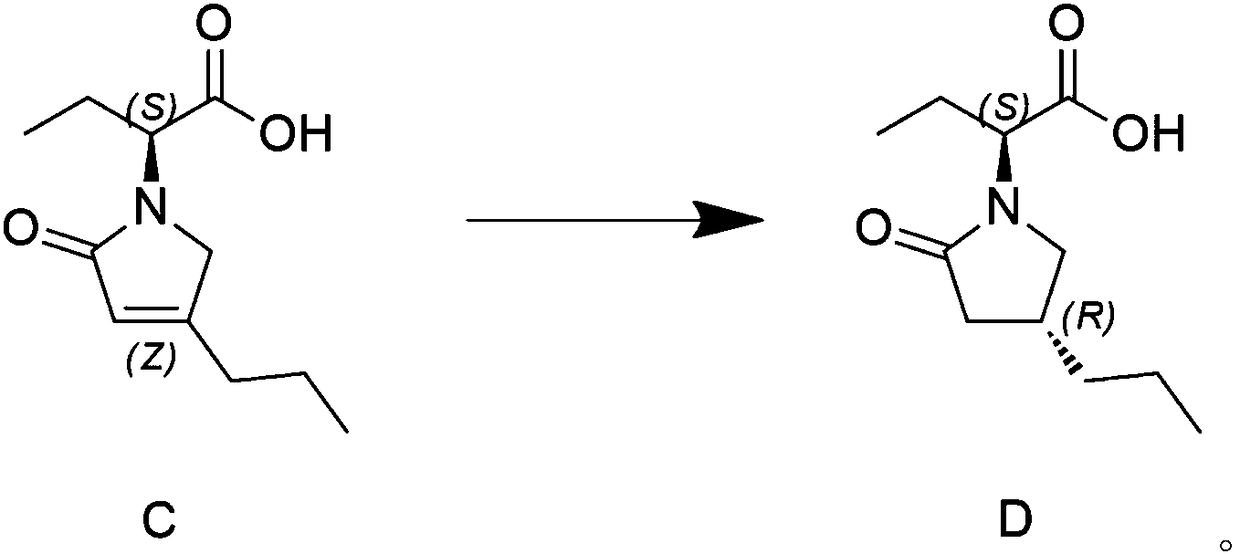
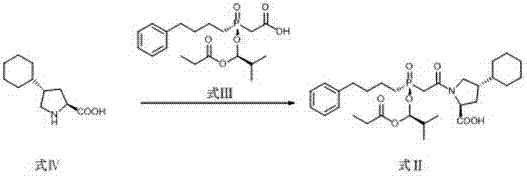
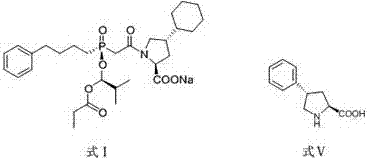
Preparation method of high chiral purity lactam intermediate and brivaracetam
InactiveCN108101823AEasy to separateLow costOrganic chemistry methodsChiral selectivityStructural formula
The invention discloses a preparation method of a high chiral purity lactam intermediate and brivaracetam. The preparation method of the lactam intermediate structural formula compound D includes thesteps of: in a solvent, by means of a heavy metal catalyst and a chiral inducer, conducting hydrogenation reduction on a compound C into the lactam intermediate D. The lactam intermediate structural formula compound D provided by the invention can be made into brivaracetam by only one step, the synthesis route is short, the reaction conditions are mild, the post-treatment is simple, the reaction yield is high, the chiral selectivity is good, and the production cost is low. In the reaction process, the conversion rate of the compound C reaches 81%, and the de value of the compound D reaches 99%or more, therefore the method is suitable for industrial production.
Owner:YANGZHOU AORUITE PHARMA CO LTD +1
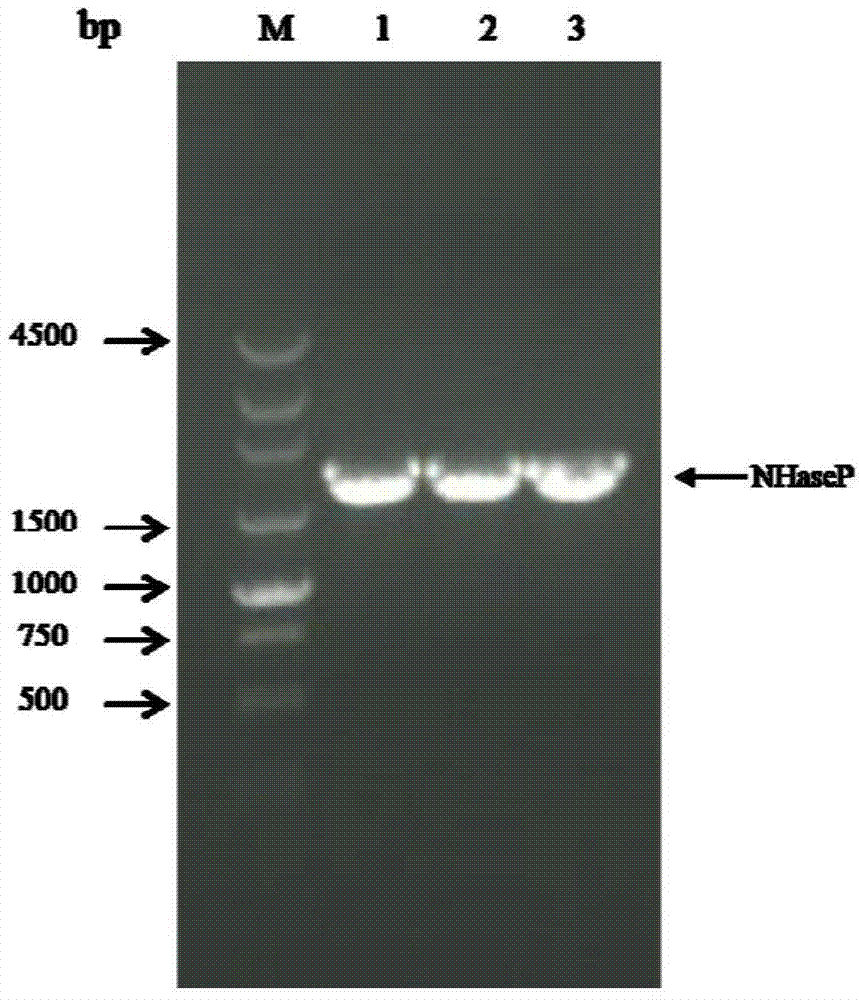
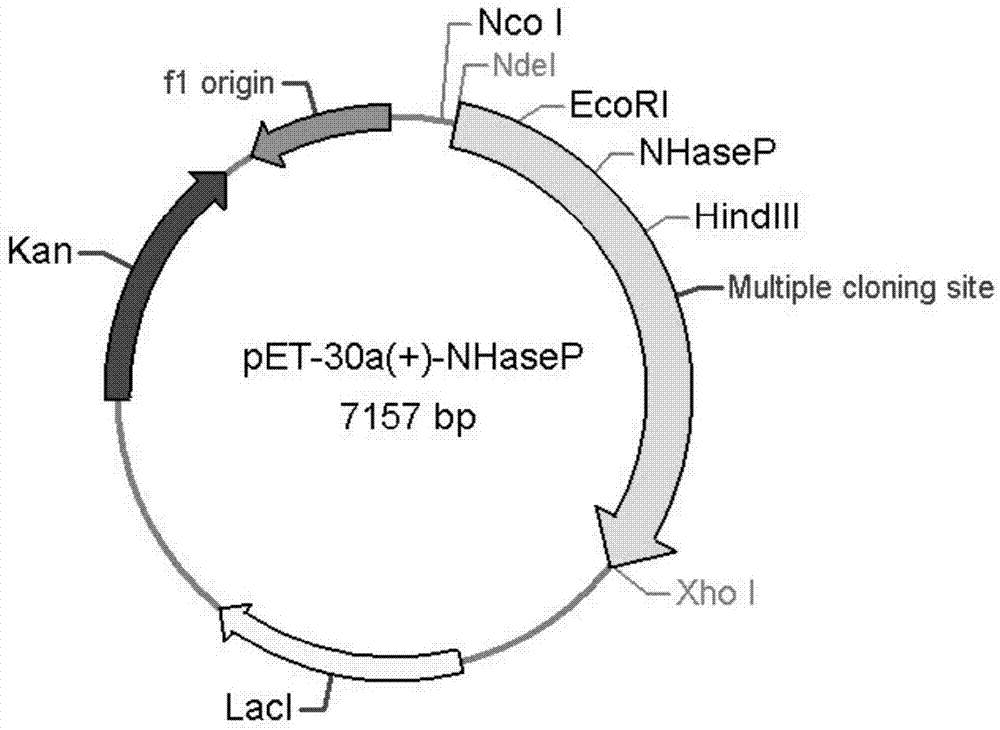
Nitrile hydratase and application thereof
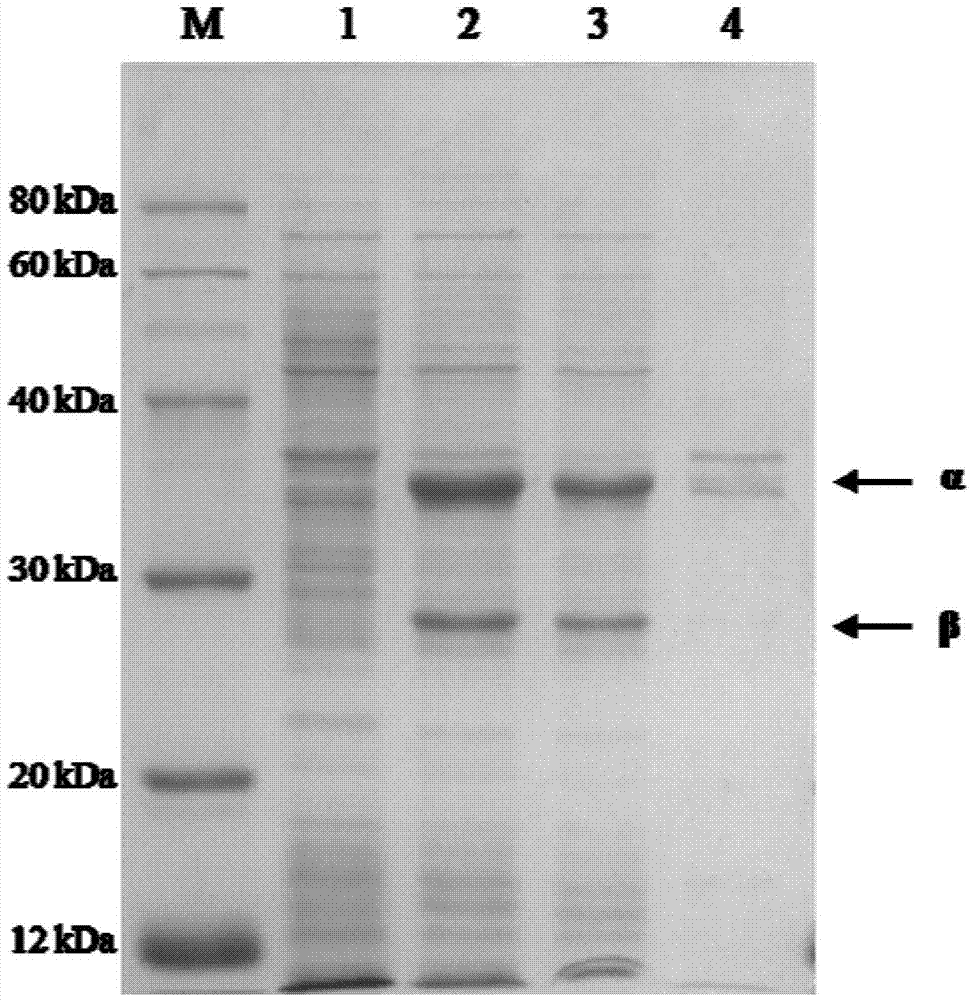
The invention discloses a nitrile hydratase and an application thereof. The nitrile hydratase is composed of an alpha subunit and a beta subunit, wherein an amino acid sequence of the alpha subunit is as shown in SEQ ID NO.6; the amino acid sequence of the beta subunit is as shown in SEQ ID NO.7. The nitrile hydratase is cloned to a nitrile hydratase gene from Burdett bacteria DSM 12804 (Bordetella petrii DSM 12804); the nitrile hydratase which is high in expression quantity, high in activity, wide in substrate spectrum and good in chiral selectivity is successfully obtained after gene expression. An existing nitrile hydratase is relatively high in activity on aliphatic acrylic compounds in general, and is low in activity on aromatic nitrile compounds in general. The nitrile hydratase disclosed by the invention has relatively high catalytic activity on the aromatic nitrile compounds, especially 2-isopropyl-4-chlorophenyl acetonitrile.
Owner:ZHEJIANG UNIV
Alanine racemase chiral binaphthol derivative with powerful hydrogen bond donor, and optical resolution and optical transformation methods using the same
InactiveUS20090023931A1Effective optical resolutionHigh yieldUrea derivatives preparationOrganic compound preparationChiral selectivityAlanine racemase
Disclosed is an alanine racemase chiral binaphthol derivative having the ability to recognize amino alcohols selectively on the basis of chirality and transform amino acids from an L-form into a D-form. Methods for the optical resolution of amino acid or amino alcohol and for the optical transformation of D- and L-forms of amino acids using the binaphthol derivative are also provided.
