Marine bacterial novel esterase, as well as preparation method and application thereof
An esterase, a consistent technology, applied in the field of marine bacterial new esterase and its preparation and application, can solve the problems of expensive commercial enzymes and unsuitable for large-scale industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the construction of the recombinant expression plasmid of esterase gene pe8 and recombinant strain
[0046] Pelagibacterium halotolerans B2 T The middle esterase gene pe8 was cloned into the expression vector, and the recombinant expression strain was constructed. Based on the core conserved sequences of all esterase families published so far, the upstream primer pe8F (5'-AGGA CATATG ACCGAACCCGTAAAG-3', Nde I) and downstream primer pe8R (5'-CGAT AAGCTT CTAGAGGATCTCGCG-3', HindIII), PCR amplification confirmed the full-length sequence of the gene. The expression plasmid was constructed by enzyme digestion cloning, that is, the PCR product was double-digested with NdeI and HindIII, the digested fragment was recovered by tapping the rubber, and it was ligated with the plasmid pET28b(+) that had also been double-digested with NdeI and HindIII. 2 The transformation method was transformed into Escherichia coli DH5α, and positive clones were screened for kana...
Embodiment 2
[0047] Embodiment 2: Utilize recombinant expression strain to express recombinant esterase gene pe8
[0048] Transfer 3ml of the constructed recombinant expression strain to 100ml of LB liquid medium containing 20μg / ml kanamycin and 34μg / ml chloramphenicol, and shake at 37°C until OD 600 When it reaches 0.6, add IPTG with a final concentration of 0.5mM to induce expression, and transfer to 25°C for shaking culture overnight. The cells were collected by low-temperature centrifugation and resuspended in PBS buffer (137mM NaCl, 2.7mM KCl, 10mM NaCl 2 HPO 4 , pH7.4), washed