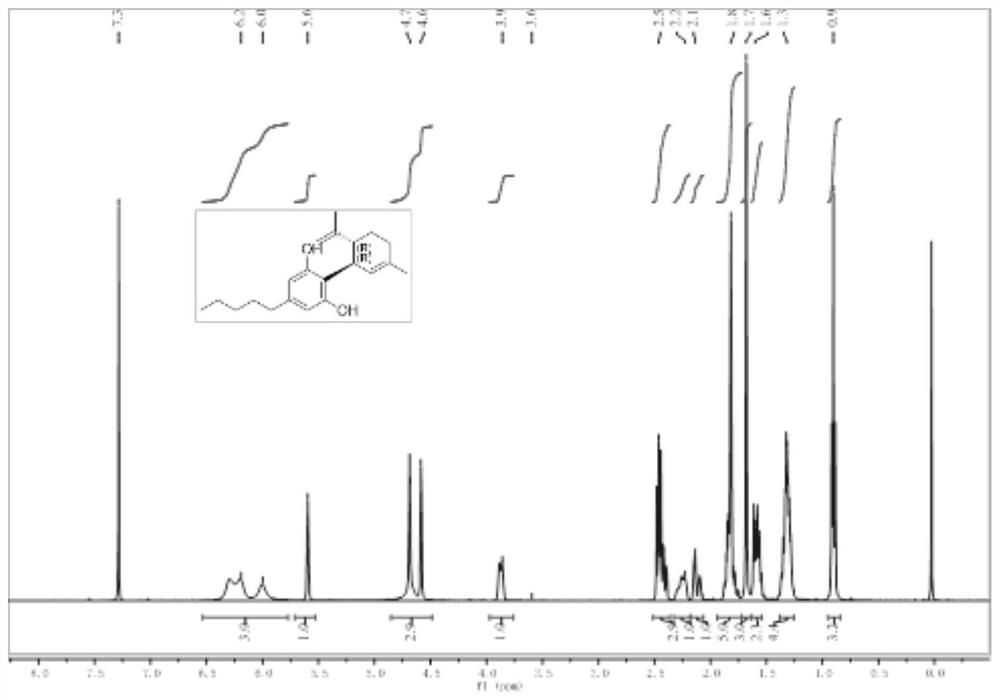
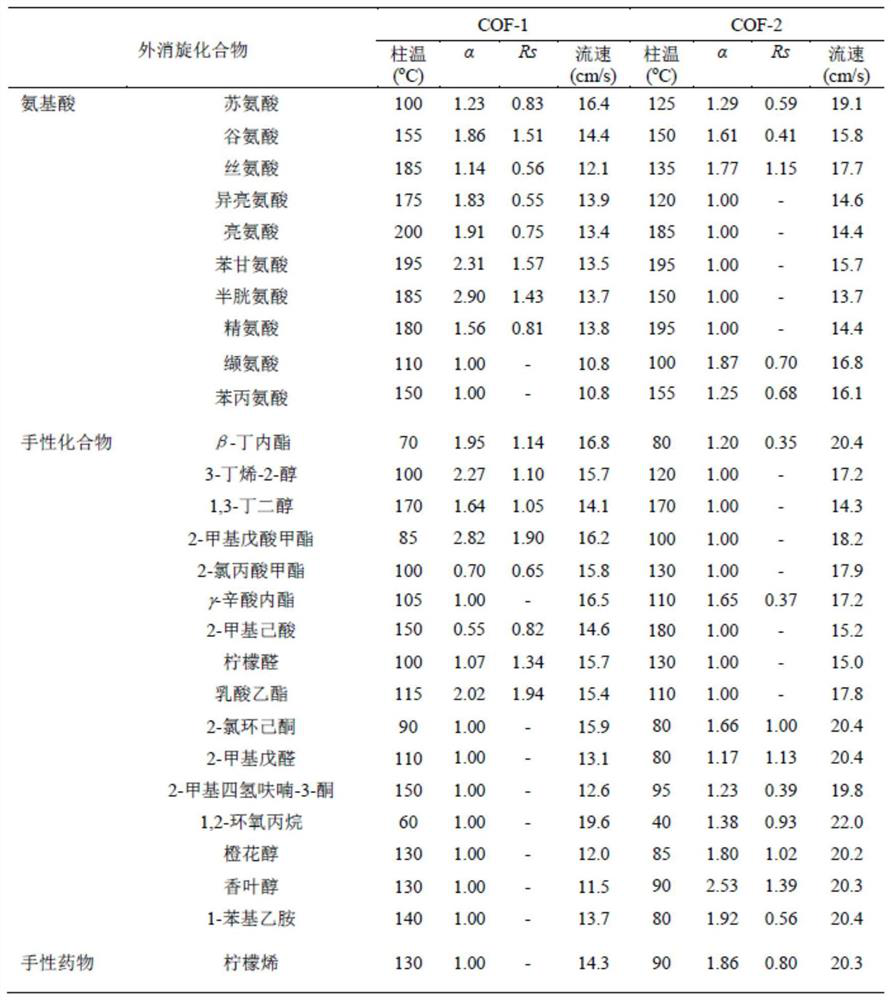
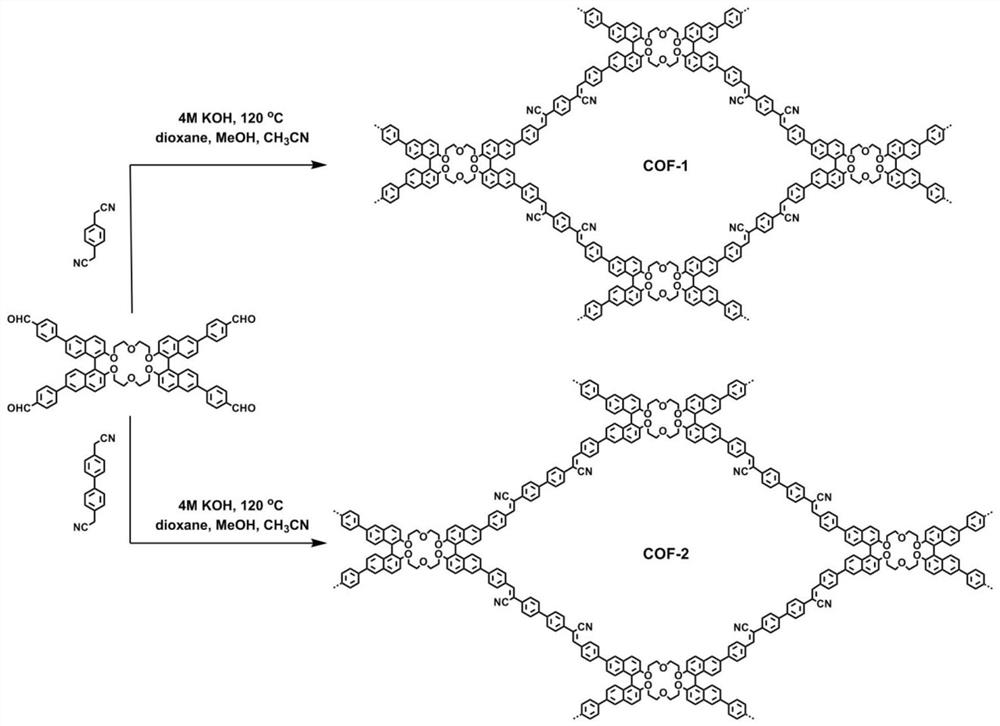
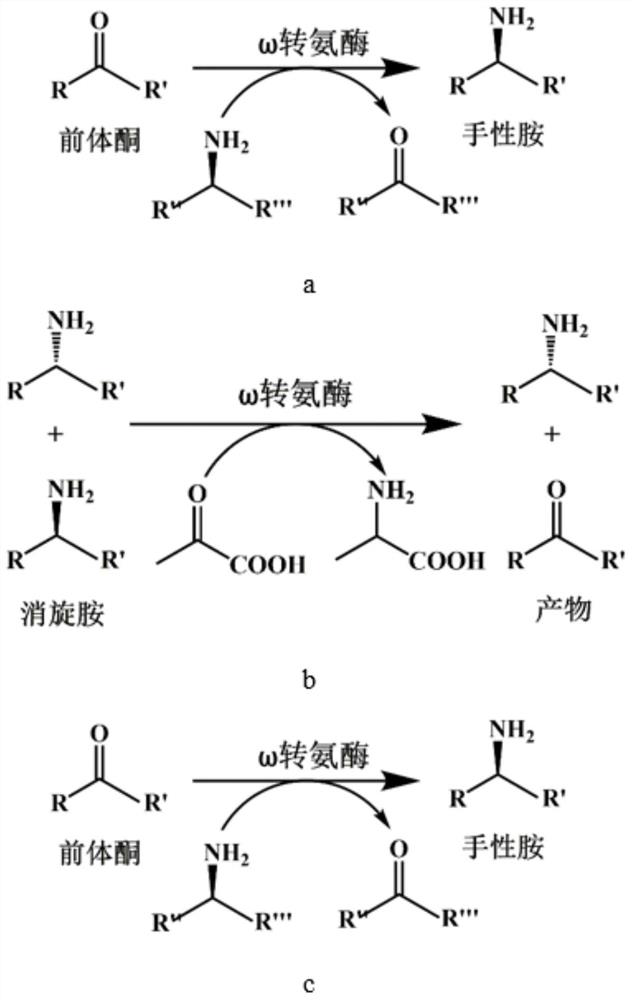
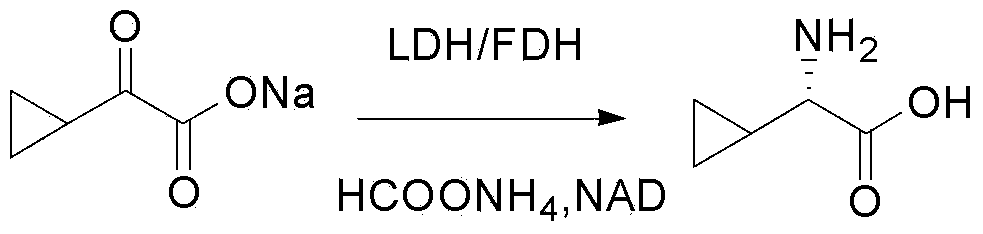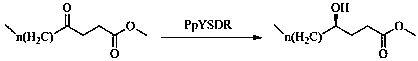Patents
Literature
47results about How to "High chiral selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Platinum/carbon nanotube catalyst and preparation method and application thereof
InactiveCN102039121AMild conditionsEasy to manufactureOrganic reductionMaterial nanotechnologyCarbon nanotubeRoom temperature
The invention relates to a platinum / carbon nanotube catalyst suitable for multiphase asymmetric hydrogenation reaction. Platinum is loaded on a carbon nanotube carrier. A preparation method comprises the following steps of: heating a purified carbon nanotube in nitric acid, washing, filtering, washing by using water until the pH value of filtrate is neutral, drying to obtain the carbon nanotube carrier, soaking in aqueous solution of chloroplatinic acid, and performing ultrasonic treatment at room temperature; and stirring and impregnating a mixture of the carbon nanotube and the aqueous solution of chloroplatinic acid, raising the temperature to 110 DEG C from room temperature, drying at the temperature of 110 DEG C, grinding into fine powder, reducing by using aqueous solution of sodiumformate with heating, filtering, washing by using deionized water and drying. The invention also provides the preparation method of the catalyst and application of the catalyst to the multiphase asymmetric hydrogenation reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
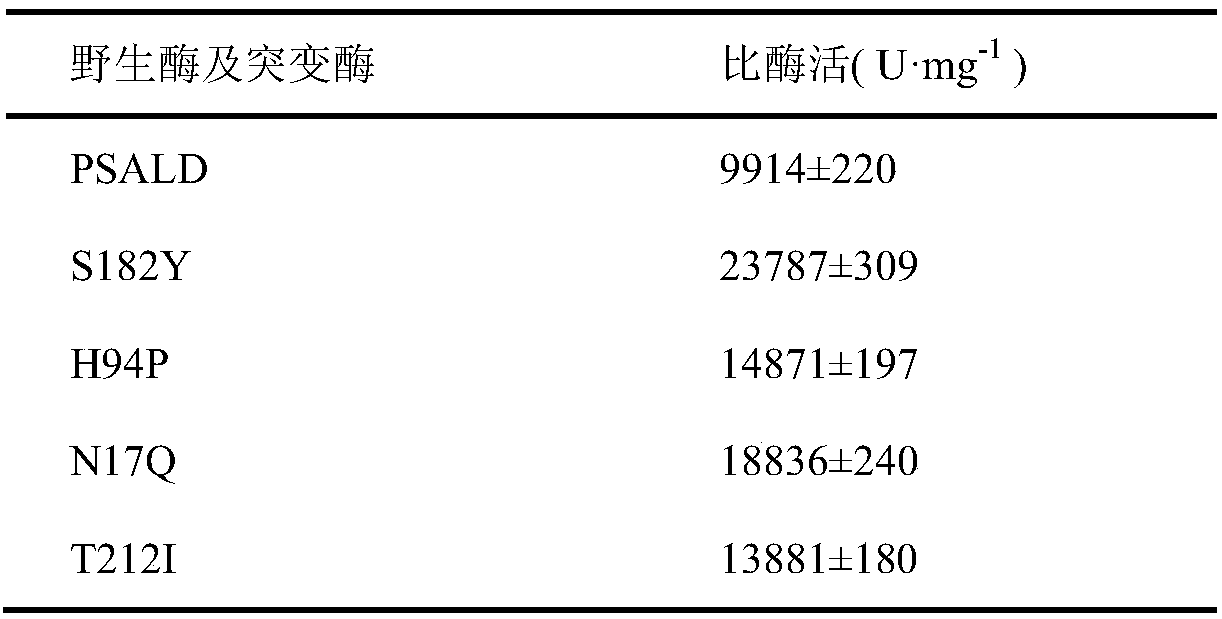
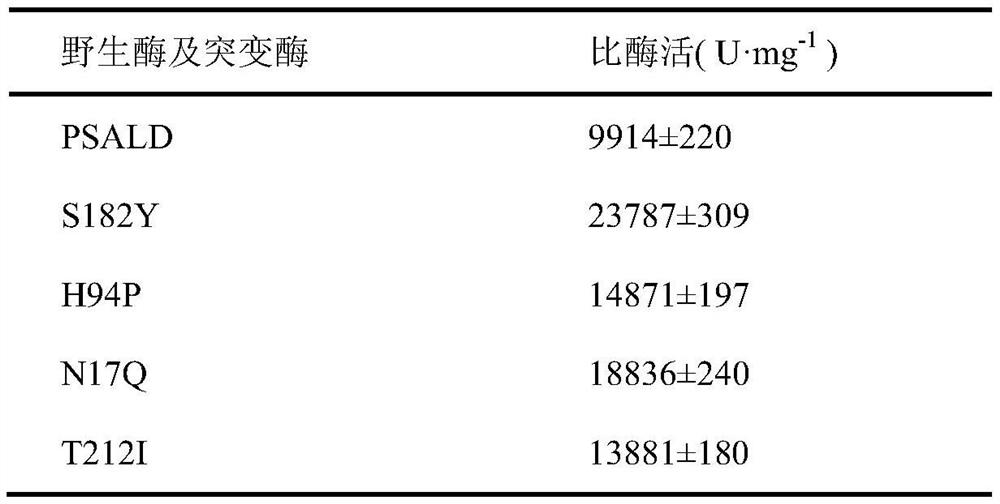
Threonine aldolase, mutant and application of threonine aldolase and mutant to preparation of substituted phenylserine derivatives
The invention discloses threonine aldolase, a mutant, encoding genes of the threonine aldolase and the mutant, a recombinant vector constructed by the encoding genes, recombinant genetic engineering strains obtained by transforming the recombinant vector and application of the threonine aldolase and the mutant in preparation of 2- / 3- / 4-substituted phenylserine derivatives. The threonine aldolase and the mutant serve as biocatalysts, and 2- / 3- / 4-substituted benzaldehyde serves as a substrate, 5-pyridoxal phosphate serves as coenzyme, glycine / glycine ester serves as an auxiliary substrate, and enzymic catalytic reaction is performed in a medium under appropriate conditions to separate and prepare a series of phenylserine derivatives with different substituents. According to the method, the total yield ranges from 76% to 99%, and the ee value of the product is greater than 99%, and the de value ranges from 70% to 99%.