Owner:EWHA UNIV IND COLLABORATION FOUND +1
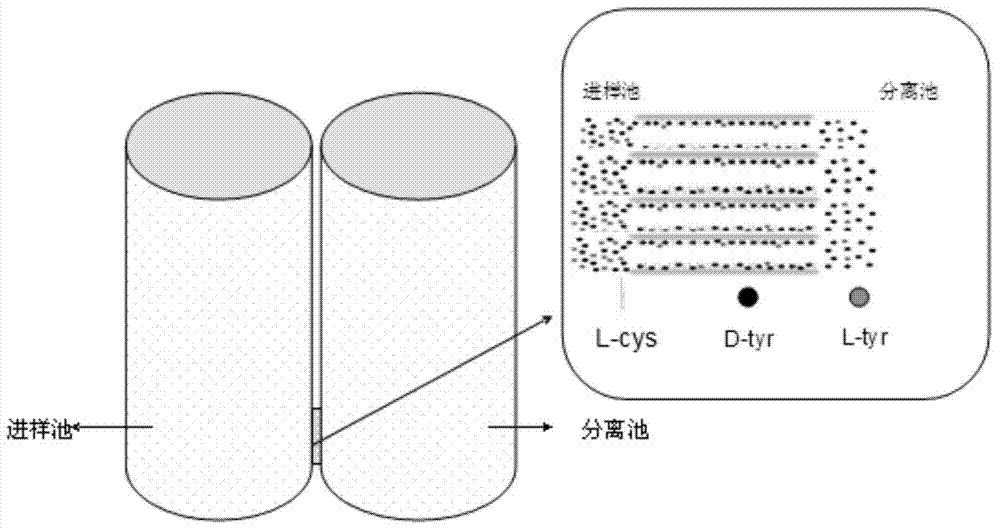
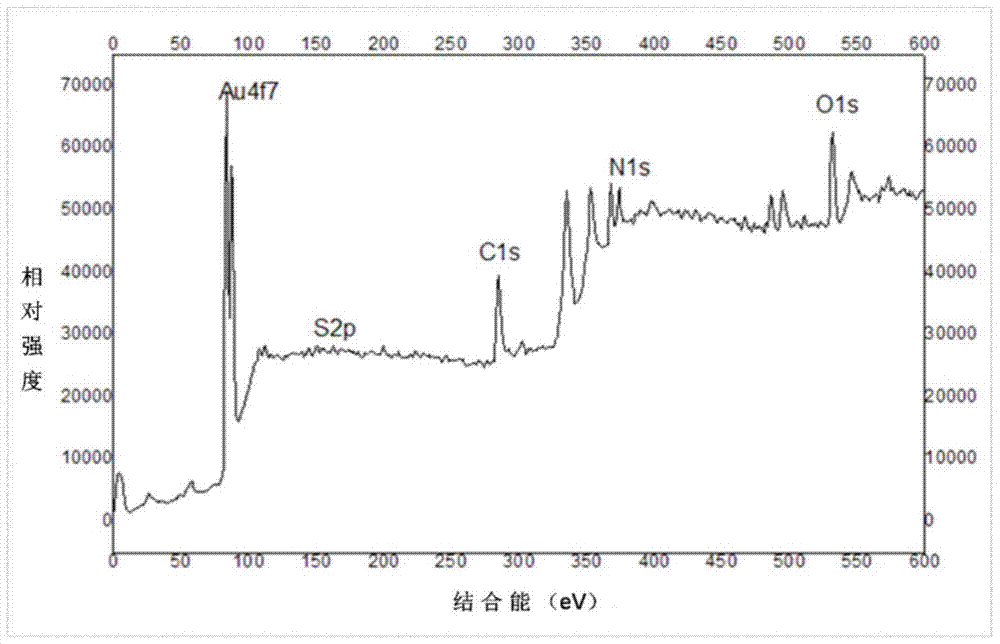
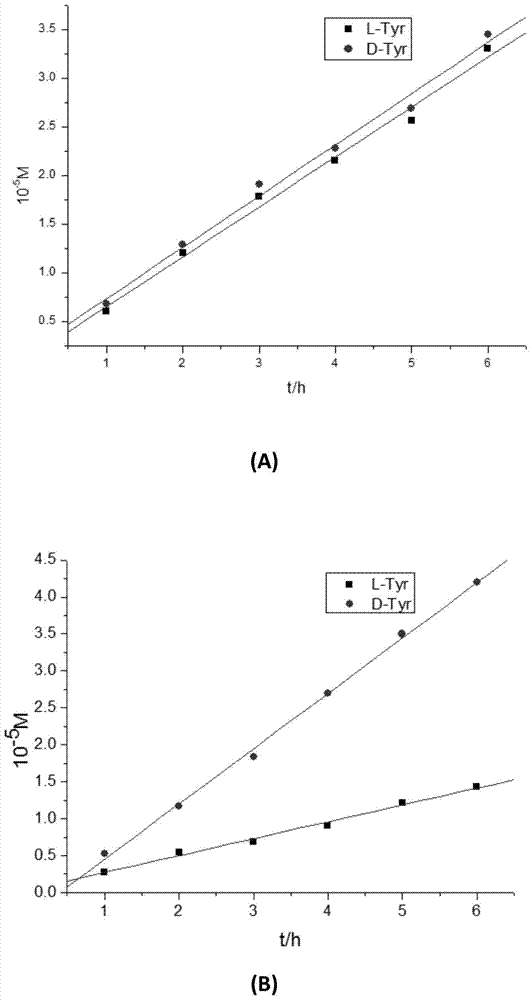
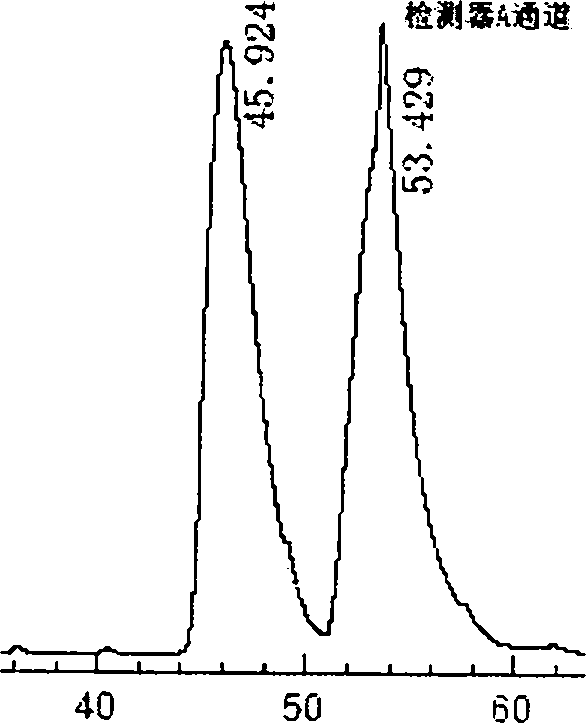
DL tyrosine chiral resolution and on-line detection method based on functional nano channel arrays
InactiveCN104119244ARealize online detectionEasy to splitOrganic compound preparationRaman scatteringChiral selectivitySulfur
The invention discloses a DL tyrosine chiral resolution and on-line detection method based on functional nano channel arrays in association with surface enhanced Raman spectrum. By using a polycarbonate film as a base film, a chemical deposition process is utilized to prepare the Au nano channel film with the diameter of 25nm or so, L-cysteine is self-assembled to the Au nano channel pore wall through the Au-S covalent bond to form a chiral selective identification film with L-cysteine on the surface. The channel subjected to chiral selective identification has a different effect on the DL tyrosine, so that the DL tyrosine permeation rate of the modification film is different, thereby separating the D-tyrosine from the L-tyrosine. By utilizing the difference of the SERS characteristic peak of the DL tyrosine and the very low detection limit, the object in the separation tank can be detected instantly. The invention provides a novel convenient method for separating and detecting the DL tyrosine which is hard to resolve.
Owner:SHANGHAI NORMAL UNIVERSITY
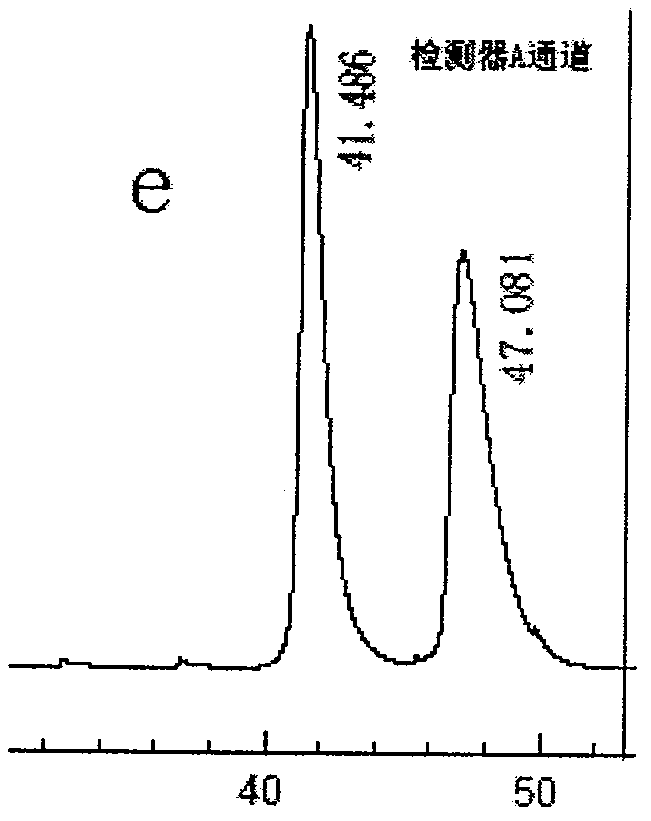
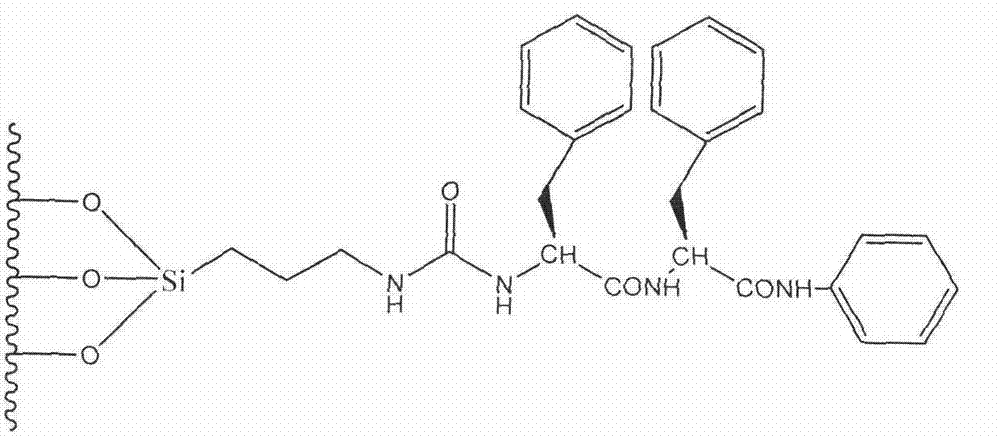
Preparation method of novel phenylalanine chiral chromatographic column stationary phase
ActiveCN104289210AOther chemical processesSolid sorbent liquid separationStationary phaseChiral selectivity
The invention provides a preparation method of a novel chiral chromatographic column stationary phase based on phenylalanine. The preparation method comprises the following steps: 1, carrying out surface treatment on a silica gel sphere; 2, preparing a phenylalanine chiral selector monomer; and 3, preparing the chiral chromatographic column stationary phase. The method has the advantages of simple process route, low cost and operation convenience, and the prepared chiral chromatographic column stationary phase has the advantages of wide chiral selectivity, wide mobile phase application range, good bonding force, difficult loss, long service life, short separation time, no tailing, high column efficiency and the like.
Owner:上海可力梅塔生物医药科技有限公司 +3
Application of magnetic molecular imprinting technique in chiral recognition of microfluidic system
The invention discloses an application of a magnetic molecular imprinting technique in a chiral recognition of a microfluidic system, provides a new method of constructing a polydimethylsiloxane (PDMS) microfluidic chip system with selective recognition reaction by using the magnetic molecular imprinting technique to a chiral material, and belongs to the field of microfluidic chip technique. The new method of constructing the chiral selective PDMS microfluidic system based on the magnetic molecular imprinting technique has a great number of imprinting sites specifically recognizing D / L-tryptophan and can realize selective, quick and efficient separation of the chiral tryptophan. The technique can be applied to the fields such as imprinting, separation and detection of biomacromolecules (protein, polypeptide and nucleotide).
Owner:NANCHANG UNIV
Alanine racemase chiral binaphthol derivative with powerful hydrogen bond donor, and optical resolution and optical transformation methods using the same
InactiveUS7847124B2Effective optical resolutionHigh yieldUrea derivatives preparationOrganic compound preparationAlcoholChiral selectivity
Disclosed is an alanine racemase chiral binaphthol derivative having the ability to recognize amino alcohols selectively on the basis of chirality and transform amino acids from an L-form into a D-form. Methods for the optical resolution of amino acid or amino alcohol and for the optical transformation of D- and L-forms of amino acids using the binaphthol derivative are also provided.
