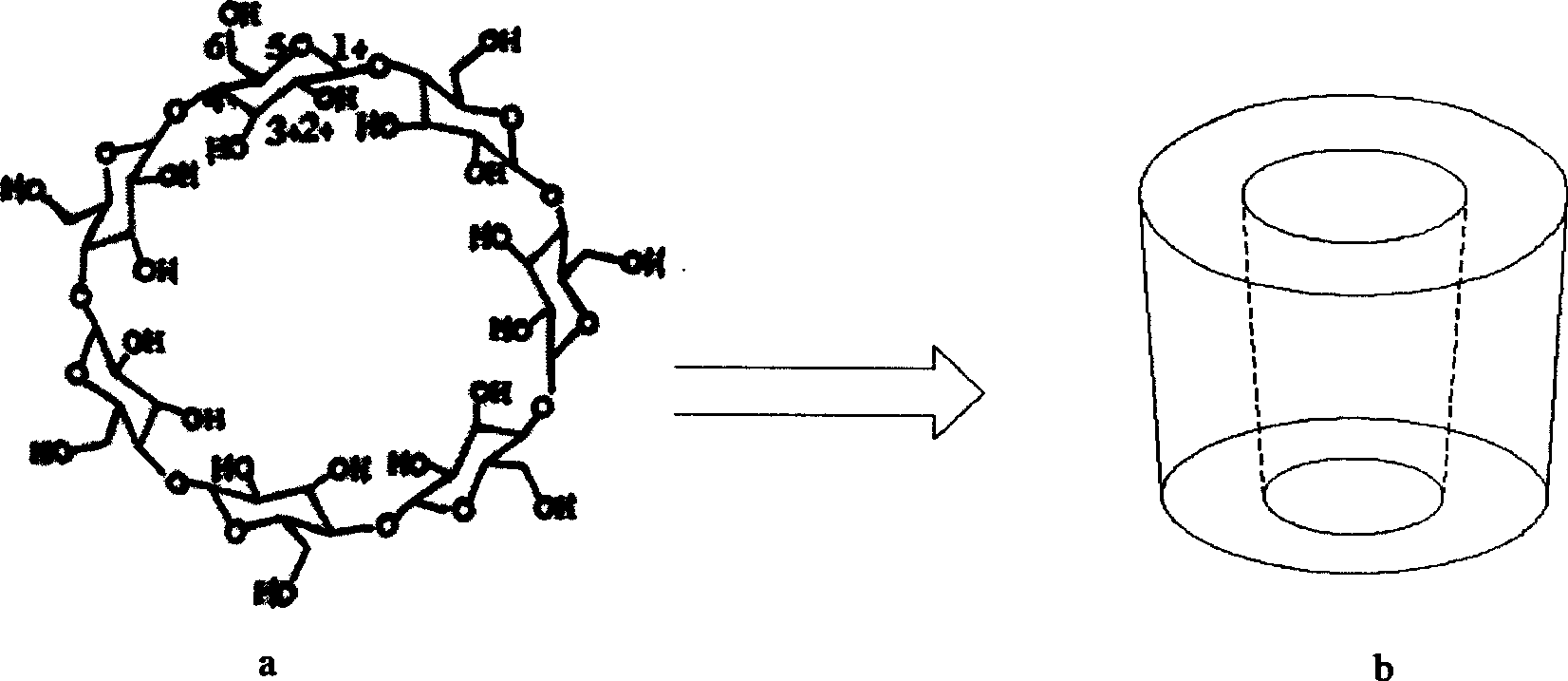
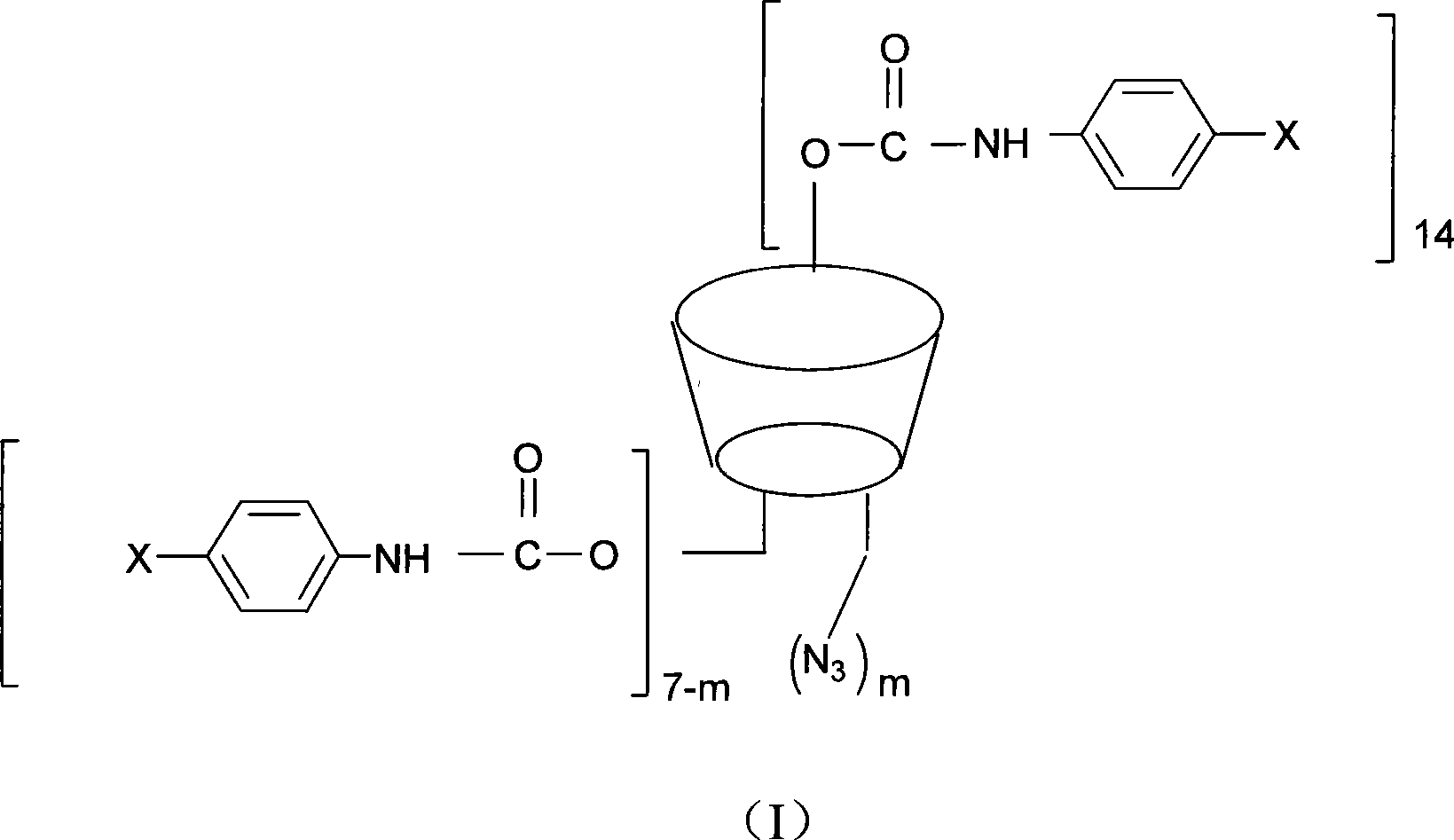
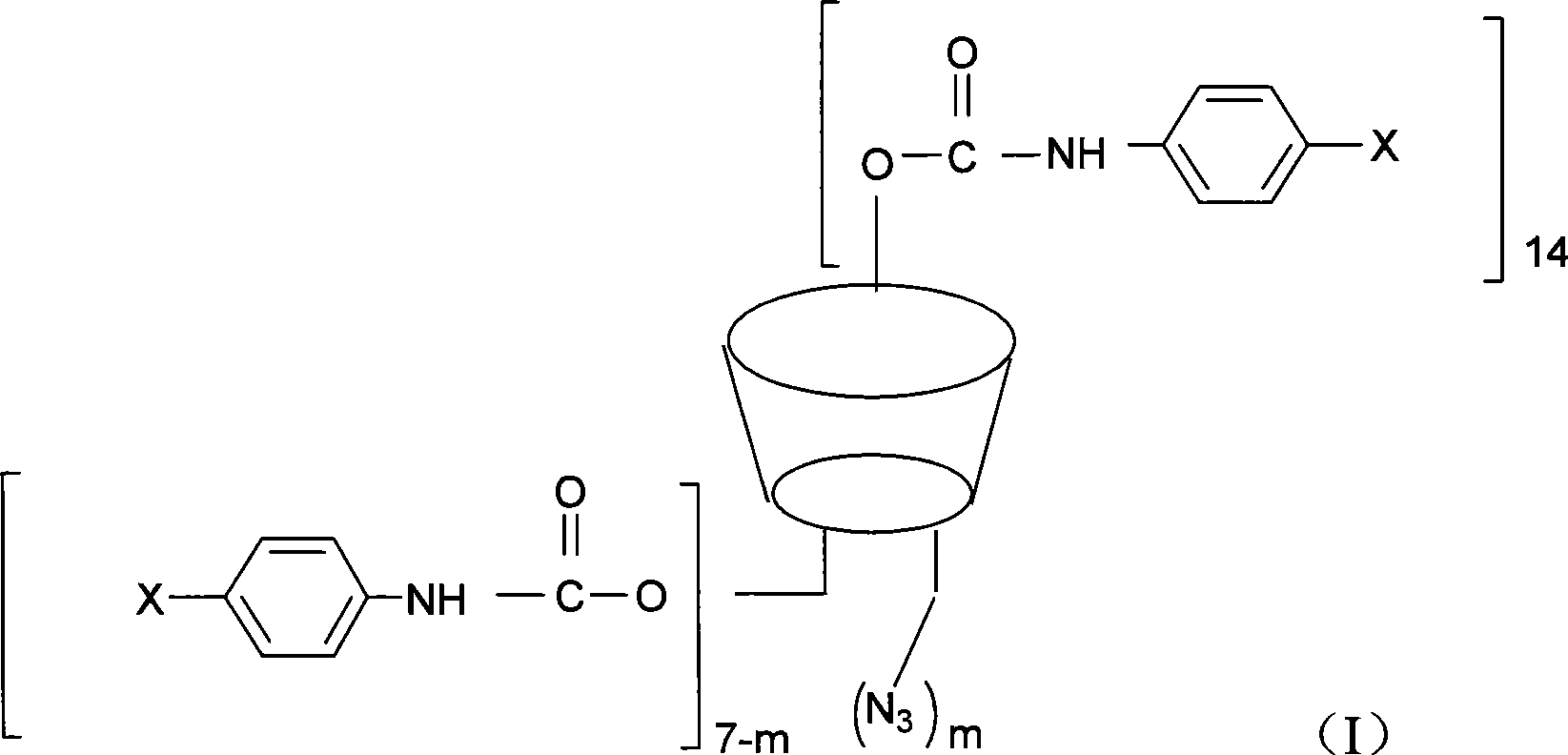
Beta-cyclodextrin derivative, preparation thereof and use as chiral selector
A technology of cyclodextrin and derivatives, which is applied in the field of fully substituted p-halogenated phenyl β-cyclodextrin derivatives and its preparation, can solve the problems of inability to separate chiral compounds and low separation efficiency, and achieve strong chiral recognition ability, wide range of raw material sources, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of SAZ-ClPh-β-CD
[0040] (1)6 A Preparation of -O-P-methylbenzenesulfonyl-β-cyclodextrin:
[0041] In a three-neck flask equipped with a magnetic stirrer and a thermometer, 30 ml of pyridine was used as a solvent, and 20 mmol of β-cyclodextrin and 16 mmol of p-toluenesulfonyl chloride were sequentially added. The reaction conditions were controlled at 25±2°C, and the reaction time was 20 hours; after the reaction was completed, pyridine was evaporated under reduced pressure, washed 3 times with 200ml acetone each time, and the white product TS-β-CD was obtained after vacuum drying, with a yield of about 8 gram;