Owner:王喆明 +1
Mutant of threonine aldolase and application thereof in preparation of substituted phenylserine derivatives
The invention discloses a mutant of threonine aldolase, a coding gene of the mutant, a recombinant vector constructed by the coding gene, a recombinant genetic engineering bacterium obtained by converting the recombinant vector, and application of the mutant of the threonine aldolase in preparation of 2- / 3- / 4-substituted phenylserine derivatives. According to the invention, the mutant of the threonine aldolase is taken as a biocatalyst, 2- / 3- / 4-substituted benzaldehyde is taken as a substrate, 5-phosphopyridoxal is taken as a coenzyme, glycine / glycine ester is taken as a cosubstrate, the enzyme catalysis reaction is performed under proper conditions and in a proper medium, a series of phenylserine derivatives with different substituent groups are prepared through separation, and the method has the total yield range of 76-99%, the product ee value of over 99%, and the de value range of 71-92%.
Owner:王喆明 +1
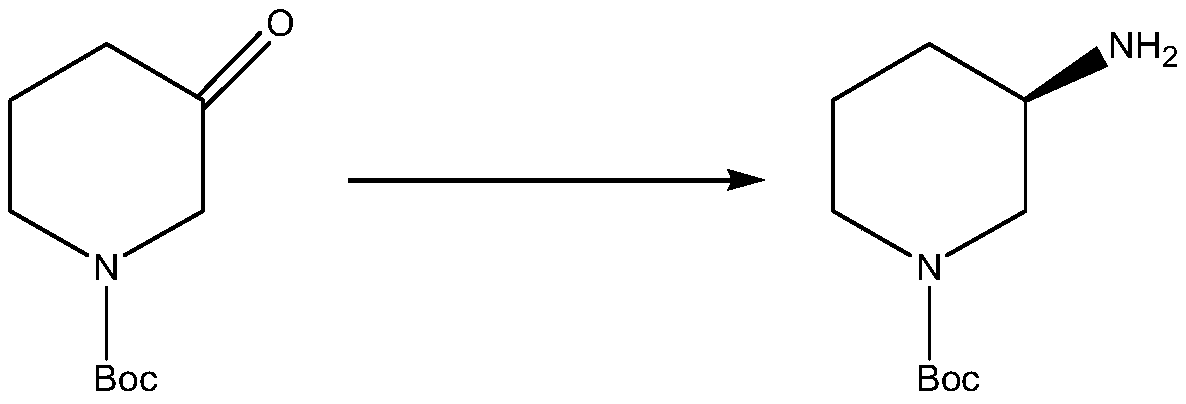
Method for synthesizing (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting transaminase catalyst and enzymatic way
ActiveCN110724675AStrong specificityHigh catalytic activityTransferasesGenetic engineeringPtru catalystTert-Butyloxycarbonyl protecting group
The invention relates to the technical field of enzyme catalysis, and specifically relates to a transaminase catalyst; and the invention further relates to a method for synthesizing (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting an enzymatic way, as well as a production method of the (R)-1-tert-butoxycarbonyl-3-aminopiperidine. The method for synthesizing the (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting the enzymatic way comprises a step of, in the presence of pyridoxal phosphate and the transaminase catalyst, allowing reaction of N-tert-butoxycarbonyl-3-piperidinone as a reaction substrate with an amino donor so as to produce the (R)-1-tert-butoxycarbonyl-3-aminopiperidine. The method for synthesizing the (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting the enzymatic way utilizes relatively few reagents, and is mild in reaction conditions, so that tedious steps needed for chemical process synthesis are greatly simplified; and moreover, a target product withan ee value up to 99.77% or above can be obtained without performance of separation. Therefore, the transaminase catalyst and the process method utilizing the transaminase catalyst to synthesize the (R)-1-tert-butoxycarbonyl-3-aminopiperidine provided by the invention have broad application prospects as well as great market values.