Owner:EWHA UNIV IND COLLABORATION FOUND +1
Synthesis process of trans-menthyl-2, 8-diene-1-ol
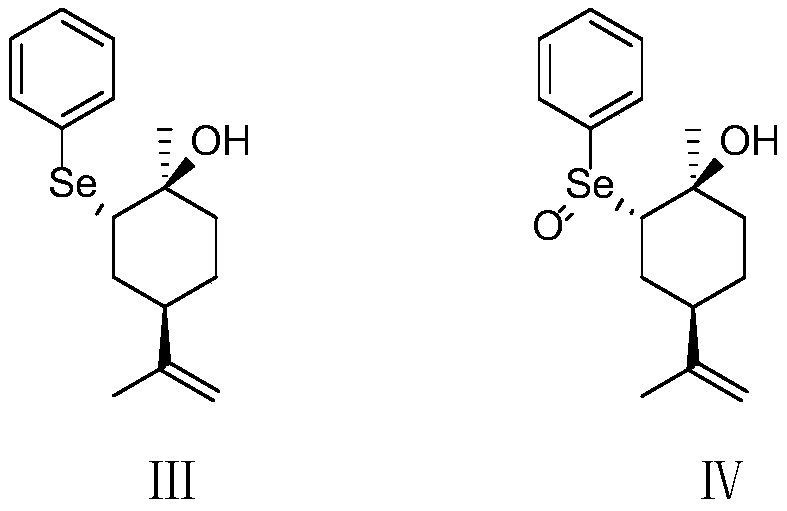
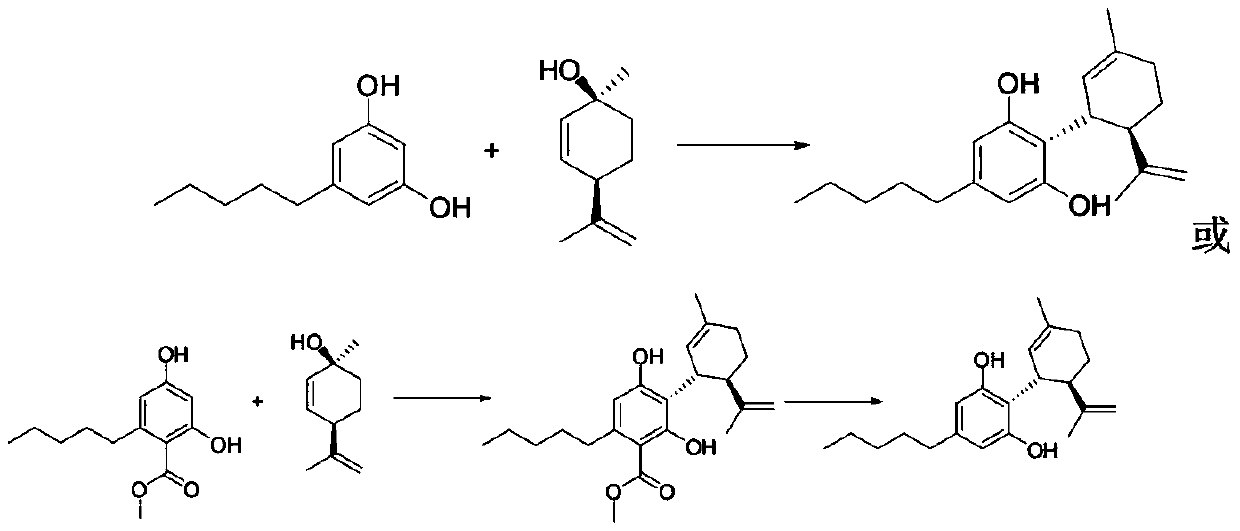
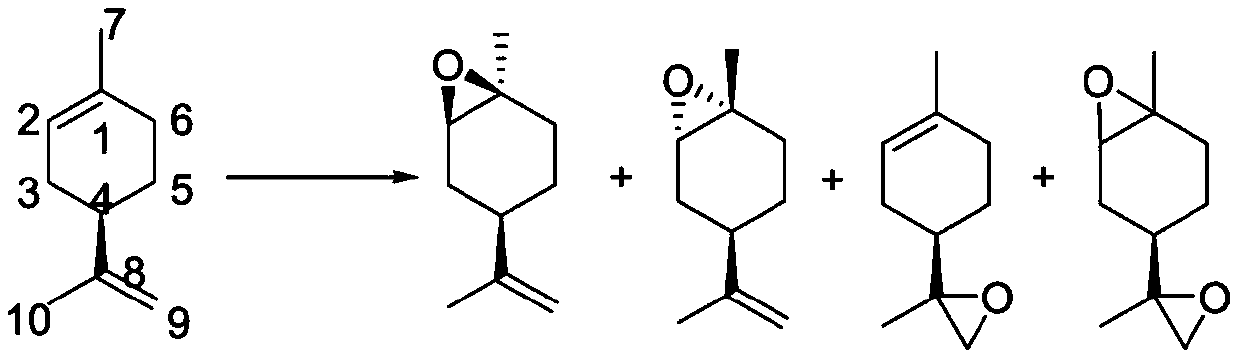
ActiveCN109734554AHigh chiral purityHigh yieldOrganic compound preparationHydroxy compound preparationEpoxyChiral selectivity
The invention belongs to the technical field of preparation of trans-menthyl-2, 8-diene-1-ol, and particularly relates to a synthesis process of trans-menthyl-2, 8-diene-1-ol. The synthesis process comprises the following steps: (1) taking limonene as a raw material, and carrying out lipase catalytic oxidation to prepare 1, 2-epoxy limonene; (2) ring-opening the 1, 2-epoxy limonene in the presenceof sodium borohydride and diphenyl diselenide to form limonene selenide; (3) forming selenium oxide under the action of an oxidizing agent by the limonene selenide and then carrying out elimination reaction to prepare the trans-menthyl-2, 8-diene-1-ol. According to the invention, the 1, 2-epoxy limonene is firstly prepared by a lipase catalysis method with high chiral selectivity, the purity of areaction intermediate is improved without a complex purification process, and the chiral purity of the final product trans-menthyl-2, 8-diene-1-ol is further improved.
Owner:JIANGSU JIMING PHARMA TECH
Intelligent nanometer chiral selector material, as well as preparation and application thereof
ActiveCN108299651AEfficient temperature sensitivityEfficient Magnetic ResponseOther chemical processesAlkali metal oxides/hydroxidesTemperature responseChiral selectivity
The invention discloses an intelligent nanometer chiral selector material, as well as preparation and application thereof. The preparation comprises the steps of firstly preparing Fe3O4 magnetic nanoparticles; then modifying the obtained Fe3O4 magnetic nanoparticles through polydopamine; and the like. The material provided by the invention has thermosensitivity, temperature-response chiral selectivity, and magnetic responsiveness, and is capable of quickly and simply decomposing an enantiomer molecule and regenerating the material after splitting an amino acid enantiomer.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Chiral chromatographic stationary phase of sandwich structure and preparation method thereof
InactiveCN105727910AAvoid churnHigh chiral separation abilityOther chemical processesStationary phaseChiral selectivity
The invention discloses a chiral chromatographic stationary phase of a sandwich structure. The chiral chromatographic stationary phase comprises a polymer layer, a nano-gold particle layer and a silica gel core, wherein the silica gel core is wrapped by the nano-gold particle layer through An-N bonds or Au-S bonds, and the polymer layer is the outermost layer and forms a stable outer layer shell by way of physical wrapping. The invention further provides a preparation method of the chiral chromatographic stationary phase of the sandwich structure. The preparation method of the chiral chromatographic stationary phase of the sandwich structure comprises the following steps: 1, preparation of gold nanoparticle sol; 2, preparation of SiO2@Au; 3, synthesis of the chiral chromatographic stationary phase of the sandwich structure. According to the chiral chromatographic stationary phase of the sandwich structure and the preparation method thereof, nanoparticles are loaded to the surface of silica gel through a layer-by-layer self-assembly method, and then the surface is coated with a layer of polymer, so that the problems that traditional bonded silica gel is not resistant to alkali, and chiral selectivity is limited are solved.
Owner:NINGXIA UNIVERSITY
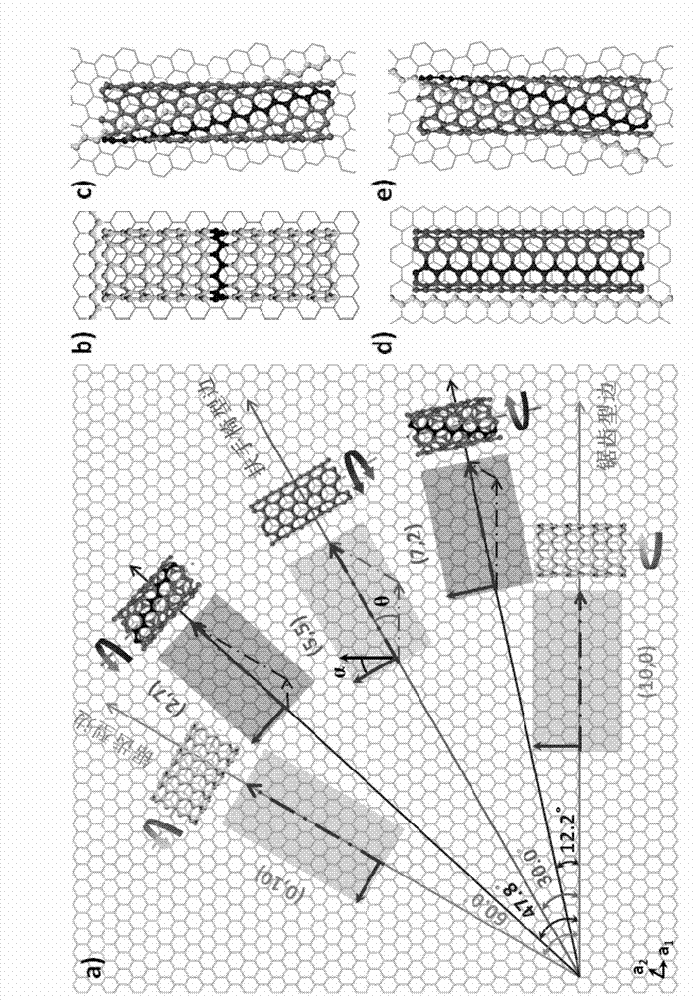
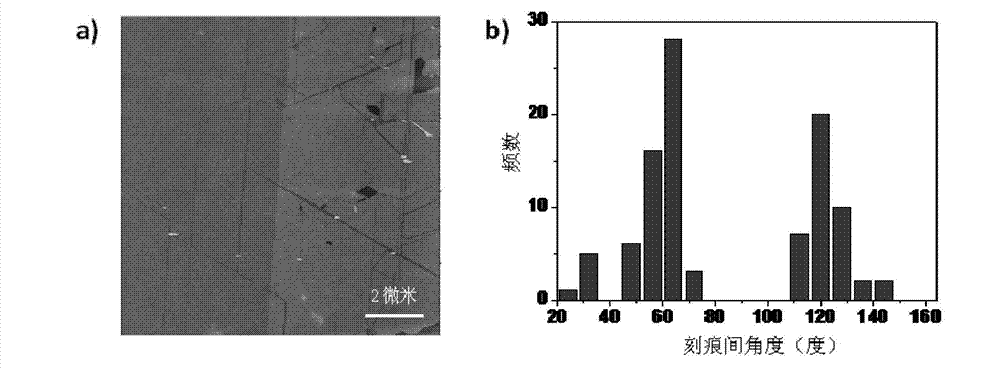
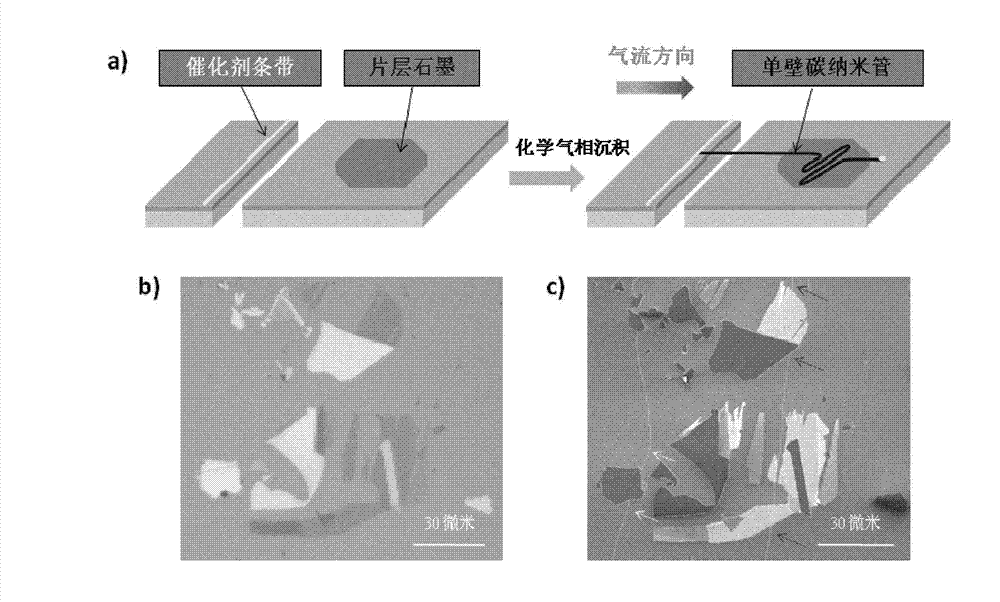
Single-walled carbon nanotube array with chiral selective orientation and method for representing chiral structure thereof
ActiveCN103086353AMaintain structureKeep shapeCarbon nanotubesNanotechnologyChiral selectivityCarbon nanotube
The invention discloses a single-walled carbon nanotube array taking graphite as a substrate and having chiral selective orientation and a method for representing the chiral structure thereof. The preparation method comprises the steps of 1) preparing a graphite substrate with regular nicks on the surface; and 2) arranging the substrate with a single-walled carbon nanotube catalyst growing catalyst and the graphite substrate in a chemical vapor deposition system in an adjacent manner; and growing a single-walled carbon nanotube on the graphite substrate by adopting an airflow orientation method to obtain a single-walled carbon nanotube array with chiral selective orientation. By combining the chiral selective orientation conclusion of the single-walled carbon nanotube on the surface of the graphite substrate with regular nicks on the graphite surface, the chiral angle and optical activity of the single-walled carbon nanotube can be measured, and the single-walled carbon nanotube with the known chiral structure is further subjected to performance test and / or device processing. The measurement method provided by the invention measures the chiral angle and the optical activity of the carbon nanotube at the same time, and can perform batch macroscopic measurement of the carbon nanotube on the sample surface.
