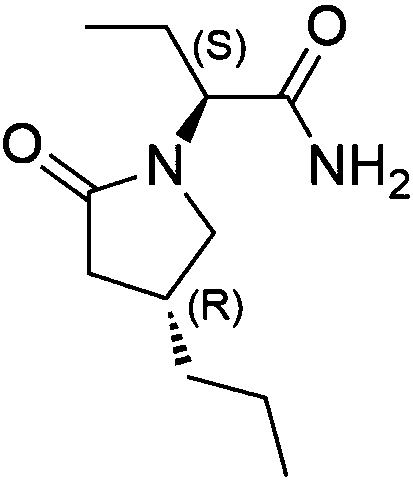
Preparation method of high chiral purity lactam intermediate and brivaracetam
A technology for intermediates and lactams, applied in the preparation of high chiral purity lactam intermediates, in the field of preparation of brivaracetam, can solve the problems of cumbersome steps, high activity of intermediates, easy occurrence of racemization, etc., and achieves simple operation. Safe, simple separation process, improved selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
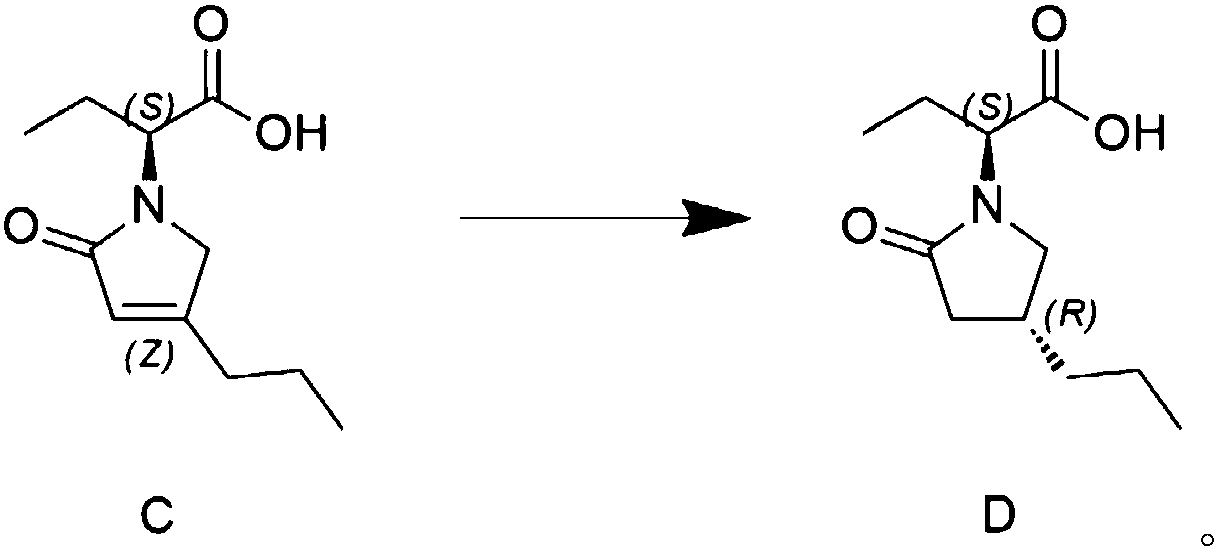
[0027] Preparation of Compound D
[0028] Add citric acid monohydrate (50g 0.237mol) to a 1L four-necked reaction flask, stir to dissolve in 500ml of water, add 5g of 5% palladium carbon, stir, add 50g (0.237mol) of the compound of chemical formula C, control the temperature at 30°C, replace with hydrogen, The hydrogen pressure was 2 bar, and the reaction was stirred. After 20 hours of reaction, the TLC was controlled until the raw materials disappeared completely, the reaction was stopped, filtered, 2M hydrochloric acid was adjusted to pH=2, the temperature was lowered to 5°C, filtered, and washed with 50ml of water to obtain 46g of white solid. 100ml of methyl tert-butyl ether was recrystallized to obtain 41g (0.192mol) of white solid, yield 81%, HPLC 99.52%, de% 99.2%.
Embodiment 2
[0030] Preparation of Compound D
[0031] Add 12g (0.115mol) of malonic acid, 250ml of water and 250ml of isopropanol into a 1L four-necked reaction flask, stir to dissolve, add 5g of 10% palladium carbon, stir, add 50g (0.237mol) of the compound of chemical formula C, and control the temperature at 30°C. Replace with hydrogen, the hydrogen pressure is 4 bar, stir the reaction, after 20 hours of reaction, TLC control until the raw material disappears completely, stop the reaction, filter, adjust the pH=2 with 2M hydrochloric acid, cool down to 5°C, filter, wash with 50ml of water, the crude product is washed with methanol 100 ml of tert-butyl ether was recrystallized to obtain 44 g (0.206 mol) of white solid, yield 87%, HPLC 99.23%, de% 99.1%.
Embodiment 3
[0033] Preparation of Compound D
[0034] Add 20g (0.095mol) of citric acid monohydrate to a 500ml reaction bottle, stir to dissolve in 100ml of water, add 0.5g of 10% palladium carbon, add 1,3,5-triazine-2,4,6-trithione trisodium Add 0.0001g of salt, stir after adding, then add 10g (0.047mol) of the compound of chemical formula C, control the temperature at 5°C, replace with hydrogen, the pressure of hydrogen is 5bar, stir and react, after 20 hours of reaction, control in TLC until the raw materials disappear completely, stop the reaction , filtered, adjusted to pH=2 with 2M hydrochloric acid, cooled to 5°C, filtered, washed with 20ml of water, and the crude product was recrystallized with 20ml of methyl tert-butyl ether to obtain 8.1g (0.038mol) of white solid, yield 82%, HPLC95 .6%, de% 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com