Owner:ENZYMASTER NINGBO BIO ENG CO LTD
Intelligent graphene nano-material with high chiral selectivity as well as preparation and application thereof
ActiveCN108299652AHigh chiral selectivityTo overcome the defects in practical applicationChiral selectivityCvd graphene
The invention discloses an intelligent graphene nano-material with high chiral selectivity as well as preparation and application thereof. The material is prepared from graphene, a temperature-sensitive component, a magnetic component and the like. The material disclosed by the invention has high chiral selectivity, temperature-sensitive performance and magnetic responsiveness; the material has the advantages of simplicity in operation, high resolution efficiency, convenience for recycling materials, and environment-friendly operation process in chiral resolution of an amino acid enantiomer, and has a good industrial application prospect.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Method for preparing nebivolol midbody
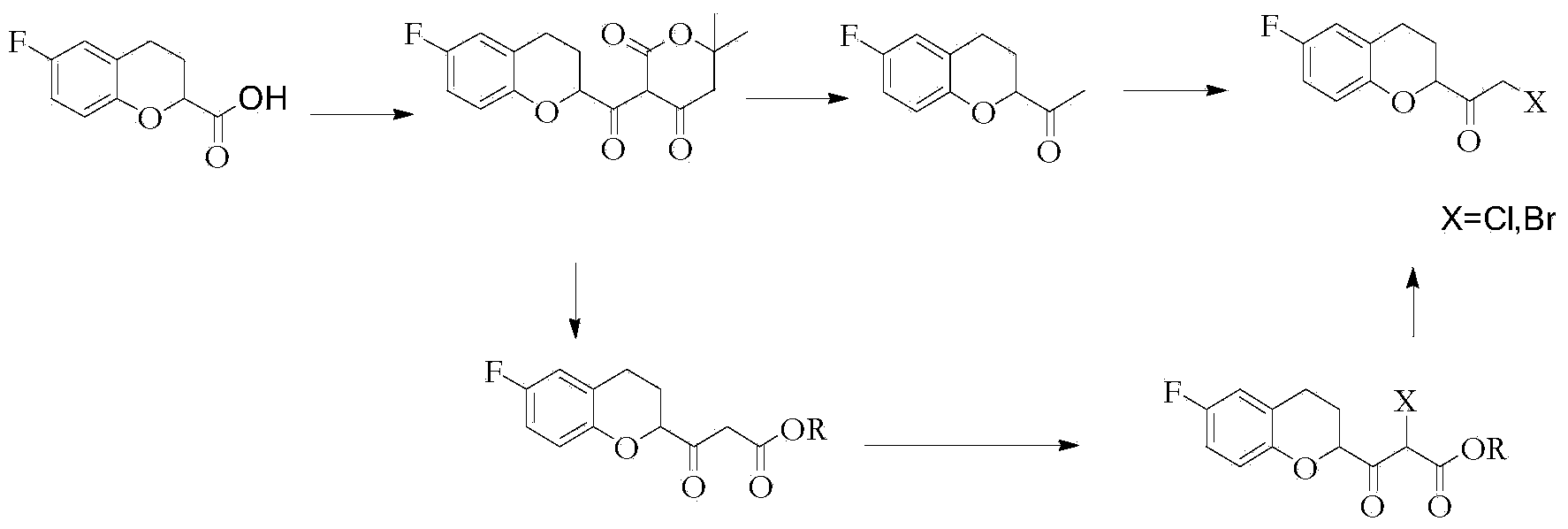
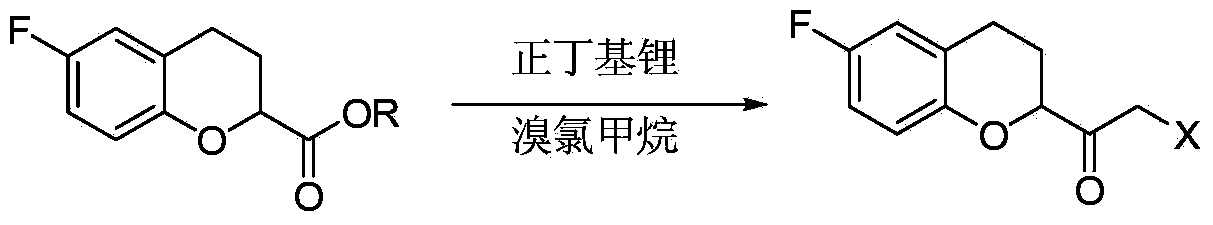
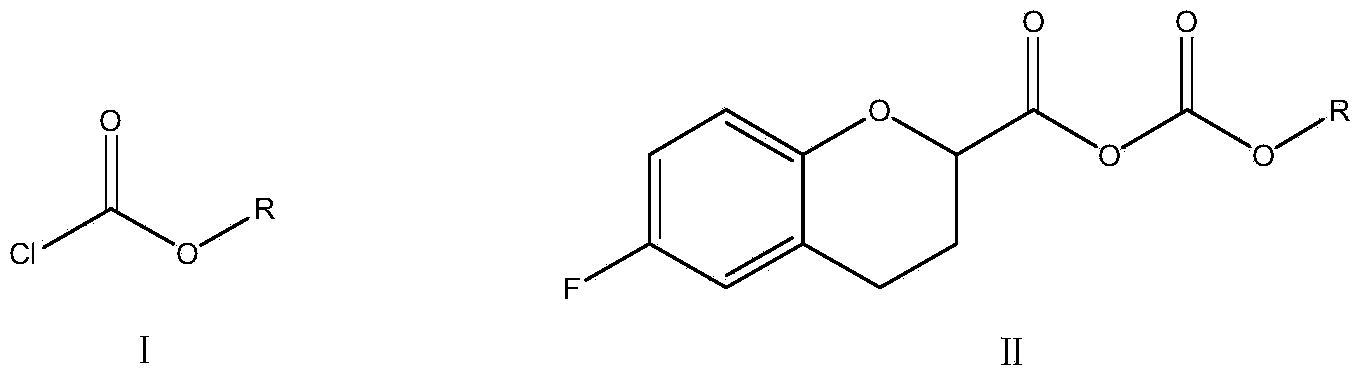
The invention relates to a method for preparing a nebivolol midbody, which belongs to the technical field of medicament synthesis, for solving the problems that the conventional raw material is high in cost, long in route and low in yield. The method comprises the following steps: enabling 6-fluorine chroman-2-formic acid as a raw material to react with chloro-carbonic ester with the presence of a deacid reagent, then adding diazomethane after the reaction is accomplished, so as to enable the midbody to react with the diazomethane to generate a reaction liquid of an intermediate product, and then adding a hydrogen halide gas or a hydrogen halide solution into the reaction liquid to perform halogenating reaction so as to obtain a compound of formula IV, namely, (6-(fluorine-3,4-dihydro-2H-benzopyran-2-methanol-2-yl) ethanone halogenate. The method provided by the invention has the advantages that the reaction route is short, the yield is high, and the used material is low in price and easy to purchase.
Owner:江苏八巨药业有限公司
Reagent for separating amino acid enantiomer
InactiveCN1670012AAchieve enantiomer separationHigh partition coefficientSolvent extractionOrganic compound preparationChemical industryEnantiomer
The invention relates to a new pattern agent for separating amino acid enantiomer, which belongs to the mass transfer isolation technique field about chemical industry, chemical and medicine. The invention discloses the new pattern agent to solve the problem which both the low selective and small flux of chiral selectors hinder the chirality enantiomer drugs industrial mass production seriously, which comprises: mixing the chirality molecule D-dimethylbenzoyl tartaric acid or L-dimethylbenzoyl tartaric acid with the mole ratio as 1:1-40 and D2EHPA, adding octanol as deflocculating agents, and stirring to get the D-dimethylbenzoyl tartaric acid, L-dimethylbenzoyl tartaric acid or D2EHPA with unreactive chiral molecules and the mixture liquid system of intermediate complex compound from the reaction formation. The mixture liquid system is the said new pattern agent for separating amino acid enantiomer.
Owner:TSINGHUA UNIV
Biological preparation method of (S)-1-(2-iodine-5-fluorophenyl) ethanol
ActiveCN109468346AMild reaction conditionsHigh substrate adaptabilityMicroorganism based processesFermentationHigh concentrationNucleotide
The invention discloses a biological preparation method of (S)-1-(2-iodine-5-fluorophenyl) ethanol. The biological preparation method comprises the following steps: taking a certain concentration of prochiral ketone 2'-iodine-5'-fluoroacetophenone as a substrate, adding a certain amount of genetically engineered bacteria, at the temperature of 20 to 50 DEG C, reacting in a reaction system converted by a buffer solution with the pH of 5.5 to 10.5, and after the reaction is completed, separating and purifying a reaction solution to obtain a corresponding product, wherein the genetically engineered bacteria are genetically engineered bacteria containing a carbonyl reductase EbSDR8 mutant encoding gene; and the nucleotide sequence of the carbonyl reductase EbSDR8 mutant encoding gene is SEQ IDNO.3. The method is mild in reaction condition, high in substrate adaptation and environmentally-friendly, and reconstitution cells of the enzyme can be used for efficiently catalyzing asymmetric reduction of high-concentration prochiral ketone in an isopropanol-containing reaction system without adding any coenzyme, so that generated chiral alcohol (ee being greater than or equal to 99%) with high optical purity has an excellent industrialized application prospect.