Owner:PEKING UNIV
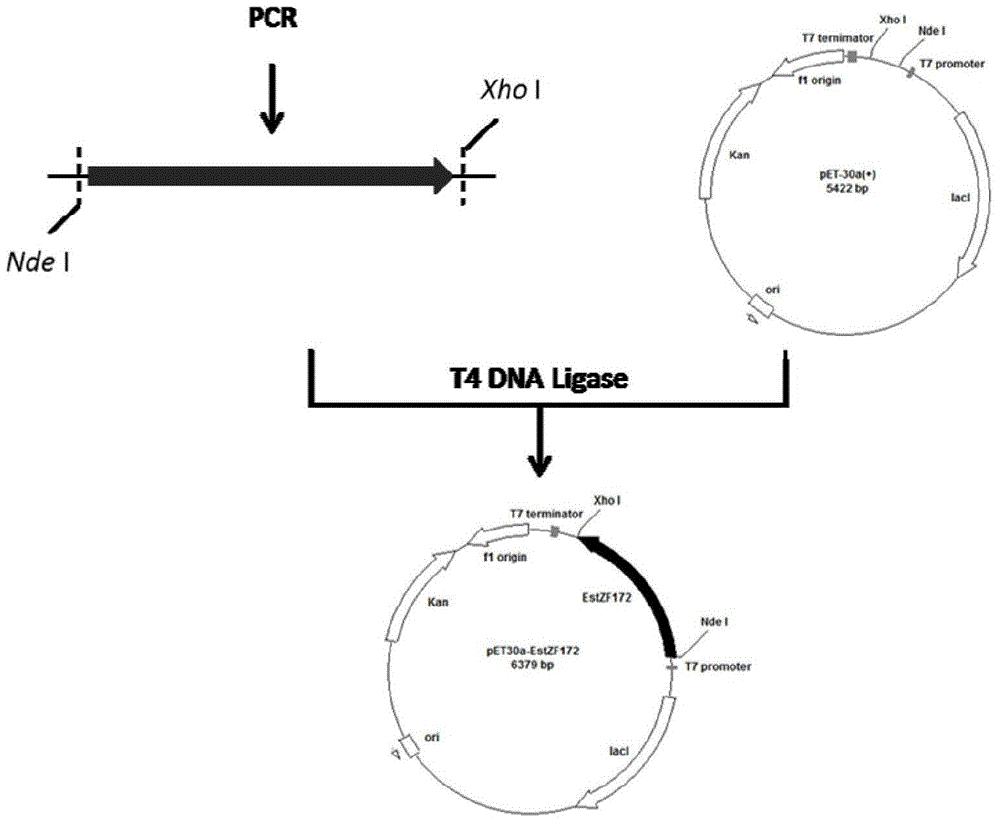
Marine bacterial novel esterase, as well as preparation method and application thereof
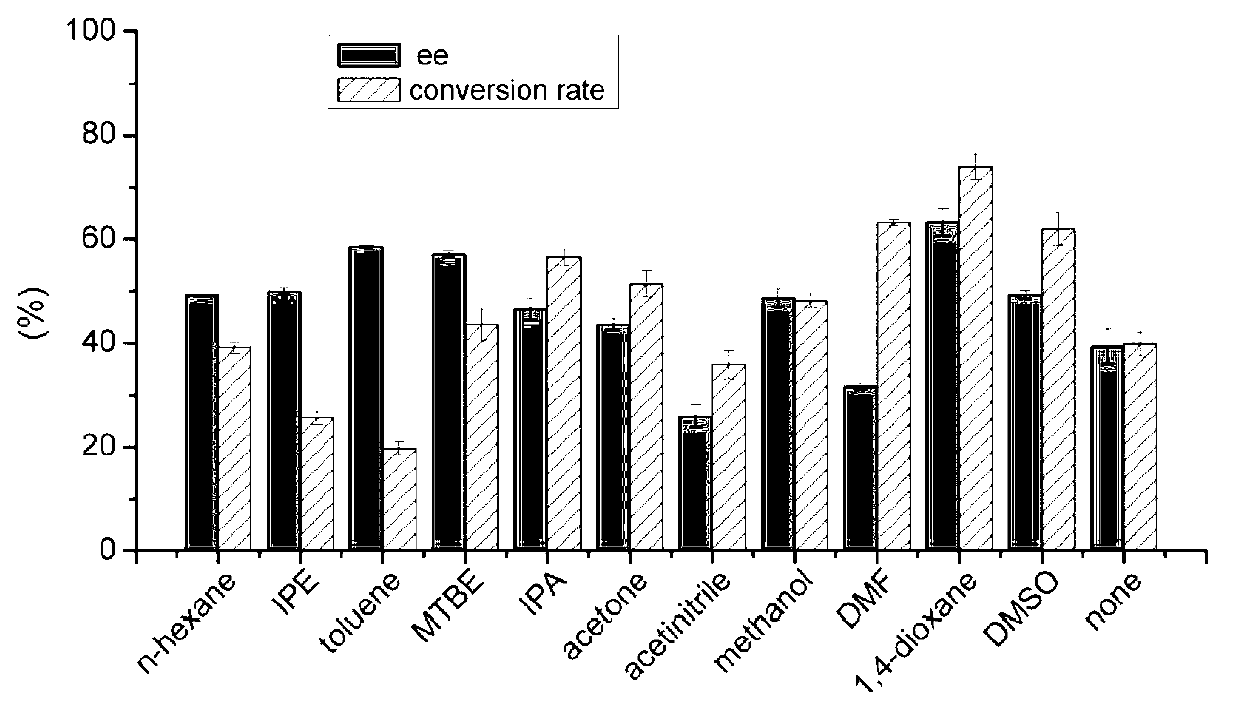
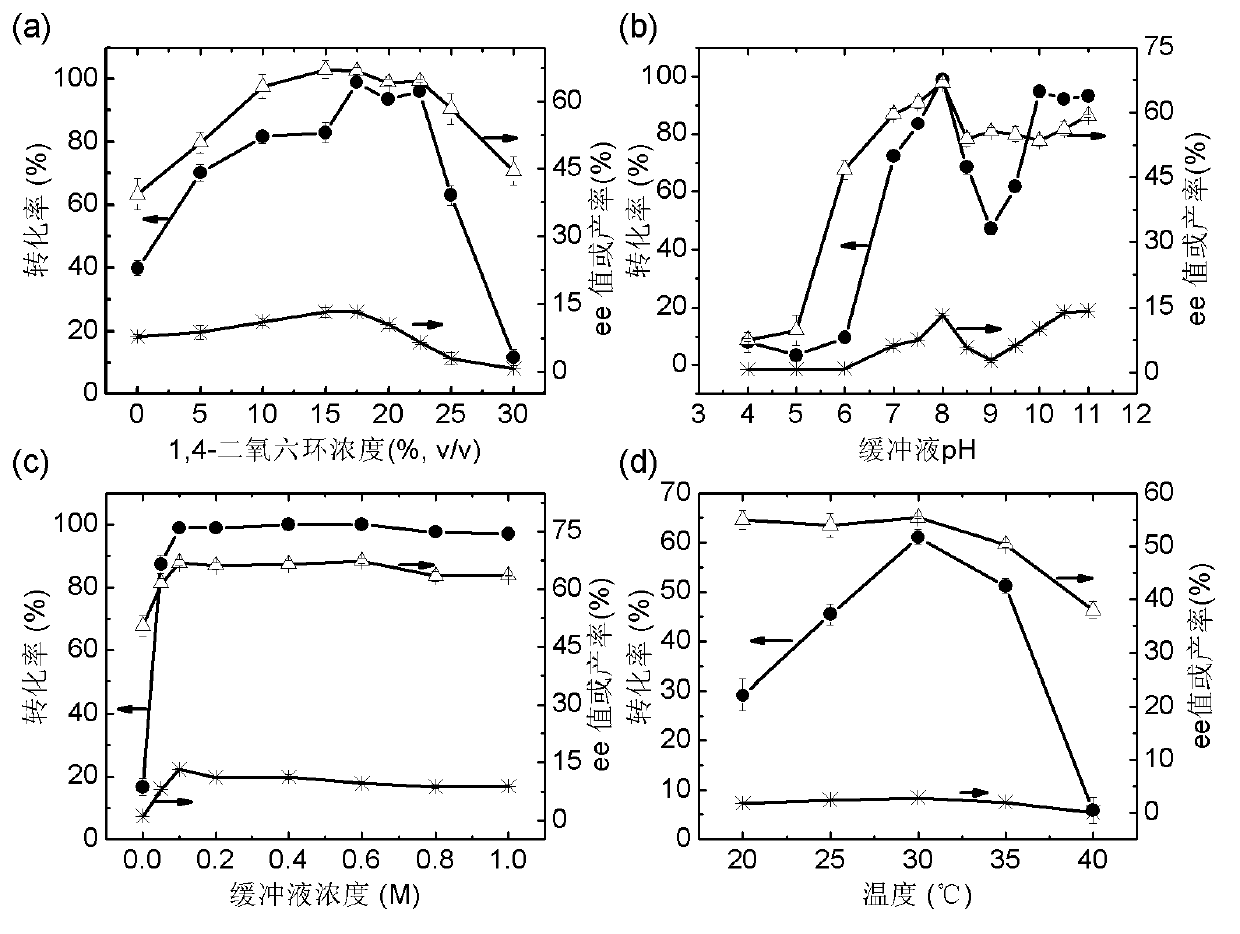
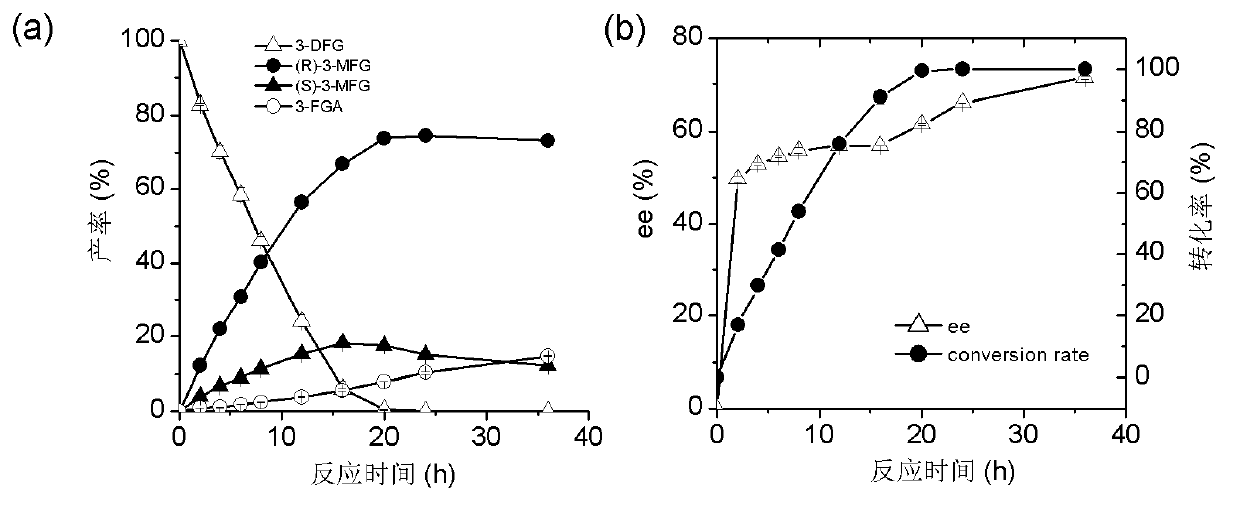
The invention discloses a marine bacterial novel esterase, and a method for producing a drug intermediate (R)-3-(4-fluorophenyl) monomethyl glutarate by chirally catalyzing 3-(4-fluorophenyl) methyl glutarate by using the esterase. The gene of the esterase is cloned to an expression plasmid to transform escherichia coli Rosetta. As the esterase can be highly and solubly expressed in an expression strain, and shows excellent salt resistant, alkali resistance and chiral selectivity, the esterase can be used as potential enzyme for industrial production of the antidepressant drug intermediate (R)-3-(4-fluorophenyl) monomethyl glutarate. As long as the reaction conditions are optimized, the esterase can be used for catalyzing 3-(4-fluorophenyl) methyl glutarate to produce the drug intermediate (R)-3-(4-fluorophenyl) monomethyl glutarate; and as a result, the transformation ratio and chiral selectivity of the esterase are greatly improved.
Owner:SECOND INST OF OCEANOGRAPHY MNR +1
Method for direct, chirality-selective synthesis of semiconducting or metallic single-walled carbon nanotubes
The present invention is a method comprising a direct chirality-selective nucleation and synthesis of single-walled carbon nanotubes from carbon-containing gases using catalytic nanoparticles of uniform size heated by ultra-short laser pulses of selected frequency to temperatures sufficient for carbon nanotube nucleation and synthesis.
Owner:CFD RES CORP
Intelligent graphene nano-material with high chiral selectivity as well as preparation and application thereof
ActiveCN108299652AHigh chiral selectivityTo overcome the defects in practical applicationChiral selectivityCvd graphene
The invention discloses an intelligent graphene nano-material with high chiral selectivity as well as preparation and application thereof. The material is prepared from graphene, a temperature-sensitive component, a magnetic component and the like. The material disclosed by the invention has high chiral selectivity, temperature-sensitive performance and magnetic responsiveness; the material has the advantages of simplicity in operation, high resolution efficiency, convenience for recycling materials, and environment-friendly operation process in chiral resolution of an amino acid enantiomer, and has a good industrial application prospect.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
The synthetic method of salidroside
InactiveCN102286036AGood chiral selectivityShort reaction pathSugar derivativesSugar derivatives preparationSalidrosidePtru catalyst
The invention discloses a chemical method for synthesizing salidroside. The synthesis steps are: tetraacetyl-β-D-glucose trichloroacetate or tetraacetyl-α-D-glucose trichloroacetate and p-hydroxybenzene Ethanol in anhydrous organic solvents, under the dehydration of molecular sieves and under the catalysis of Lewis acids such as tin tetrachloride, glycosides to generate tetraacetyl salidroside; In the solution, the acetyl group is removed to obtain salidroside. Compared with the traditional synthetic method, the method of the present invention has high reaction yield, good chiral selectivity, easy to obtain raw materials, few reaction steps, can use cheap Lewis acid catalyst, greatly reduces the cost, and is applicable to the production of salidroside mass production.