[0042] (2)6 A -Azide-6 A - Preparation of deoxy-β-cyclodextrin:
[0043] Take TS-β-CD 6mmol obtained in step (1) and sodium azide 124mmol, add 400ml of ion-free water, and in a three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, control the reaction temperature to be 60-65 °C, the reaction time...
Embodiment 2
[0046] Example 2 Preparation of SAZ-ClPh-β-CD
[0047] (1)6 A Preparation of -O-P-methylbenzenesulfonyl-β-cyclodextrin:
[0048] In a three-necked flask equipped with a magnetic stirrer and a thermometer, 30 ml of pyridine was used as a solvent, and 20 mmol of β-cyclodextrin and 18 mmol of p-toluenesulfonyl chloride were sequentially added. The reaction conditions were controlled at 25±2°C, and the reaction time was 24 hours. After the reaction, the pyridine was evaporated under reduced pressure, washed three times with 200ml acetone each time, and about 8.8 grams of white product TS-β-CD was obtained after vacuum drying.
[0049] (2)6 A -Azide-6 A - Preparation of deoxy-β-cyclodextrin:
[0050] Take 6 mmol of TS-β-CD obtained in step (1) and 132 mmol of sodium azide, add 400 ml of ion-free water, and in a three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, control the reaction temperature to be 85 to 90 °C, the reaction time is 4h; ...
Embodiment 3
[0055] Example 3 Preparation of MAZ-ClPh-β-CD)
[0056] (1) Seven replaces 6 A - Preparation of I-β-cyclodextrin:
[0057] In a three-necked flask equipped with a magnetic stirrer and a thermometer, 60 ml of dichloroethane was used as a solvent, and 20 mmol of β-cyclodextrin, 160 mmol of iodine, 140 mmol of triphenylphosphine, and 140 mmol of imidazole were sequentially added. Control the reaction condition to be 25±2°C, and the reaction time is 3h under the protection of nitrogen; after the reaction is completed, filter and remove the filtrate, wash the solid with 80ml ether 3 times each time, and vacuum dry to obtain the yellow product heptasubstituted 6 A -I-β-CD11 grams or so.
[0058] (2) Seven replaces 6 A -Azide-6 A - Preparation of deoxy-β-cyclodextrin:
[0059] Take step (1) gained seven to replace 6 A -I-β-CD 6mmol and sodium azide 848mmol were added into 600ml deionized water, and in a three-necked flask equipped with a magnetic stirrer, a thermometer and a re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com