Owner:杭州馨海生物科技有限公司
L-cyclic alkylamino acid synthesis method and medicinal composition containing L-cyclic alkylamino acid
ActiveCN103361388AHigh chiral selectivityRaw material conversion rate is highOrganic active ingredientsPeptide/protein ingredientsFormate dehydrogenaseLeucine dehydrogenase
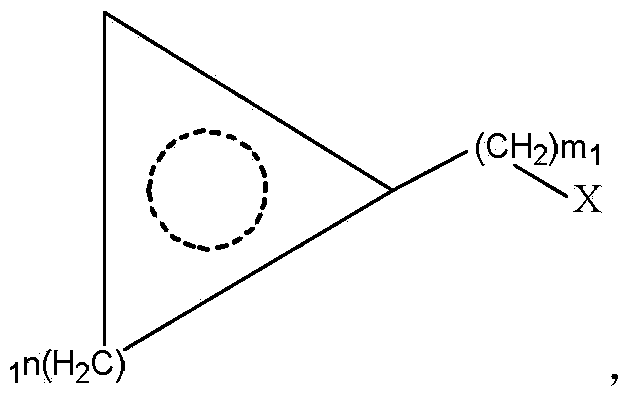
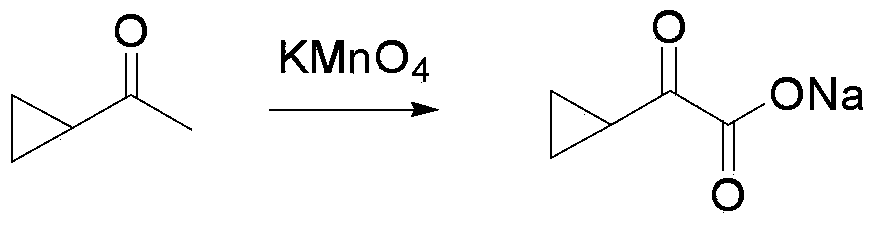
The invention provides an L-cyclic alkylamino acid synthesis method and a medicinal composition containing L-cyclic alkylamino acid. The synthesis method comprises the following steps: 1, preparing cyclic alkyl ketonic acid or cyclic alkyl ketonate having a structure represented by formula (I) or formula (II); and 2, mixing the cyclic alkyl ketonic acid or cyclic alkyl ketonate with ammonium formate, a leucine dehydrogenase, a formate dehydrogenase and a coenzyme NAD<+>, and carrying out a reduction amination reaction to generate the L-cyclic alkylamino acid, wherein n1 in the formula (I) is not lower than 1, m1 in the formula is not lower than 0, and M1 is H or a univalent cation; n2 in the formula (II) is not lower than 0, m2 in the formula (II) is not lower than 0, and M2 is H or a univalent cation; and the amino acid sequence of the leucine dehydrogenase is represented by SEQ ID No.1. The method utilizes specific leucine dehydrogenase, the formate dehydrogenase and the coenzyme NAD<+> to carry out the reduction amination reaction of the cyclic alkyl ketonic acid or cyclic alkyl ketonate, and has a high raw material conversion rate and a high chiral selectivity.
Owner:ASYMCHEM LAB TIANJIN +4
Biological preparation method of chiral hydroxy acid ester
ActiveCN111454998AHigh activityImprove efficiencyOxidoreductasesFermentationEnzymatic synthesisChiral selectivity
The invention discloses a biological preparation method of a chiral hydroxy acid ester. The method comprises the following steps: (1) oxidizing a fatty acid by ketolase spheroidene monooxygenase to generate a 4-oxo fatty acid; and (2) asymmetrically reducing the 4-oxo fatty acid by short-chain dehydrogenase PpYSDR-M85Q / L136A to obtain an R-hydroxy acid ester. In the biological preparation method of a chiral hydroxy acid ester of the invention, a short-chain dehydrogenase mutant is constructed, the short-chain dehydrogenase mutant greatly improves the activity and chiral selectivity for keto acids compared with the wild type, the efficiency of the enzyme in catalyzing keto acids to prepare chiral lactones can be improved, and thereby an application value of the enzyme is fully explored. Atthe same time, the enzymatic synthesis of the chiral hydroxy acid ester has the characteristics such as simple process, environmental friendliness, and high benefit.
Owner:黄山科宏生物香料股份有限公司
Preparation method of moxifloxacin intermediate
InactiveCN111100125AEasy to operateSimple processOrganic chemistry methodsChiral selectivityCinoxacine
The invention discloses a preparation method of a moxifloxacin intermediate. The invention provides a preparation method of a compound as shown in a formula III, which comprises the following step: ina solvent, in the presence of alkali, carrying out cyclization reaction as shown in the specification on a compound as shown in a formula II to obtain the compound as shown in the formula III. The method is simple to operate, high in chiral selectivity, simple in process, high in yield and high in purity.
Owner:XIAMEN GINPOSOME PHARM CO LTD
Method for preparing racemic cis-8-benzyl-2, 8-diazabicyclo [4, 3, 0] nonane
The invention provides a method for preparing racemic cis-8-benzyl-2, 8-diazabicyclo [4, 3, 0] nonane. The method comprises the following steps of: step S1, preparing a platinum dehydrogenation catalyst; S2, catalyzing piperidine cyclic dehydrogenation reaction by adopting the platinum dehydrogenation catalyst; and S3, carrying out a catalytic hydrogenation reaction by using a chiral brominated acid catalyst. The method for preparing the racemic cis-8-benzyl-2, 8-diazabicyclo [4, 3, 0] nonane of the invention is high in raceme purity and high in reaction yield.
Owner:SHAYANG QINJIANG CHEM
Transaminase catalyst and method for enzymatic synthesis of (R)-1-naphthylethylamine
ActiveCN111411096AEasy to getHigh chiral selectivityTransferasesMicroorganism based processesEnzymatic synthesisEscherichia coli
The invention relates to the technical field of medical intermediates, specifically to a transaminase catalyst and a method for enzymatic synthesis of (R)-1-naphthylethylamine. The method comprises the following steps: adding recombinant escherichia coli wet thalli expressing the transaminase catalyst and 1-acetophenone into a reaction container, carrying out heating in a water bath, and carryingout mechanical stirring; adding an organic solvent or an aqueous solution of water, an amino donor and pyridoxal phosphate under stirring; and after a reaction is finished, carrying out post-treatmentso as to obtain the (R)-1-naphthylethylamine. The transaminase catalyst disclosed by the invention is easy to obtain and high in chiral selectivity; meanwhile, the method for enzymatic synthesis of the (R)-1-naphthylethylamine is simple in operation, mild in reaction conditions and stable in reaction completion, and can directly generate the (R)-1-naphthylethylamine with an optical purity of 99%or above, so tedious chemical resolution steps and a tedious chemical reaction route are avoided.
Owner:ENZYMASTER NINGBO BIO ENG CO LTD
Synthetic method of L-heterocyclic amino acid and pharmaceutical composition with L-heterocyclic amino acid
ActiveCN103276025AThe synthesis process is simpleAdapt to industrial mass productionOrganic active ingredientsFermentationAlkyl transferPhenylalanine dehydrogenase
The invention provides a synthetic method of L-heterocyclic amino acid and a pharmaceutical composition with L-heterocyclic amino acid. The synthetic method comprises the steps that A, heterocyclic ketonic acid is prepared, wherein heterocycle in heterocyclic ketonic acid is selected from any one of pentabasic single heterocycle, hexabasic single heterocycle, heptabasic single heterocycle, pentabasic alkylation single heterocycle, hexabasic alkylation single heterocycle and heptabasic alkylation single heterocycle, and a structural formula of ketonic acid base in heterocyclic ketonic acid is as shown in the specification, and is positioned in any carbon position of heterocycle; and B, heterocyclic ketonic acid is mixed with ammonium formate, phenylalanine dehydrogenase, formate dehydrogenase and coenzyme NAD<+> to perform a reduced amination reaction to generate L-heterocyclic amino acid, wherein an amino acid sequence of phenylalanine dehydrogenase is SEQ ID No. 1 (sequence identifier number 1). Since special phenylalanine dehydrogenase, formate dehydrogenase and coenzyme NAD<+> are used for allowing heterocyclic ketonic acid to perform the reduced amination reaction to generate L-heterocyclic amino acid, the raw material conversion rate is high and the chiral selectivity is high.
Owner:ASYMCHEM LAB TIANJIN +4
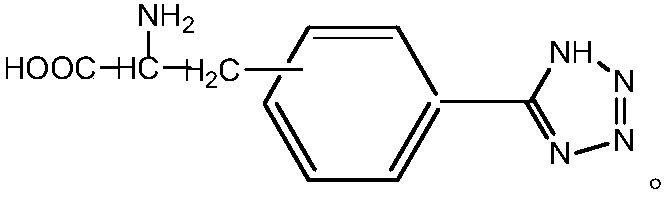
Method for preparing chiral amino acid tetrazole compound
InactiveCN107602495AEasy to separate and recycleChiral selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTetrazoleSodium azide
The invention discloses a method for preparing a chiral amino acid tetrazole compound. The method is characterized by synthesizing and preparing the chiral amino acid tetrazole compound by taking cyanophenylalanine and sodium azide as raw materials, dimethyl formamide as a solvent and imidazole polymer ferric salt ionic liquid as a catalyst, and specifically comprises the following steps: mixing the cyanophenylalanine, the sodium azide, the imidazole polymer ferric salt ionic liquid and the dimethyl formamide which are in proportional amounts, heating to 110 to 130 DEG C while stirring, reacting for 20 to 30 hours at a constant temperature, cooling to room temperature, filtering and separating an imidazole polymer ferric salt ionic liquid catalyst, regulating pH (Potential of Hydrogen) ofa separating solution to be neutral with hydrochloric acid, adding an ethyl acetate for extracting and separating liquid, taking a supernatant organic phase, rotationally drying an organic solvent after washing, obtaining white solid, and carrying out vacuum drying, thus obtaining the chiral amino acid tetrazole compound.