Owner:WUHAN SYNCHALLENGE UNIPHARM INC
Method for selecting and separating single-walled carbon nanotubes with specific diameter and chirality, and application thereof
ActiveCN107285298ALow costSimple methodSolid-state devicesSemiconductor/solid-state device manufacturingDispersed mediaChiral selectivity
The invention discloses a method for selecting and separating single-walled carbon nanotubes with specific diameter and chirality, and application thereof. The method comprises the following steps: mixing single-walled carbon nanotube raw materials and a conjugated polymer into a dispersing medium uniformly to form a dispersing solution; and separating the dispersing solution to form a solid phase part and a liquid phase part, and enriching the single-walled carbon nanotubes with specific diameter and chirality at the liquid phase part, wherein the conjugated polymer is selected from an alternating copolymer of meta-position pyridine and dialkyl fluorene. The method is simple and efficient, diameter and chiral selectivity is extremely high, and low-cost and large-scale batched preparation of single-walled carbon nanotubes with specific diameter and chirality can be realized; furthermore, the obtained single-walled carbon nanotubes with specific diameter and chirality can be used for preparing large-area uniform films easily and have excellent performance in the application aspect of micro-nano electronic devices.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
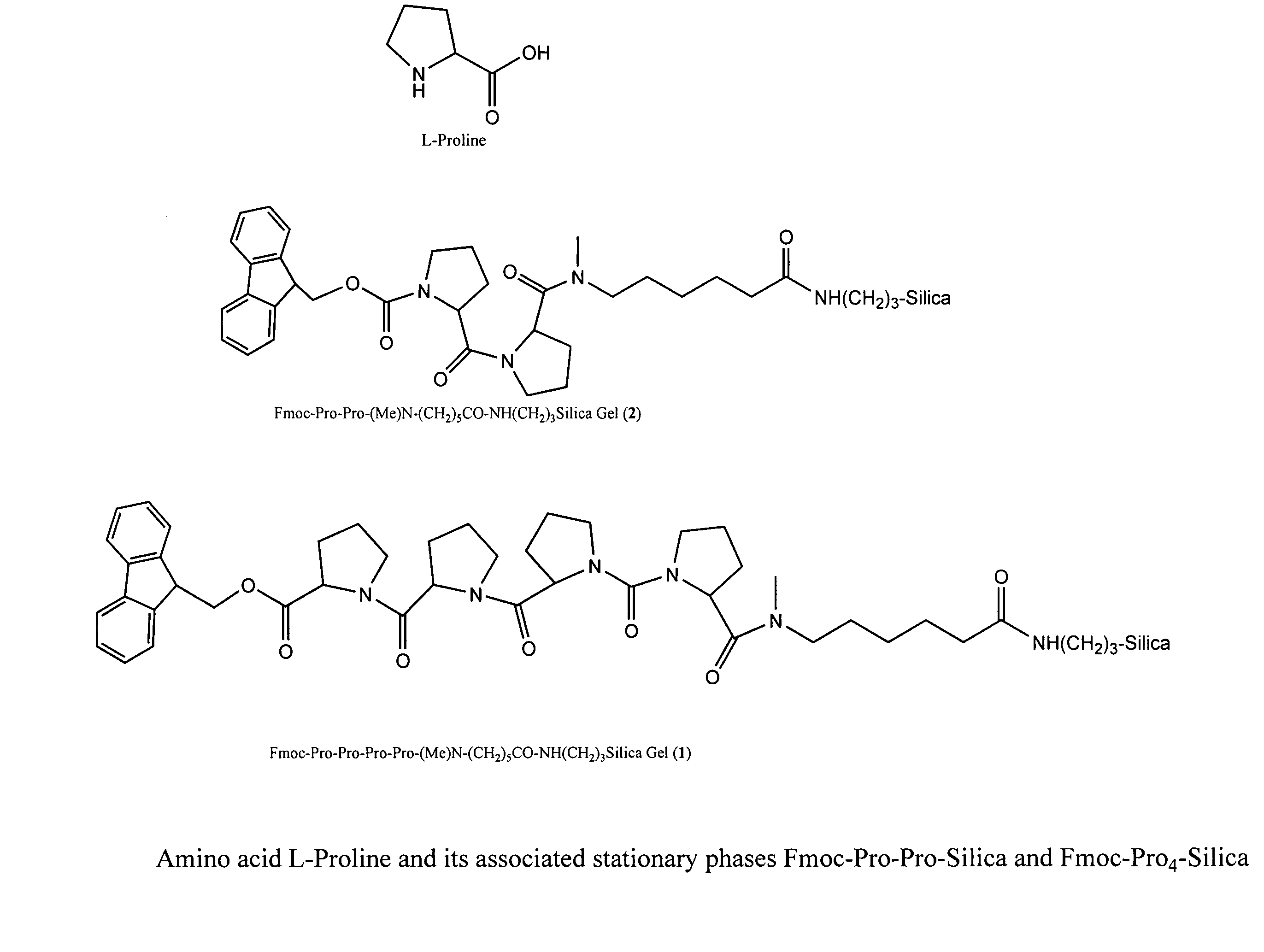
New chiral columns with broad chiral selectivity
InactiveCN101052656APeptide preparation methodsCarrier-bound/immobilised peptidesStationary phaseChiral selectivity
Owner:VANDERBILT UNIV
Enzymatic resolution method of chiral substance
ActiveCN109628508AEasy to purifyEasy to recycleHydrolasesMicroorganism based processesLiquid layerChiral selectivity
The invention belongs to the technical field of bioengineering and food, and discloses an enzymatic resolution method of a chiral substance. The method comprises the following steps that (1) an enzymesolution with the lipase concentration of 1-3000 U / mL is prepared, and soluble salt, a hydrophilic solvent and a hydrophobic solvent are added into the enzyme solution to prepare a three-liquid phasesystem; the hydrophobic solvent contains an ester or amide compound composed of a racemic chiral substance; (2) the three-liquid phase system is subjected to an enzymatic catalytic reaction under a stirring condition, after the reaction is completed, standing or centrifugation is carried out so that the three-liquid phase system is distributed in the three layers of an upper liquid layer, a middle liquid layer and a lower liquid layer from top to bottom in sequence, the hydrolyzed single optically chiral product is mainly gathered in the middle liquid layer or the lower liquid layer, and an upper liquid layer product is another ester or amide product containing the single optically chiral product. The method has the advantages that the energy consumption is small, the raw material utilization rate is high, the reaction condition is mild, and the problems are solved that an existing enzymatic chiral resolution is low in efficiency, poor in chiral selectivity, low in recovery rate and difficult in industrialization.
Owner:SOUTH CHINA UNIV OF TECH
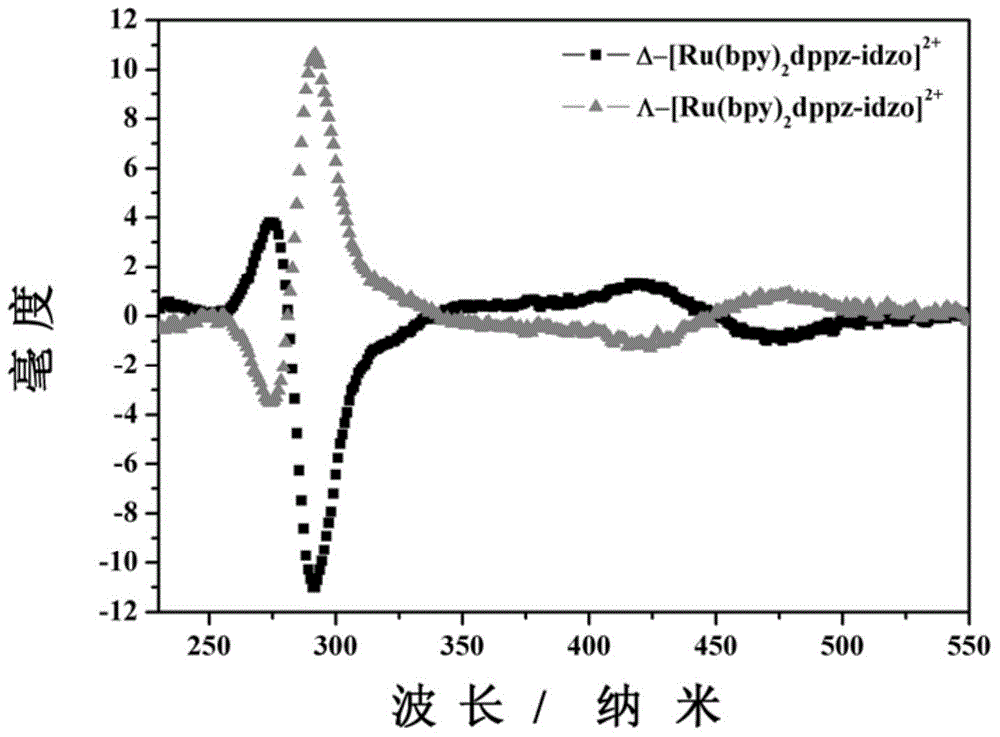
Polypyridine chiral ruthenium (II) complex and preparation method and application thereof
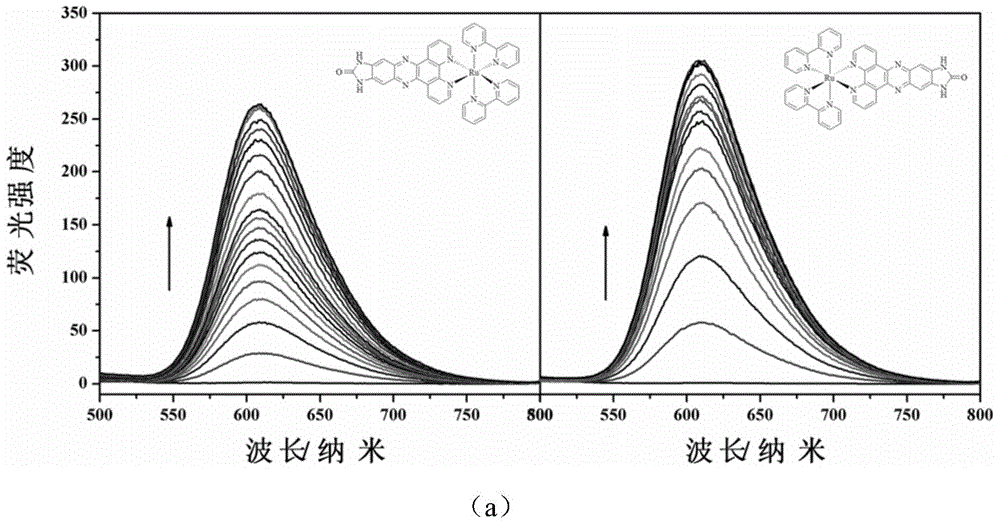
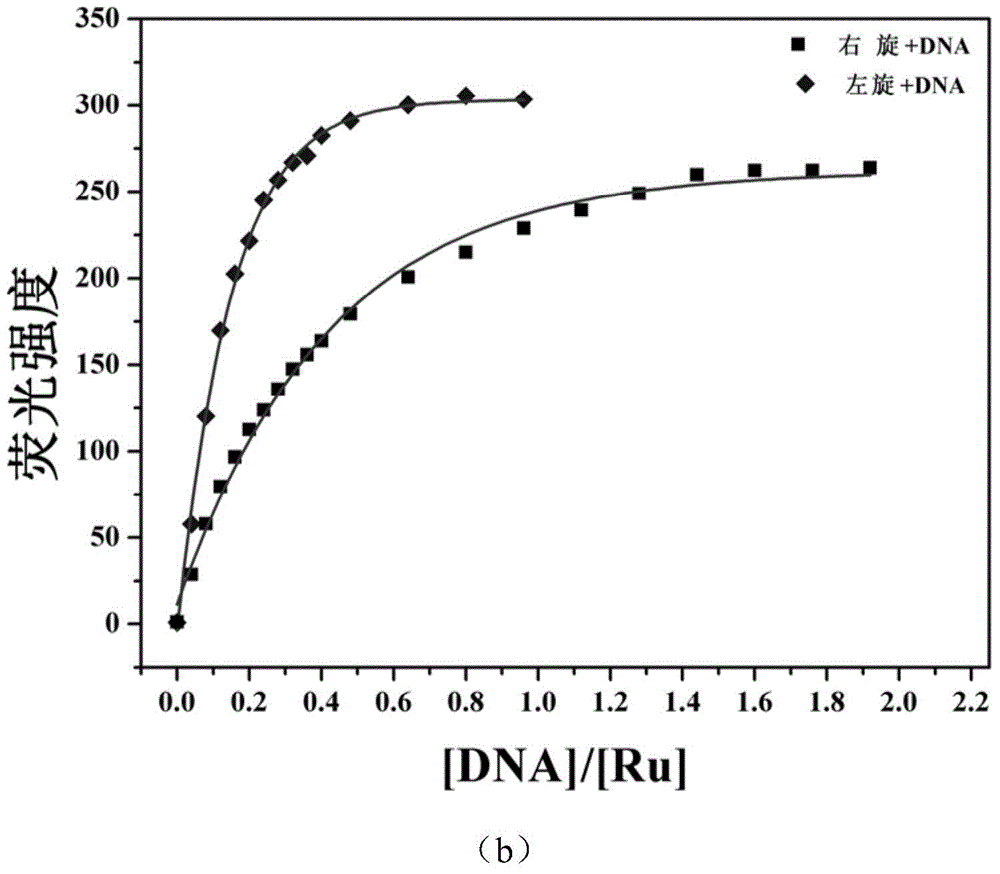
InactiveCN104876968AImprove bindingImprove stabilityGroup 8/9/10/18 element organic compoundsFluorescence/phosphorescenceChiral selectivityFluorescence
The invention relates to a polypyridine chiral ruthenium (II) complex and a preparation method and application thereof. An imidazolone (imidazolone, idzo) is introduced into a main ligand dppz of the ruthenium complex [Ru(bpy)2(dppz)]<2+>, so as to prepare two chiral ruthenium complexes which mutually are geometric isomers. The affinities of the two chiral symmetrical ruthenium complexes on different G-quadruplexes and the recognition capability on the structure are researched by applying means such as fluorescence titration, combination ratio, thermal deformation and circular dichroism spectrum (CD). Compared with the prior art, the chiral ruthenium complex prepared by the method has obvious chiral selectivity on the action of telomere G-quadruplexes of sodium ion-induced antiparallel conformation; and the bonding and stabilizing capabilities of a levorotatory enantiomer on the telomere G-quadruplexes of the sodium ion-induced antiparallel conformation are obviously higher than those of a dextrorotatory ruthenium complex.