twice, and ultrasonicated on ice. The supernatant was collected by low-temperature centrifugation, and freeze-dried for 24 hours to obtain PE8 esterase powder.
Embodiment 3
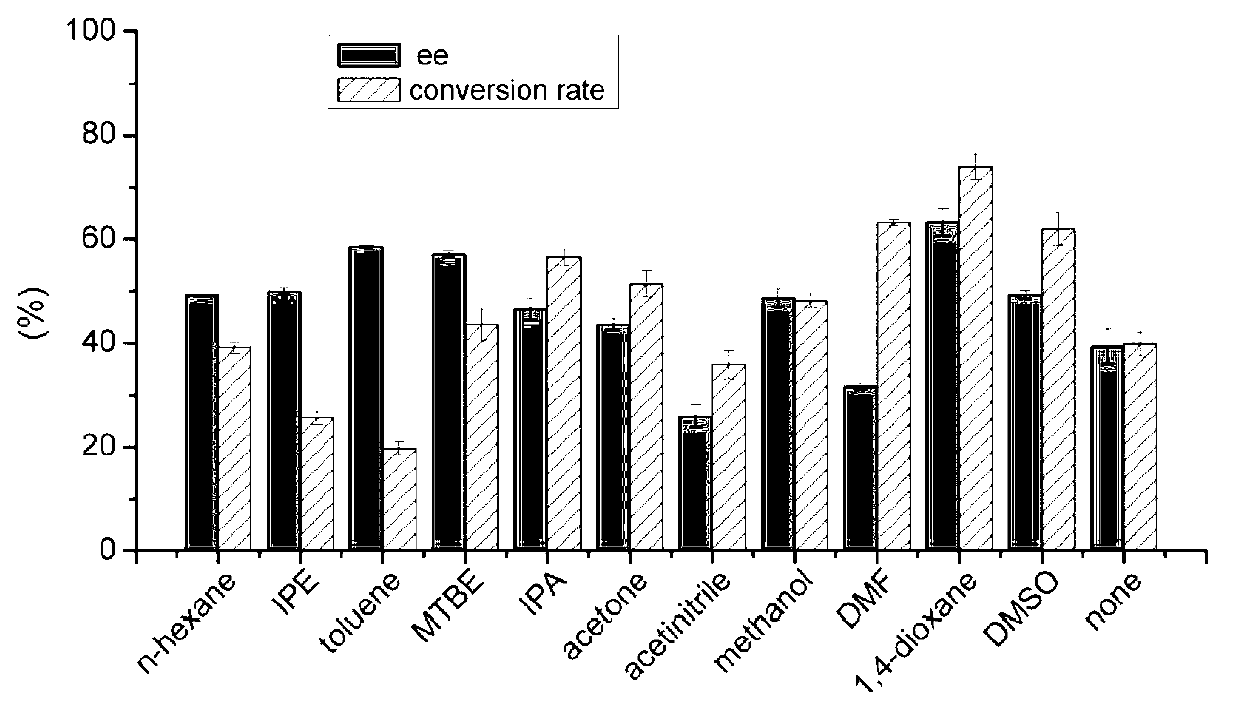
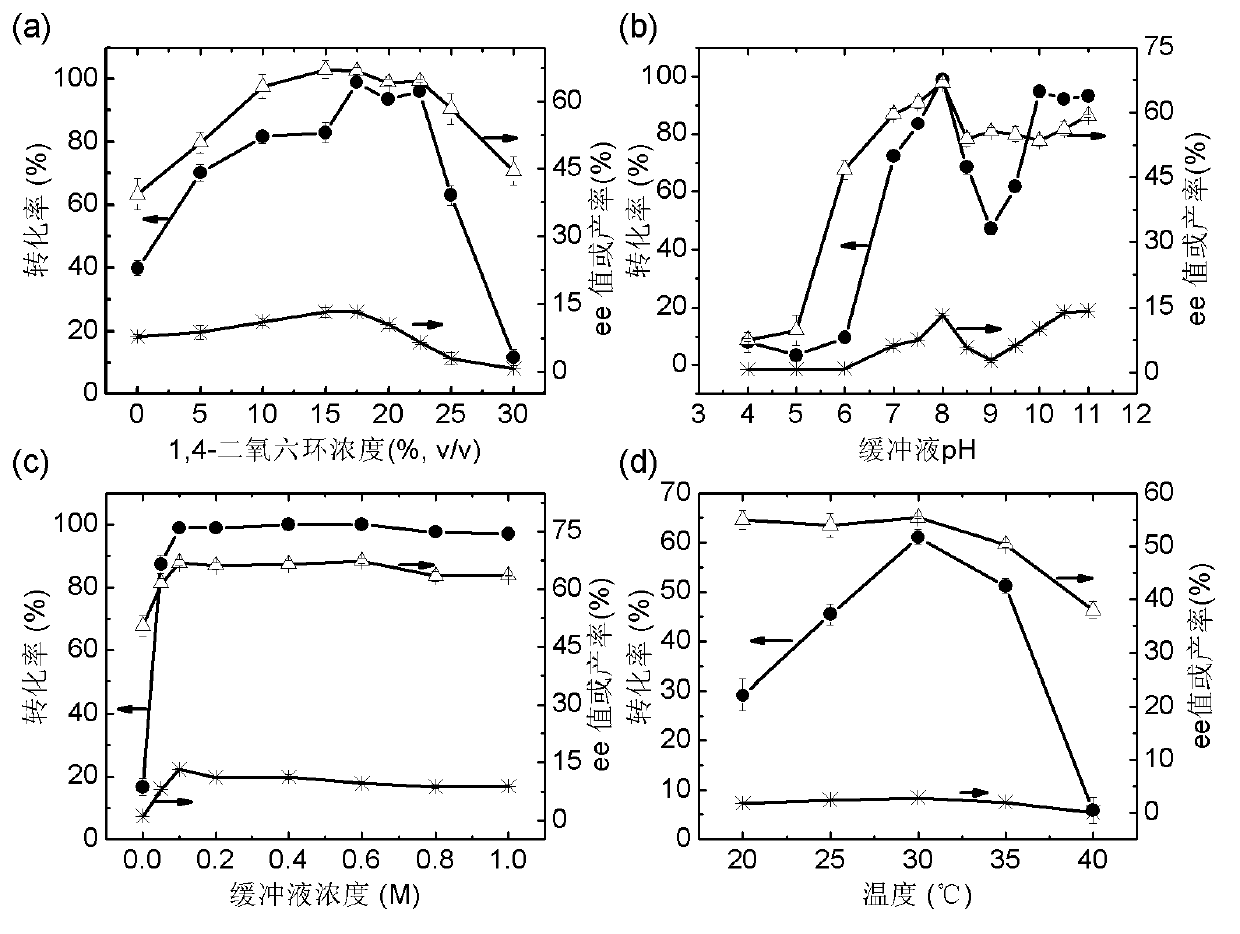
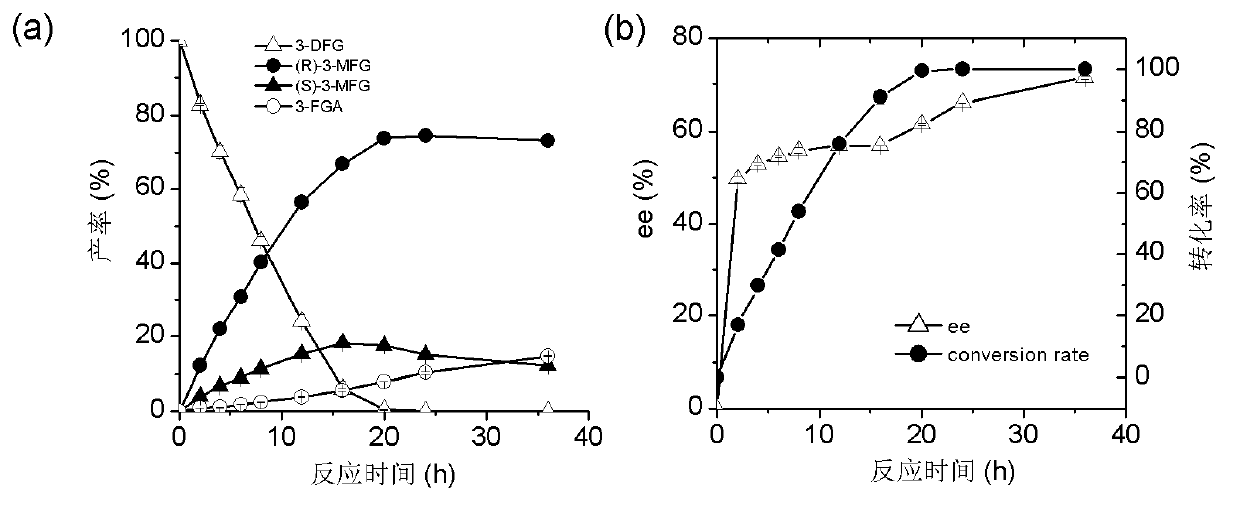
[0049] Example 3: Hydrolysis of 3-(4-fluorophenyl) dimethyl glutarate by esterase PE8
[0050] Use esterase PE8 to hydrolyze 3-(4-fluorophenyl) dimethyl glutarate, specific operation: Include 40mM 3-(4-fluorophenyl) dimethyl glutarate, 5mg PE8 in a 0.5ml reaction system Enzyme powder, 30°C, 200rpm shaking reaction for 24h, adjust the pH to 2.0 with 5M hydrochloric acid to terminate the reaction, add 0.5ml ethyl acetate to extract twice, vacuum dry to remove ethyl acetate, then add 300μl isopropanol to dissolve the precipitate, high-efficiency solution The product components were analyzed by phase chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com