Owner:YANCHENG INST OF TECH
Preparation method of high-purity capecitabine key intermediate
ActiveCN109369736AMild method conditionsHigh yieldEsterified saccharide compoundsSugar derivativesOrganic solventAcetylation
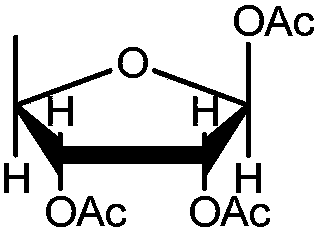
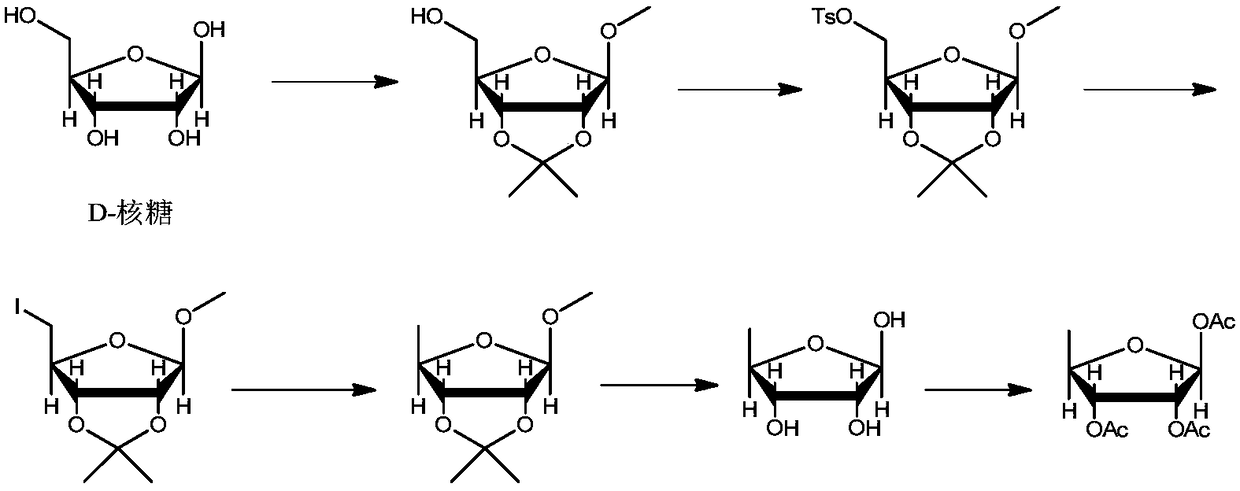
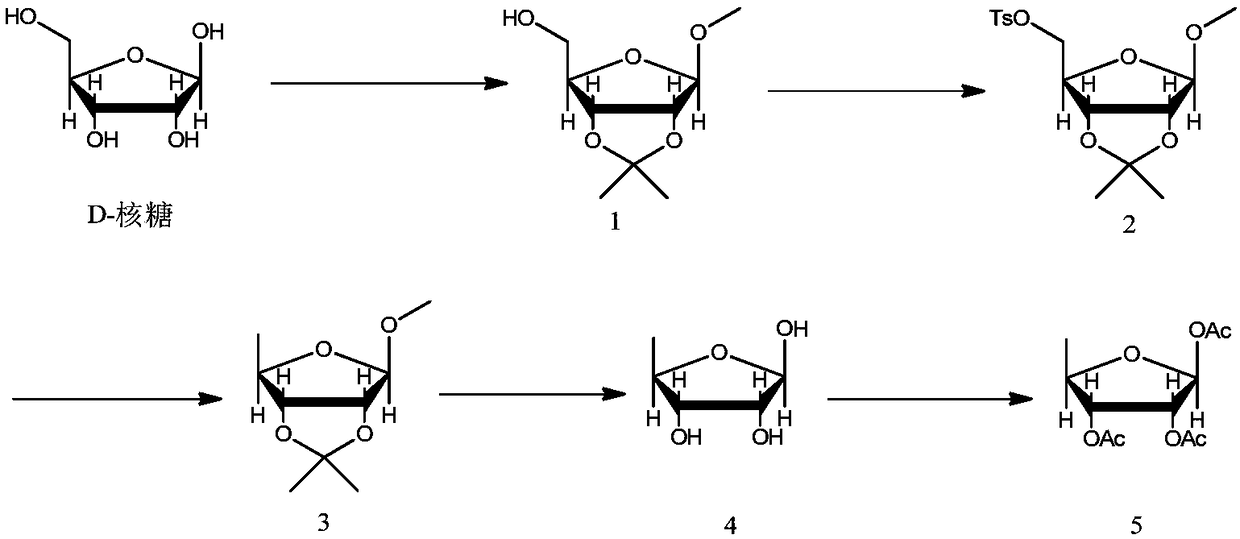
The invention discloses a preparation method of a high-purity capecitabine key intermediate. The preparation method comprises the following steps: taking D-ribose as an initial raw material, performing hydroxyl protection, 5-site tosylation, reduction, deprotection and acetylation to obtain high-purity 1,2,3-O-triacetyl-5-deoxo-D-ribose, wherein the 5-site tosylation reaction is carried out in anorganic solvent 1 by adopting inorganic base 1. Meanwhile, the acetylation reaction is carried in the presence of alkali 2 by taking water as a reaction solvent and taking 4-dimethylamiopryidine as acatalyst. The preparation method disclosed by the invention is mild in reaction conditions, high in yield, economic and effective, the purity of the prepared 1,2,3-O-triacetyl-5-deoxo-D-ribose can reach 99.0%, the alpha-isomer is small in content even is not detected, and the preparation method is applicable to large-scale industrial production.
Owner:广安凯特制药有限公司
Preparation method of cannabidiol compound
ActiveCN112047815AAvoid generatingHigh chiral selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsEster prodrugLithium chloride
The invention discloses a preparation method of a compound with a general formula I or pharmaceutically acceptable salts, stereoisomers, esters, prodrugs and solvates thereof, which comprises the following steps: (1) by using a compound A and (1S, 4R) 1methyl 4 (1methyl vinyl) 2cyclohexene 1alcohol as raw materials, carrying out coupling under the action of a chiral imidazolone salt catalyst to obtain a compound B; carrying out sodium sulfite dehalogenation and alkali washing to obtain an intermediate 1; (2) heating the intermediate 1 to 80100 DEG C under the action of DMSO, lithium chloride and an antioxidant, reacting, and recrystallizing to obtain a compound with a general formula I;.
Owner:CHENGDU HYPERWAY PHARM CO LTD
Preparation method of 2-deoxy-2, 2-difluoro-D-red-3, 5-dibenzoate
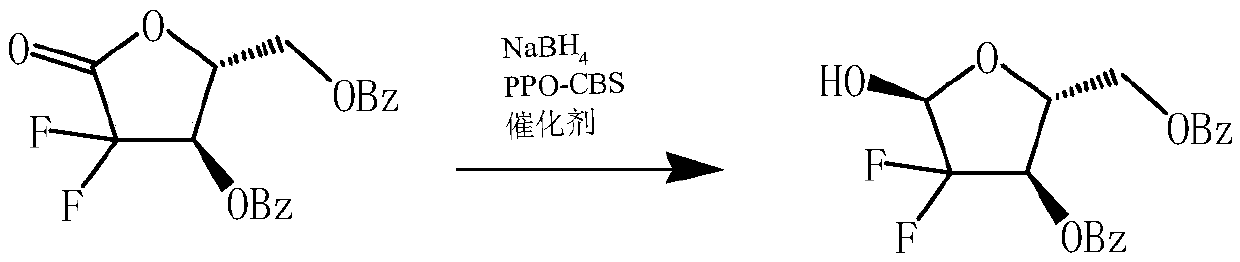
ActiveCN109651460AHigh total purityConfiguration highSugar derivativesOrganic chemistry methodsChiral selectivityRedox
The invention relates to a preparation method of 2-deoxy-2, 2-difluoro-D-red-3, 5-dibenzoate and belongs to the technical field of medicine intermediate synthesis. In order to solve the problems of poor selectivity and safety in the prior art, the invention provides the preparation method of the 2-deoxy-2, 2-difluoro-D-red-3, 5-dibenzoate, which comprises carrying out redox reaction on 2-deoxy-2,2-difluoro-D-red-1-ketofuranose-3, 5- dibenzoate under the combined action of immobilized catalysts of sodium borohydride and PPO-CBS to convert to the product of 2-deoxy-2, 2-difluoro-D-red-3, 5-dibenzoate. The method has the advantage of high chiral selectivity, improves the integral conversion rate, plays a better asymmetric reduction role, and achieves the purpose that the purity of the isomer is more than 95%.