Owner:TONGJI UNIV
Chiral inorganic-organic composite porous material and method for preparing the same
InactiveUS7906096B2High activityHigh selectivitySolid sorbent liquid separationGlass/slag layered productsChiral selectivityIon exchange
The present invention provides a chiral inorganic-organic composite porous material in which cationic chiral organic molecules are present as charge-balancing cations in a porous material containing charge-balancing cations, as well as a method for preparing the same by an ion exchange process. The chiral inorganic-organic composite porous material according to the present invention is excellent in stability, selectivity and durability, and thus, will be useful as a chiral-selective catalyst or a material of separating an isomeric mixture.
Owner:CHIROLITE INC
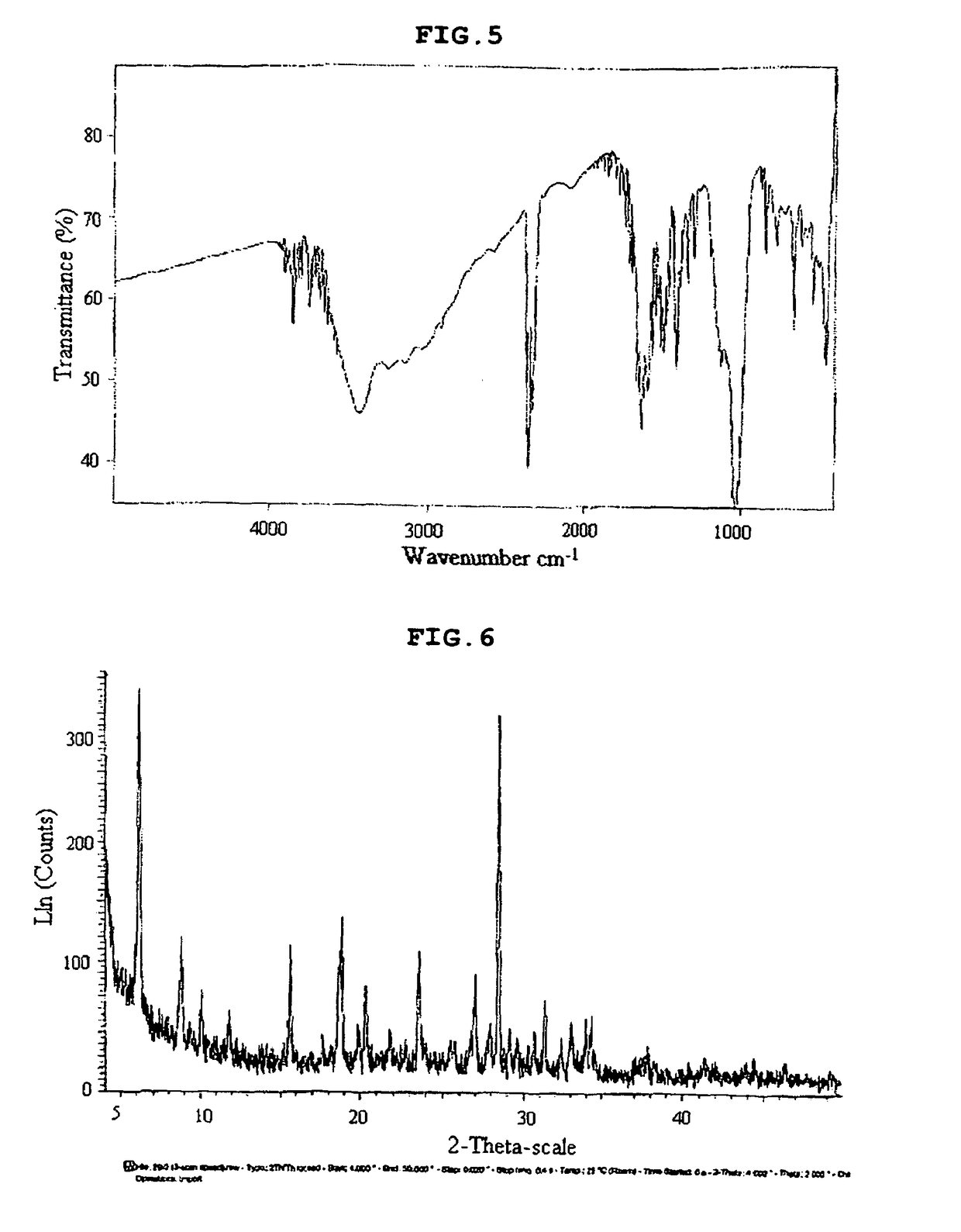
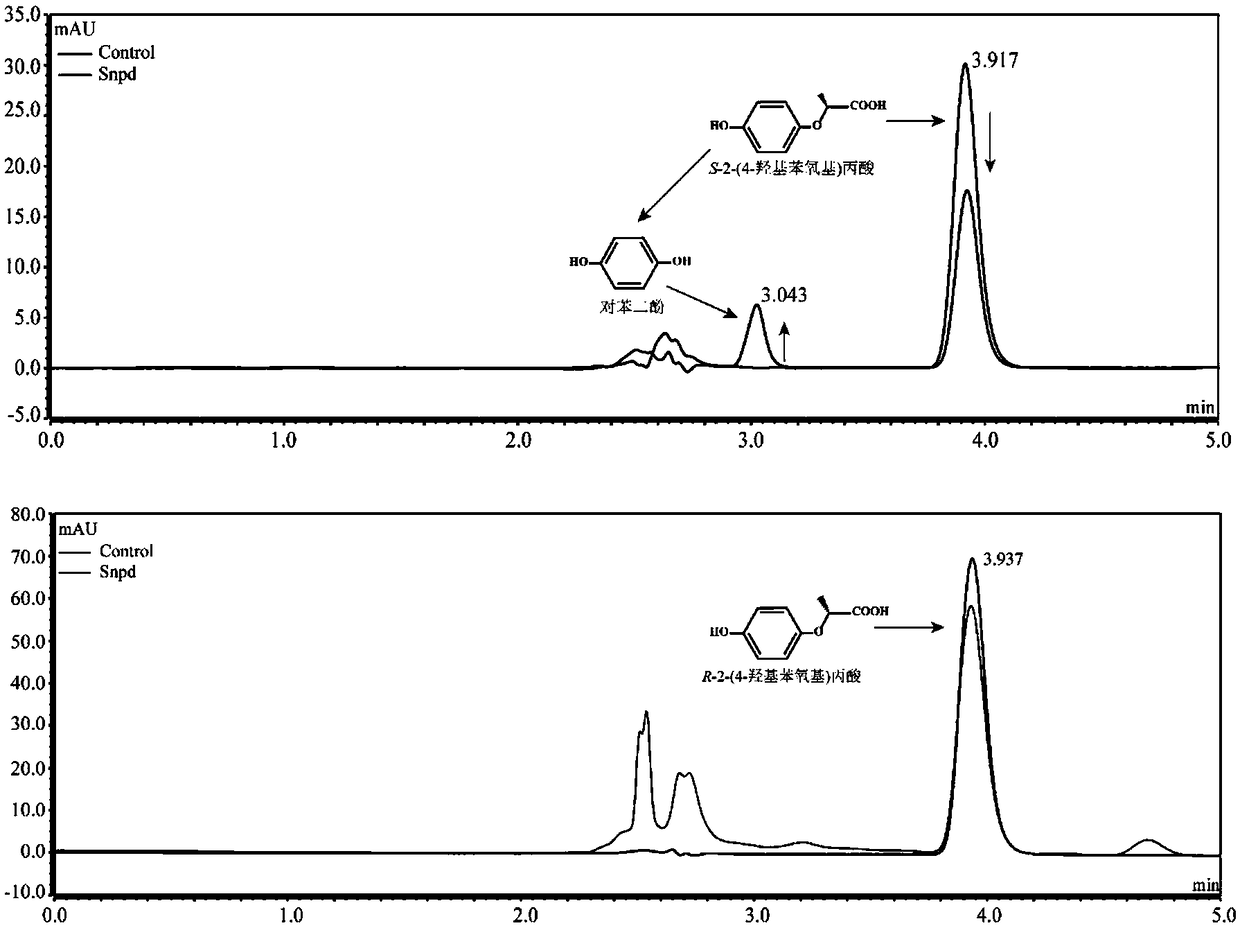
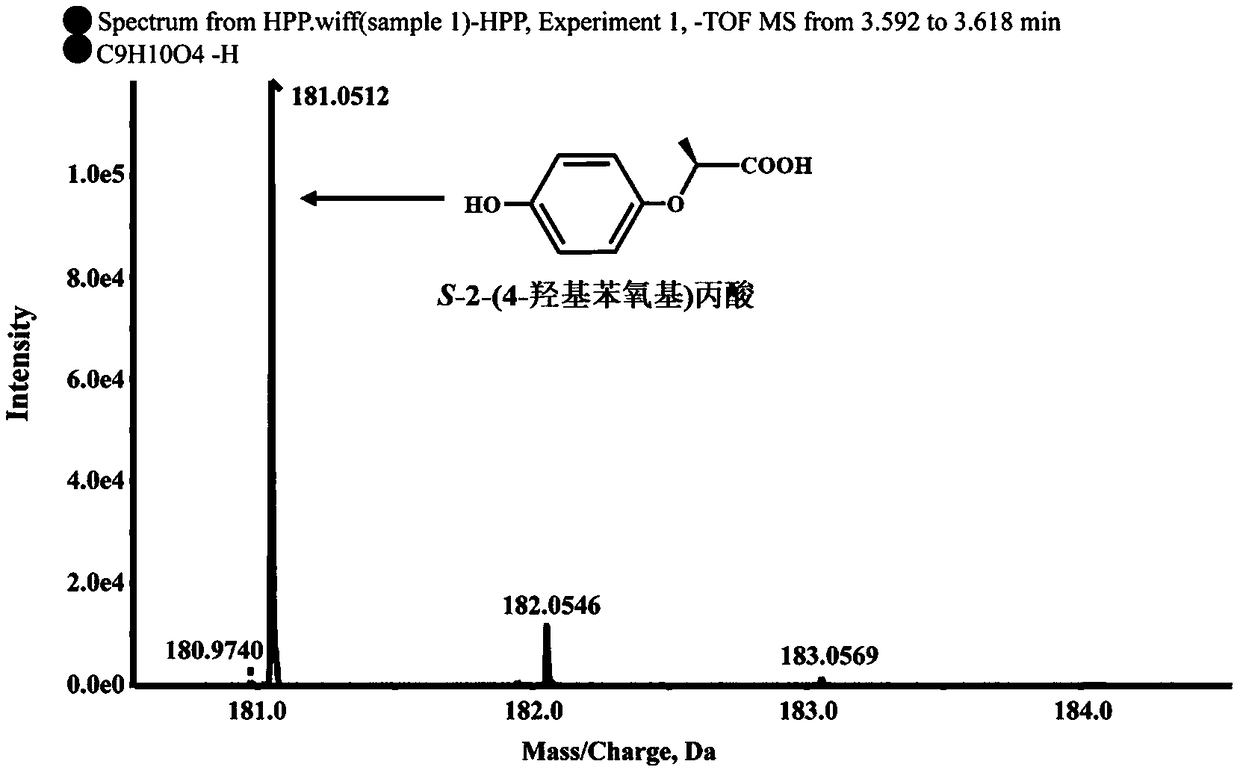
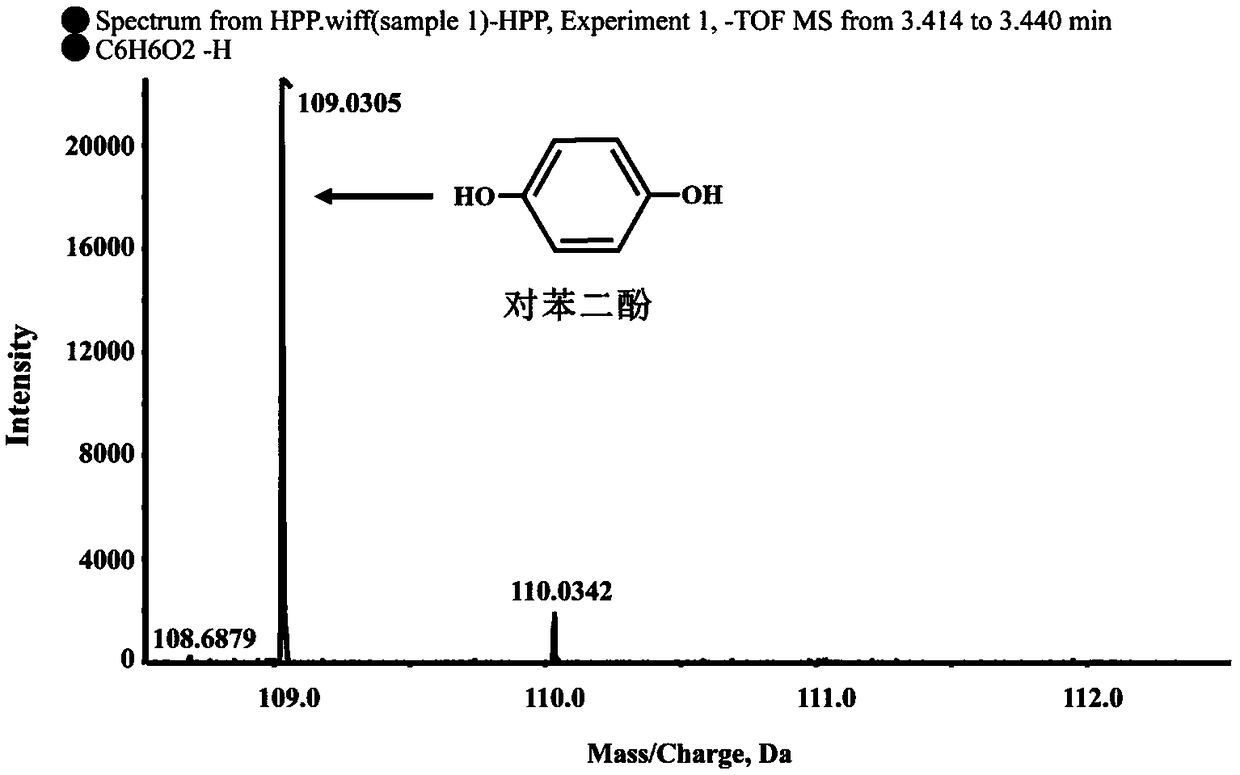
Dioxygenase Snpd with chiral selectivity to intermediate of aromatic oxyphenoxypropionic acid herbicide, and encoding gene and application thereof
The invention discloses a dioxygenase Snpd with chiral selectivity to an intermediate of an aromatic oxyphenoxypropionic acid herbicide, and an encoding gene and application thereof. The dioxygenase gene Snpd with stereoselectivity has the nucleotide sequence of SEQ ID NO.1; and the dioxygenase Snpd encoded by the dioxygenase gene Snp has the amino acid sequence of SEQ ID NO.2. An alpha-ketoglutarate dependent chiral selective dioxygenase gene snpd is successfully cloned through whole-genome sequencing and genetic comparison methods; and the gene is the first disclosed dioxygenase which can degrade S-2-(1-naphthoxy)propionic acid and S-2-(2,4-dichlorophenoxy)propionic acid, S-2-(2,4-hydroxyphenoxy)propionic acid, and herbicide 2,4-dichlorphenoxyacetic acid intermediate, and has very important theoretical value and application prospect in producing an R-type herbicide with high optical purity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Esterase as well as encoding genes and application thereof
The invention discloses esterase as well as encoding genes and application thereof. The amino acid sequence of the esterase is shown in SEQ ID NO.2. The esterase genes are obtained by cloning from pseudomonas Pseudomonas CGMCC NO.4184, and the esterase expressed by the genes has the characteristics of high expression quantity, high selectivity and chiral selectivity. The engineering bacteria containing the esterase genes are applied to a method for catalyzing hydrolysis of rac-2-carboxyethyl-3-cyano-5-methyl ethyl caproate so as to prepare 2-carboxyethyl-(S)-3-cyano-5-methyl ethyl caproate, and when the conversion rate of the reaction is controlled to exceed 50 percent, the unhydrolyzed high-purity 2-carboxyethyl-(S)-3-cyano-5-methyl ethyl caproate can be obtained.