Owner:江苏八巨药业有限公司
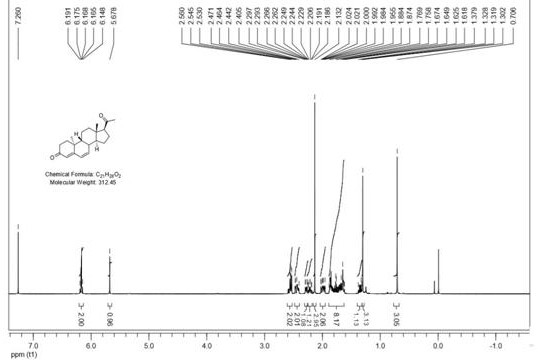
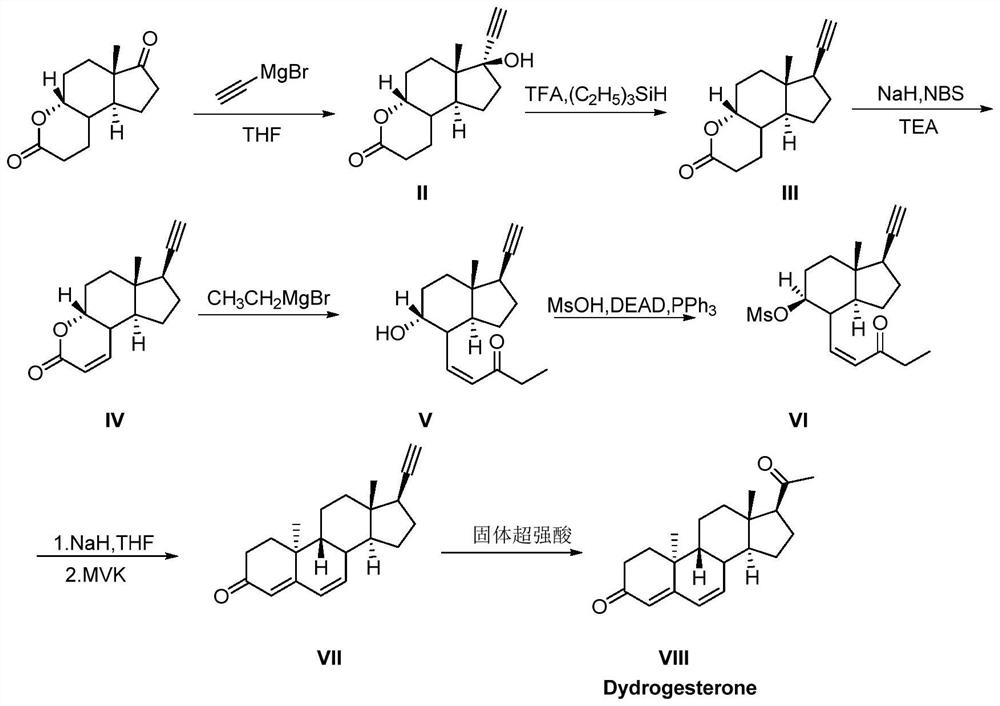
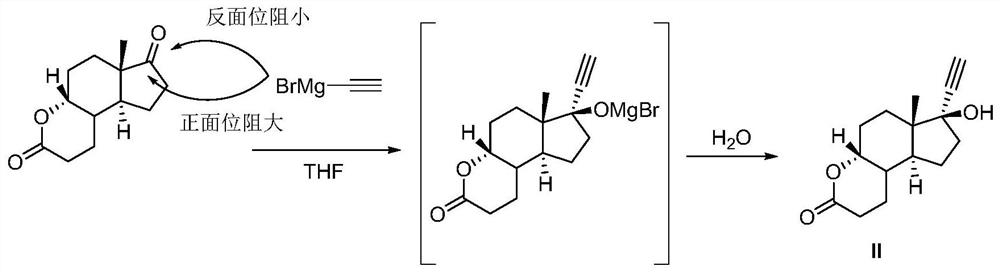
Synthetic method of dehydroprogesterone
The invention relates to the field of medicine preparation, in particular to a synthetic method of dehydroprogesterone, The synthetic method comprises the following steps: by using an A-ring degradation product as a raw material, carrying out addition reaction, dehydroxylation reaction, bromination reaction, elimination reaction, Grignard reaction, Mitsunobu reaction, twice Robinson ring-increasing reaction and water addition rearrangement reaction to generate a dehydroprogesterone product. The method has the advantages of cheap and easily available initial raw materials, few isomer impuritiesin the reaction process, easy purification, high product yield and the like, and has the advantages of short steps, simple operation, low equipment requirements, simple and easily available reagentsand easy realization of industrial production.
Owner:厦门欧瑞捷生物科技有限公司
Threonine aldolase, mutants and their application in the preparation of substituted phenylserine derivatives
ActiveCN109402098BImprove stabilityHigh chiral selectivityFungiBacteriaEnzyme catalysisEngineered genetic
The invention discloses threonine aldolase, mutants, threonine aldolase and mutant coding genes, recombinant vectors constructed by coding genes, recombinant genetically engineered bacteria obtained by transformation of recombinant vectors, and threonine aldolase and the application of mutants in the preparation of 2- / 3- / 4-position substituted phenylserine derivatives. The present invention uses threonine aldolase and mutants as biocatalysts, 2- / 3- / 4-position substituted benzaldehyde as substrate, pyridoxal 5-phosphate as coenzyme, glycine / glycine ester as Auxiliary substrate, carry out enzyme-catalyzed reaction in appropriate conditions and media, separate and prepare a series of phenylserine derivatives with different substituents, the total yield of this method is in the range of 76-99%, the ee value of the product is greater than 99%, and the de value is greater than 99%. The range is 70‑99%.
Owner:王喆明 +1
A kind of cellulose chiral derivative and its preparation method and use
InactiveCN107641156BWith chiral separation functionImproved chiral separation capabilityIon-exchange process apparatusIon-exchanger regenerationDouble bondChiral stationary phase
The invention discloses a cellulose chiral derivative, and a preparation method and an application thereof, belonging to the field of chiral stationary phases. The cellulose chiral derivative providedby the invention is a structure with oxidized cellulose bonding with a nitrogen atom through a carbon-nitrogen double bond and with the nitrogen atom connecting with a chiral carbon atom. The preparation method comprises the following steps: through a chemical oxidation process, oxidizing cellulose so as to form a new carbonyl group, then carrying out a Schiff base reaction with an amino containing chiral compound, and introducing a new chiral group onto the cellulose so as to obtain the cellulose chiral derivative. The cellulose chiral derivative provided by the invention can be used for separation and / or purity detection of chiral compounds. According to the invention, under the actions of an original chiral system and a newly added chiral system, the improvement of a cellulose chiral separation function is realized, and the effect of cellulose chiral separation is greatly improved.
Owner:WUHAN UNIV
A kind of method for preparing chiral amino acid tetrazole compound
InactiveCN107602495BEasy to separate and recycleChiral selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSodium azideEthyl acetate
Owner:YANCHENG INST OF TECH
Synthetic method of l-cyclic alkyl amino acid and pharmaceutical composition having same
ActiveCN103361388BThe synthesis process is simpleHigh chiral selectivityOrganic active ingredientsPeptide/protein ingredientsChiral selectivityKetonic acids
The invention provides an L-cyclic alkylamino acid synthesis method and a medicinal composition containing L-cyclic alkylamino acid. The synthesis method comprises the following steps: 1, preparing cyclic alkyl ketonic acid or cyclic alkyl ketonate having a structure represented by formula (I) or formula (II); and 2, mixing the cyclic alkyl ketonic acid or cyclic alkyl ketonate with ammonium formate, a leucine dehydrogenase, a formate dehydrogenase and a coenzyme NAD<+>, and carrying out a reduction amination reaction to generate the L-cyclic alkylamino acid, wherein n1 in the formula (I) is not lower than 1, m1 in the formula is not lower than 0, and M1 is H or a univalent cation; n2 in the formula (II) is not lower than 0, m2 in the formula (II) is not lower than 0, and M2 is H or a univalent cation; and the amino acid sequence of the leucine dehydrogenase is represented by SEQ ID No.1. The method utilizes specific leucine dehydrogenase, the formate dehydrogenase and the coenzyme NAD<+> to carry out the reduction amination reaction of the cyclic alkyl ketonic acid or cyclic alkyl ketonate, and has a high raw material conversion rate and a high chiral selectivity.