Owner:ZHEJIANG UNIV
Chiral columns with broad chiral selectivity
InactiveUS7141160B2Improve artIon-exchange process apparatusOther chemical processesStationary phaseChiral selectivity
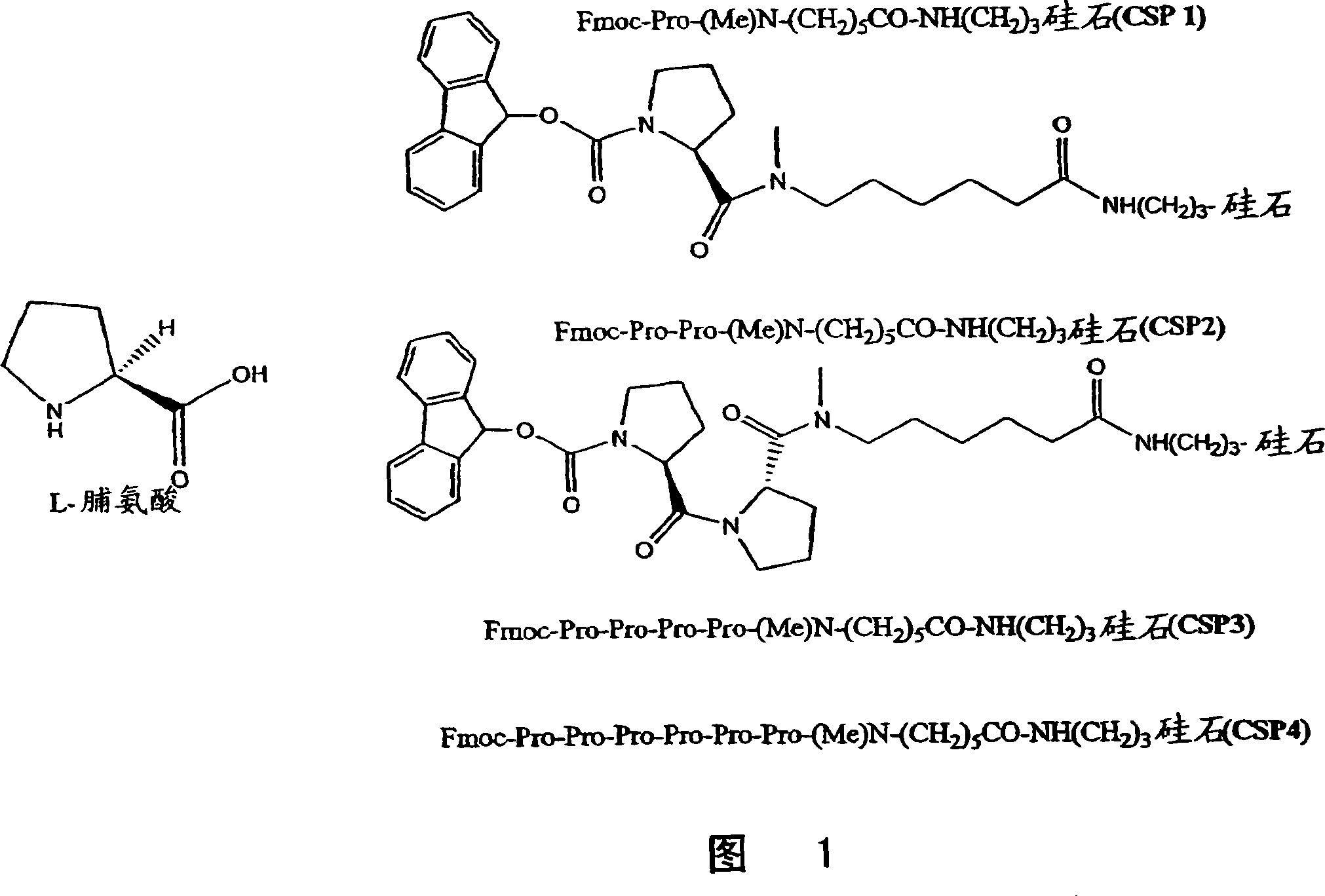
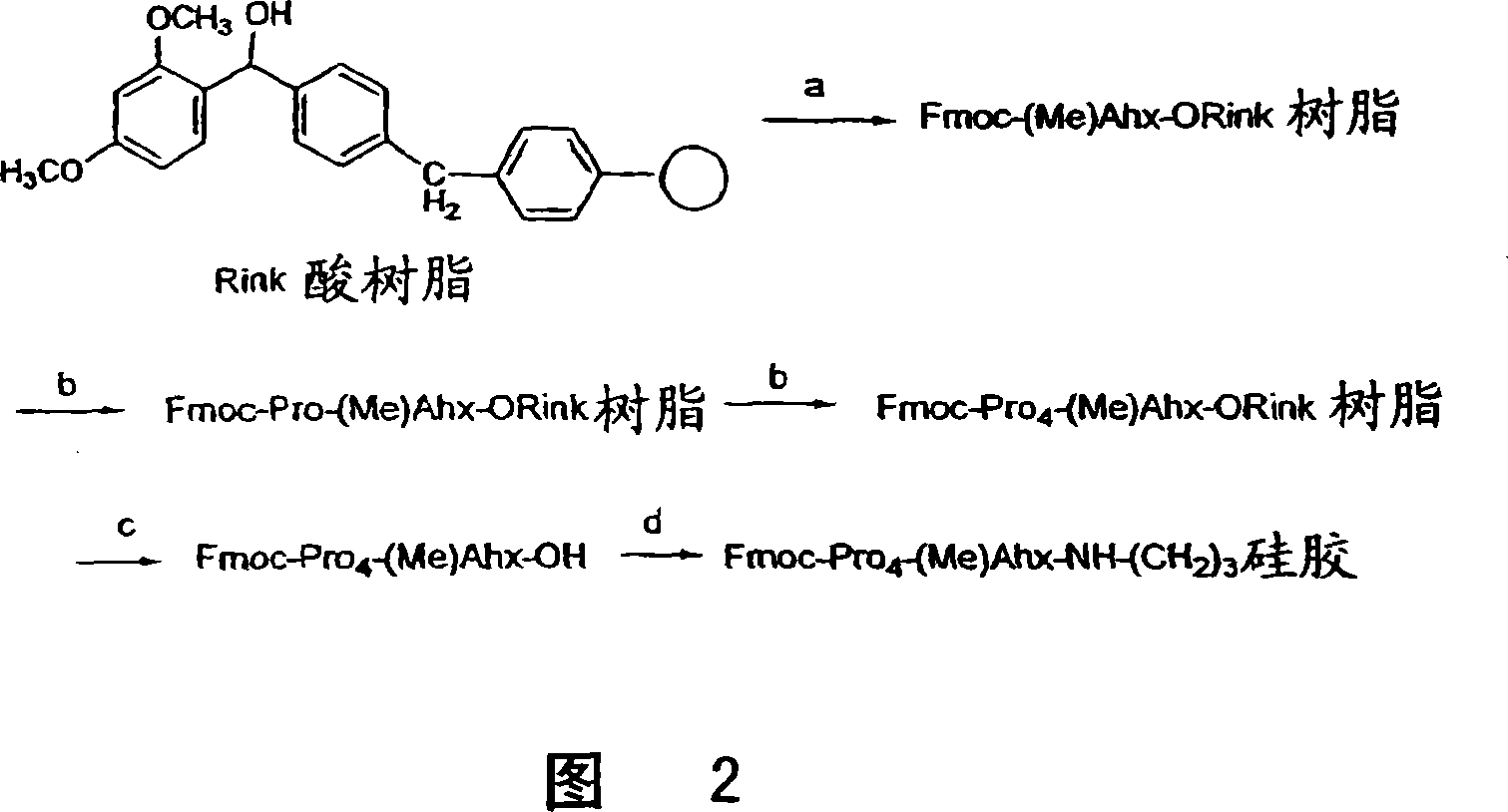
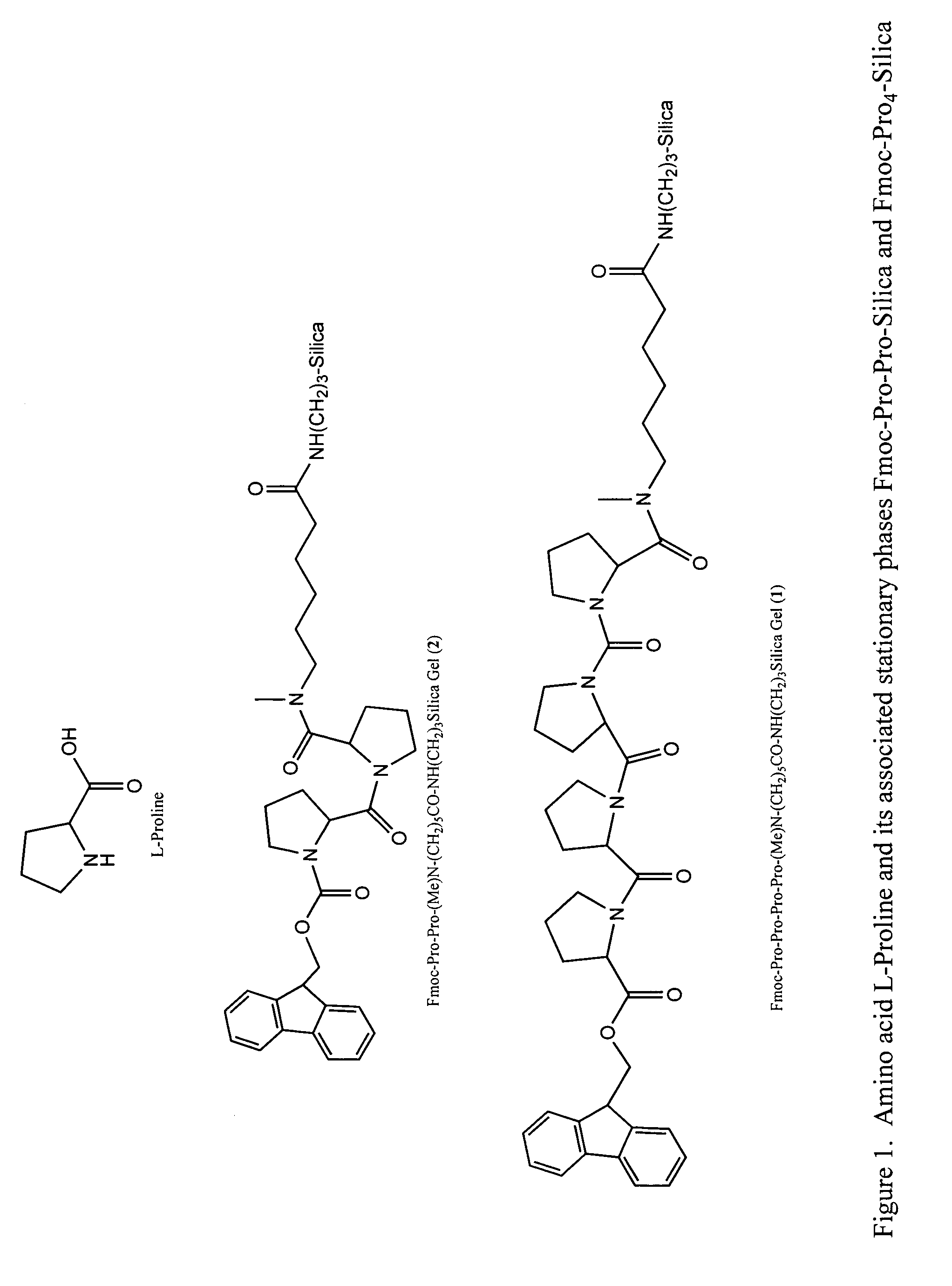
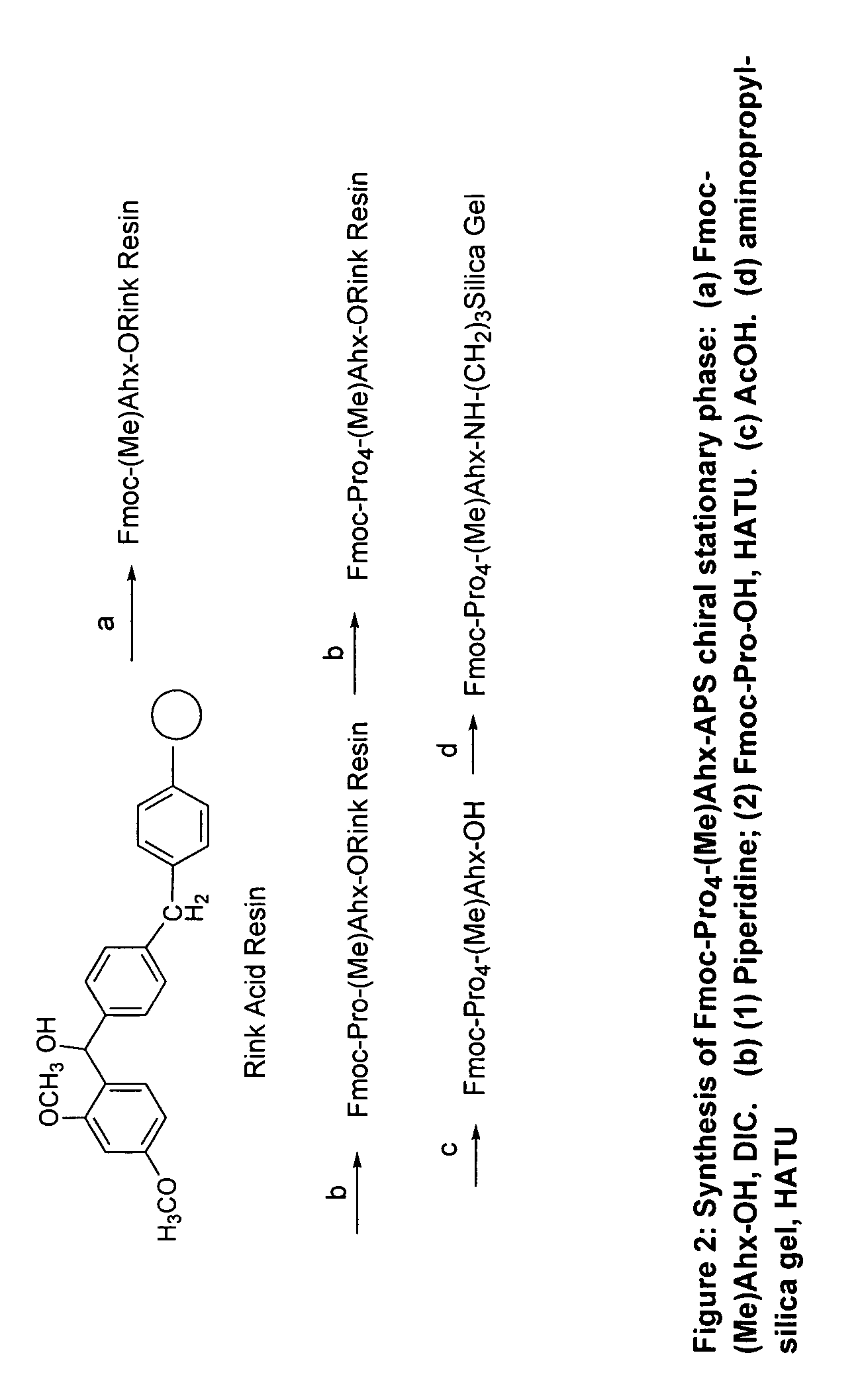
A general chiral column with a multipleproline-based chiral stationary phase. Embodiments include chiral stationary phases of the following formula:wherein n is any integer of 2 or greater, and analogs and isomers thereof.