Owner:ASYMCHEM LAB TIANJIN +4
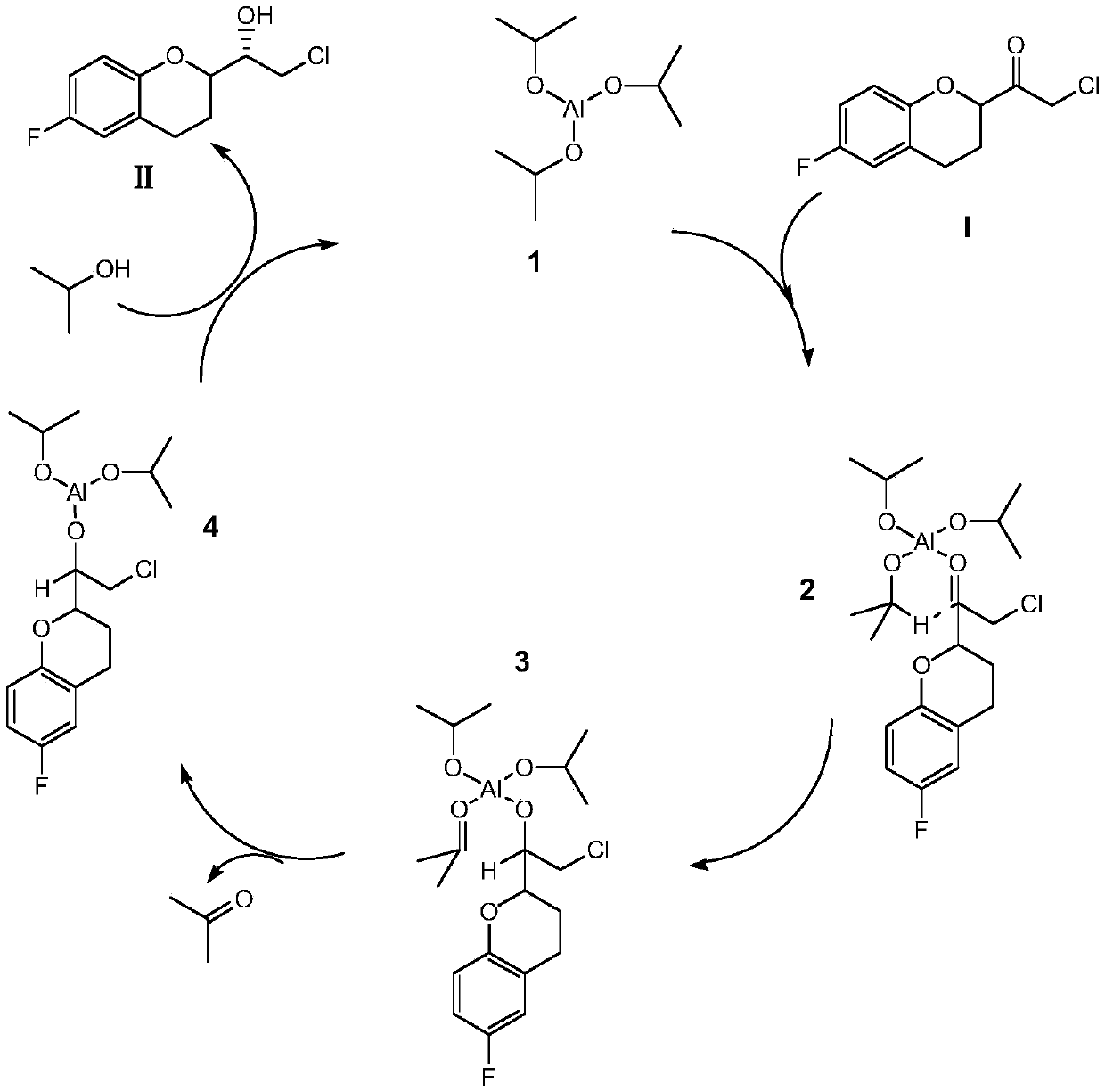
A kind of preparation method of (s)-2-chloro-1-(6-fluoro-1-chroman-2-yl)-ethanol
The invention relates to a preparation method of (S)-2-chloro-1-(6-fluoro-1-chromanone-2-yl)-ethanol and belongs to the technical field of synthesis of drug intermediates. Aiming at solving the existing problems that the chiral purity is low and the separation is difficult, the invention provides the preparation method of the (S)-2-chloro-1-(6-fluoro-1-chromanone-2-yl)-ethanol; the preparation method comprises the following step: carrying out catalytic reduction reaction on a substrate in a mixed solvent of isopropyl alcohol and a non-proton weak-polarity solvent under the catalysis effect oforganic aluminum salt, so as to obtain the product (S)-2-chloro-1-(6-fluoro-1-chromanone-2-yl)-ethanol. According to the preparation method provided by the invention, an isopropyl alcohol and non-proton weak-polarity solvent system is combined and the organic aluminum salt is used for carrying out the catalytic reaction, so that the high chiral selectivity can be realized, and the preparation method has the effects of high chiral purity and high yield.
Owner:江苏八巨药业有限公司
Application of C=C bond connection-based COF material in preparation of chiral chromatographic stationary phase
ActiveCN113813939ABroaden design ideasHigh chiral selectivityOther chemical processesDispersed particle separationChromatographic columnKnoevenagel condensation
The invention relates to application of aC=C bond connection-based COF material in preparation of a chiral chromatographic stationary phase. The preparation method comprises the following steps: synthesizing chiral COF connected with a C=C bond by using a crown ether functionalized dinaphthalene tetraaldehyde monomer on the basis of a Knoevenagel condensation reaction; compounding COF and silica gel by using a net package method to prepare a high performance liquid chromatography chiral stationary phase; uniformly coating COF on a gas chromatographic column by using a dynamic coating method so as to be used as a gas chromatographic chiral stationary phase; and applying the high performance liquid chromatographic column or gas chromatographic column to separate various racemic compounds. Compared with the prior art, due to the high stability of the synthesized C=C bond connection COF, the liquid chromatographic column and the gas chromatographic column prepared in the invention both show good separation capability and durability in the chiral separation process, so that the problem that the existing chiral stationary phase is only suitable for a specific chromatography is solved, a novel high-performance chiral separation material which can be used for liquid chromatography and gas chromatography at the same time is developed, and potential application prospects are achieved.
Owner:SHANGHAI JIAO TONG UNIV
Omega transaminase as well as mutant, recombinant plasmid, genetically engineered bacterium and application thereof
ActiveCN112481229AHigh stereoselectivityMild reaction conditionsBacteriaTransferasesHeterologousChiral selectivity