Owner:LI TINGYU
Biological preparation method of chiral hydroxy acid ester
ActiveCN111454998AHigh activityImprove efficiencyOxidoreductasesFermentationEnzymatic synthesisChiral selectivity
The invention discloses a biological preparation method of a chiral hydroxy acid ester. The method comprises the following steps: (1) oxidizing a fatty acid by ketolase spheroidene monooxygenase to generate a 4-oxo fatty acid; and (2) asymmetrically reducing the 4-oxo fatty acid by short-chain dehydrogenase PpYSDR-M85Q / L136A to obtain an R-hydroxy acid ester. In the biological preparation method of a chiral hydroxy acid ester of the invention, a short-chain dehydrogenase mutant is constructed, the short-chain dehydrogenase mutant greatly improves the activity and chiral selectivity for keto acids compared with the wild type, the efficiency of the enzyme in catalyzing keto acids to prepare chiral lactones can be improved, and thereby an application value of the enzyme is fully explored. Atthe same time, the enzymatic synthesis of the chiral hydroxy acid ester has the characteristics such as simple process, environmental friendliness, and high benefit.
Owner:黄山科宏生物香料股份有限公司
Preparation methods of antihypertensive Fosinopril sodium and key intermediate thereof
ActiveCN107365268AOrganic chemistry methodsGroup 5/15 element organic compoundsChiral selectivityPhenyl-L-proline
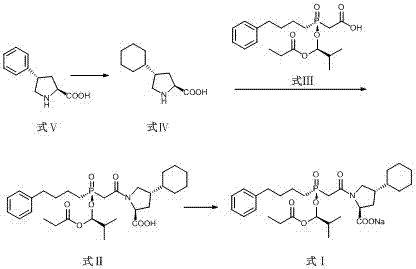
The invention relates to preparation methods of an antihypertensive Fosinopril sodium and a key intermediate thereof trans-4-phenyl-L-proline suitable for industrial production. Synthesis of the key intermediate trans-4-phenyl-L-proline has high chiral selectivity, and the method is simple and easy to operate.
Owner:CHANGZHOU PHARMA FACTORY
Method for preparing L-tertiary leucine based on biological brick tandem double enzymes
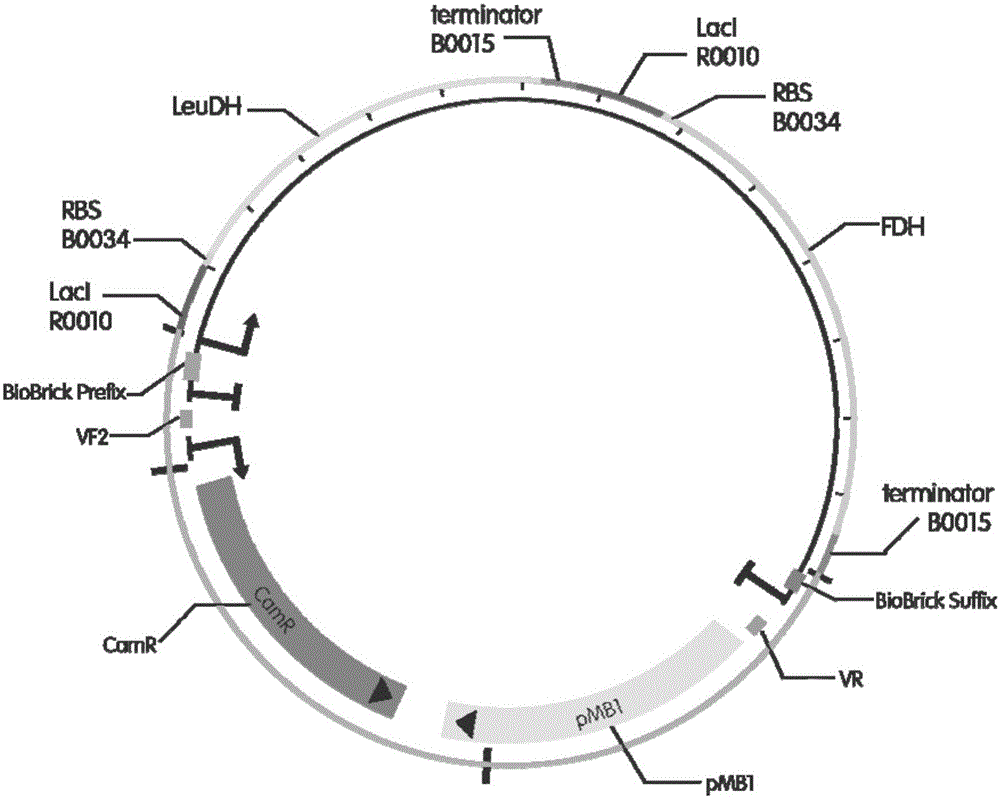
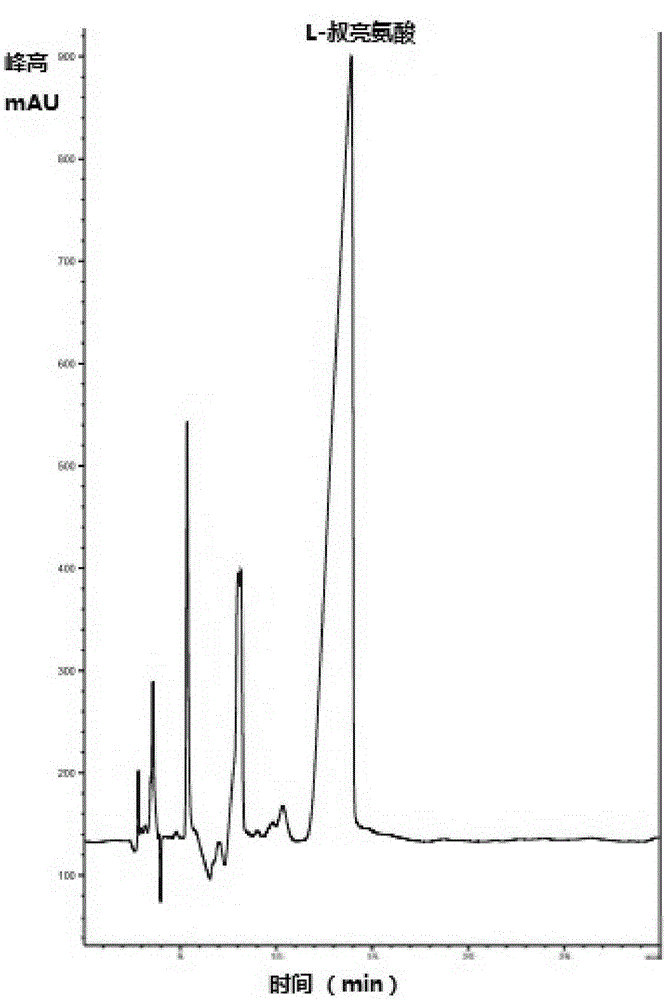
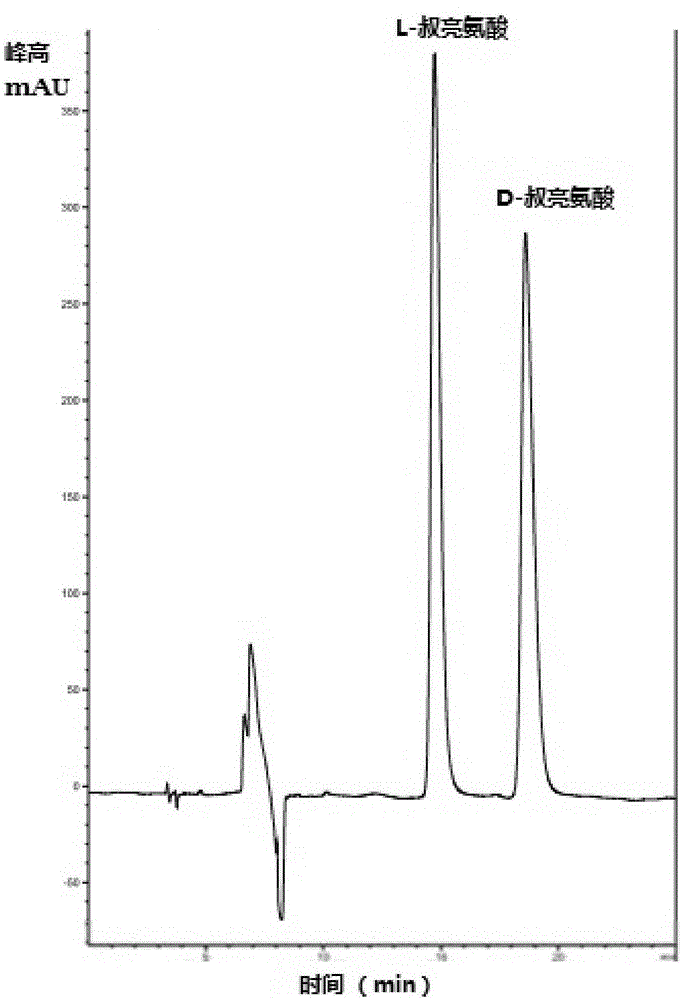
InactiveCN105154488AImprove conversion rateGood chiral selectivityBacteriaFermentationEscherichia coliBrick
The invention discloses a method for preparing L-tertiary leucine based on biological brick tandem double enzymes. The method comprises the following steps: (1) constructing a tandem biological brick element capable of realizing tandem expression of leucine dehydrogenase and formate dehydrogenase; (2) introducing the tandem biological brick element into Escherichia coli (E.coli) so as to construct E.coli engineering bacteria based on tandem expression of leucine dehydrogenase and formate dehydrogenase; (3) inoculating the E.coli engineering bacteria into a liquid enlarged culture medium containing chloromycetin for carrying out culture and induced expression to obtain fermentation liquid, carrying out refrigerated centrifugation to obtain cells, and re-suspending and washing with a buffer solution so as to obtain cell sap; and (4) putting the cell sap, trimethylpyruvic acid, amino donors, coenzymes and cosubstrates for regeneration of the coenzymes in a buffer system for carrying out vibration reaction, and carrying out whole-cell catalysis asymmetric reductive amination to obtain the product, namely L-tertiary leucine. The method disclosed by the invention has the advantages that the product conversion rate is high, the chiral selectivity is better, the reaction conditions are mild, the operation is simple, the expensive coenzymes can be regenerated, and the cost is saved by expressing the double enzymes with single cells.
Owner:XIAMEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com