The invention provides omega transaminase as well as a mutant, a recombinant plasmid, a genetically engineered bacterium and application thereof. The amino acid sequence of the omega transaminase is shown as SEQ ID No: 2, wherein the omega transaminase mutant is obtained by mutation of the amino acid sequence of the omega transaminase and has at least 90% of homology with the SEQ ID No: 2. According to the invention, the omega transaminase gene is extracted from Burkholderia phymatum, the heterologous expression of the omega transaminase gene is realized, and the generated omega transaminase strain shows excellent catalytic activity on a series of aryl alkyl substrates. The omega transaminase is mutated to obtain a series of omega transaminase mutants, and the omega transaminase mutants are coupled with different enzymes to be applied to the production of asymmetric synthesis of (S)-1-amphetamine and other chiral amine products by a biological enzyme method, so that the method has thecharacteristics of economy, environmental protection and high chiral selectivity; thus, the invention provides a potential choice for industrial application of transaminase.
Owner:EAST CHINA UNIV OF SCI & TECH
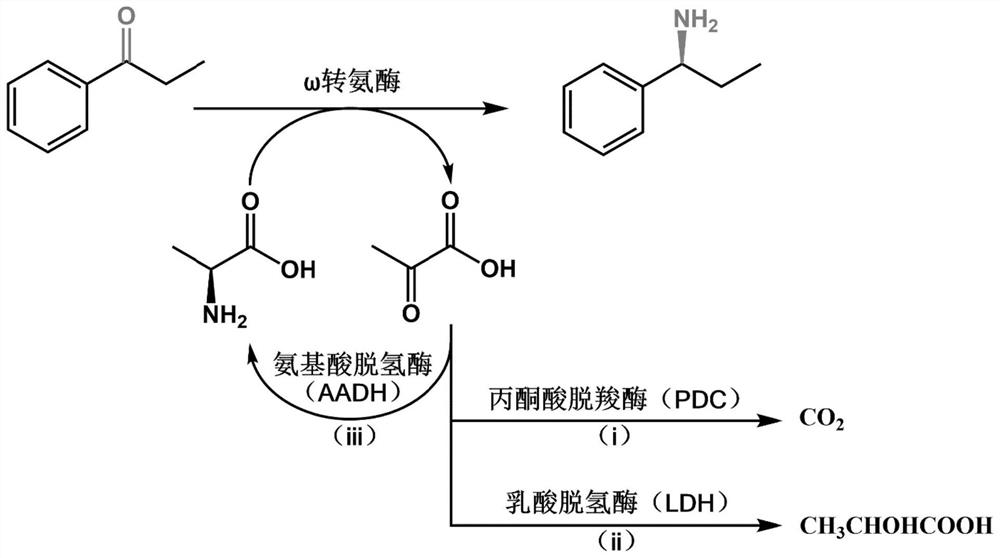
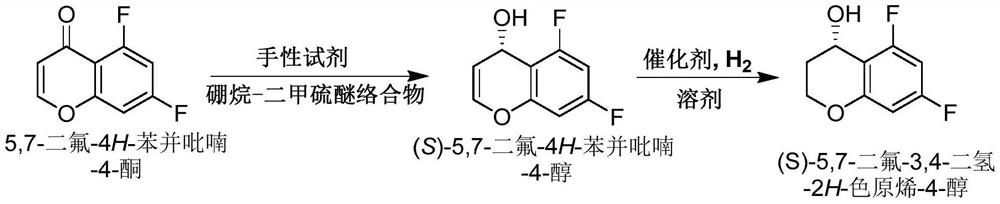
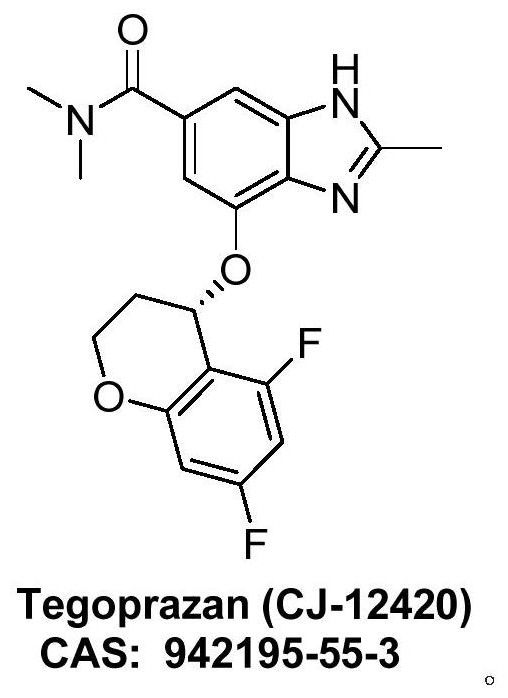
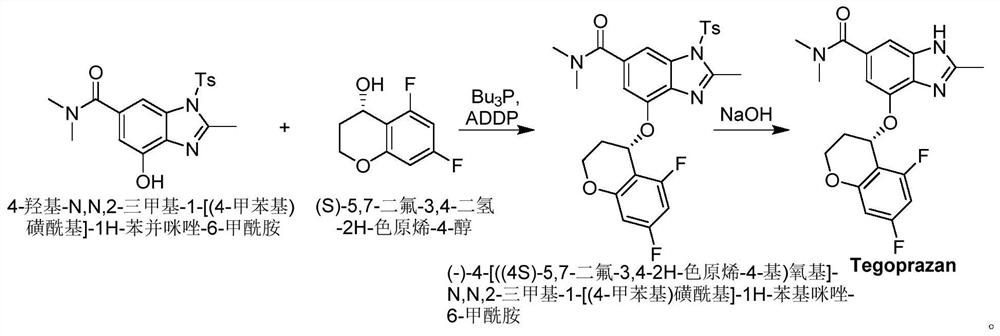
Method for synthesizing Tegrazan chiral alcohol
ActiveCN109320485BThe synthesis method is simpleConvenient sourceOrganic chemistry methodsBenzopyranAlcohol
Owner:WISDOM PHARM CO LTD
Synthesis process of S-(-)-nadifloxacin chiral intermediate
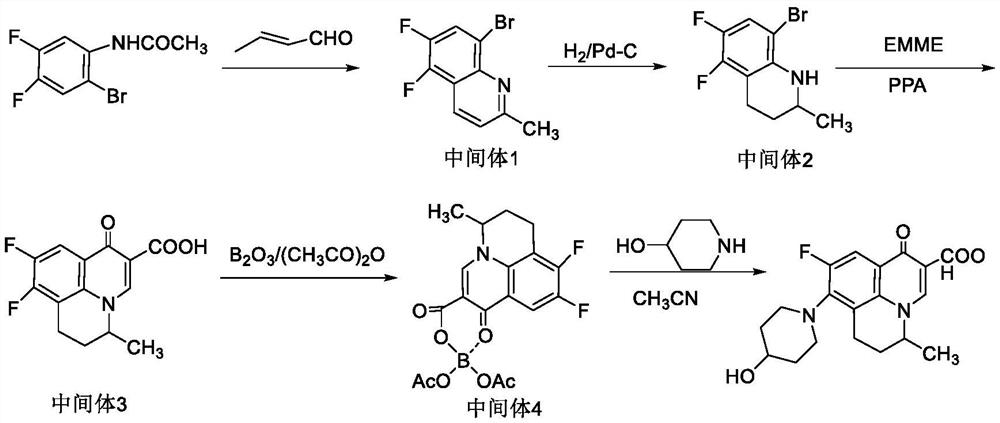
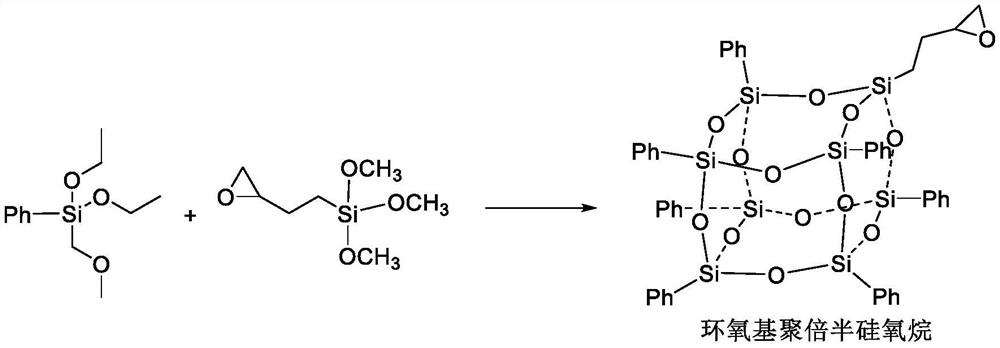
ActiveCN113527358AHigh chiral selectivityImprove solubilitySilicon organic compoundsOrganic chemistry methodsEpoxyNadifloxacin
The invention discloses a synthesis process of an S-(-)-nadifloxacin chiral intermediate, and belongs to the technical field of chemical pharmacy. The synthesis process comprises the following steps: 1, synthesizing an intermediate 1; and 2, synthesizing the S-(-)-chiral intermediate. R binaphthylenediamine is used as a matrix of a chiral ligand, epoxy polysilsesquioxane is grafted on a molecule of N-Boc-L-histidine to form the chiral ligand, on one hand, the ee value is increased by utilizing the chiral characteristic of N-Boc-L-histidine, and on the other hand, the ee value is increased by utilizing the characteristics of nanoscale size and solubility of the epoxy polysilsesquioxane, so that the solubility of the catalyst is increased, and the catalyst can be mixed with the reactant in the nanometer size so as to improve the catalytic performance of the chiral catalyst and improve the reaction yield, such that the chiral ligand and the metal ruthenium are subjected to coordination to form the chiral catalyst so as to provide the good chiral amplification effect;.
Owner:湖南中威制药有限公司
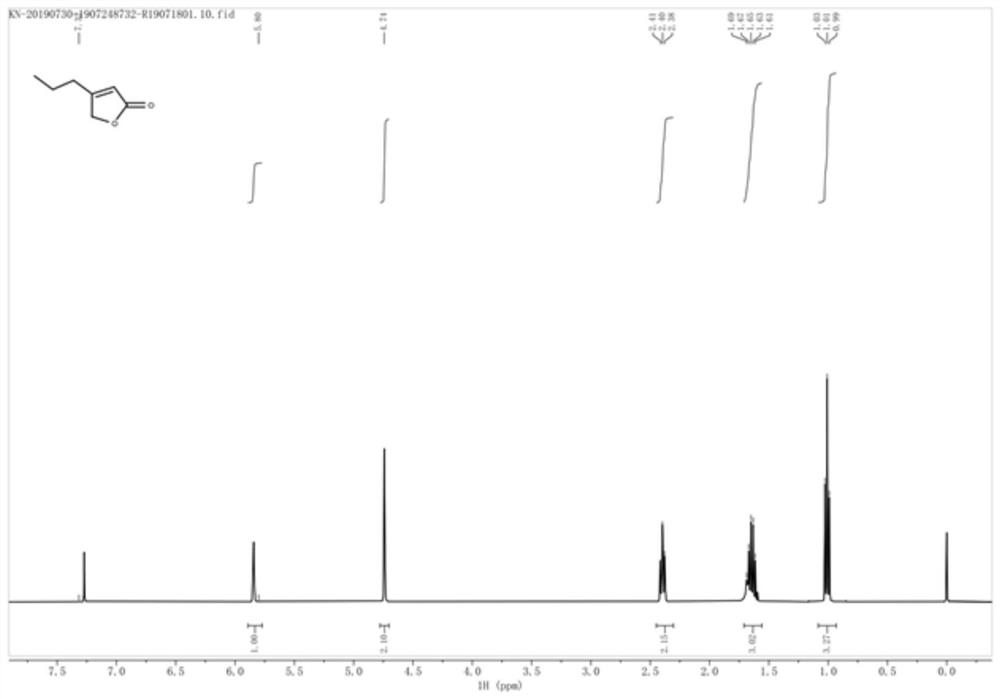
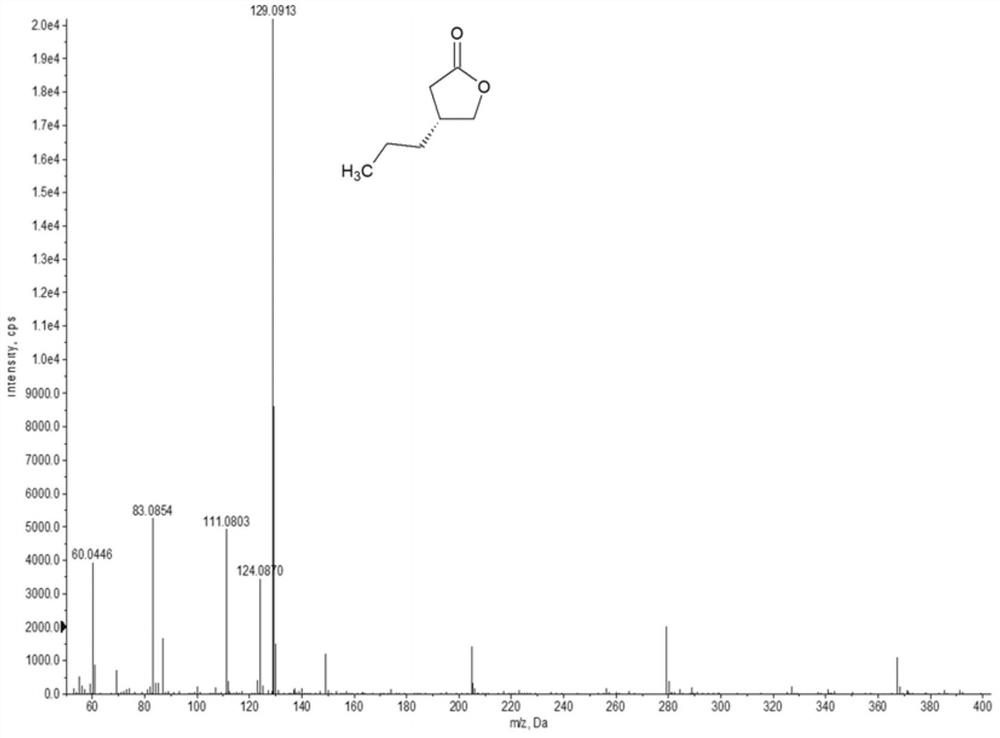
A kind of enone reductase and a kind of preparation method of buvaracetam intermediate
The invention relates to the technical field of pharmaceutical intermediates, in particular to a method for preparing an enone reductase and a buvaracetam intermediate, the preparation method comprising the following steps: under the catalysis of morpholine, valeraldehyde and glyoxylic acid The reaction generates 5-hydroxyl-4-n-propyl-2(5H)-furanone; under the catalysis of sodium borohydride, 5-hydroxyl-4-n-propyl-2(5H)-furanone dehydroxylates to generate 4 ‑n-propyl‑2(5H)‑furanone; in the presence of enone reductase, 4‑n-propyl‑2(5H)‑furanone undergoes a reduction reaction to generate the target product brivaracetam intermediate; the ene The nucleotide sequence of ketoreductase is shown in SEQ ID NO.1, and its amino acid sequence is shown in SEQ ID NO.2. The preparation method of the brivaracetam intermediate of the present invention only needs 3 steps of reaction to prepare the brivaracetam intermediate without additional chemical resolution, the overall process is green and environmentally friendly, the cost is low, and it is easy to implement.
Owner:三明旻和医药科技有限公司
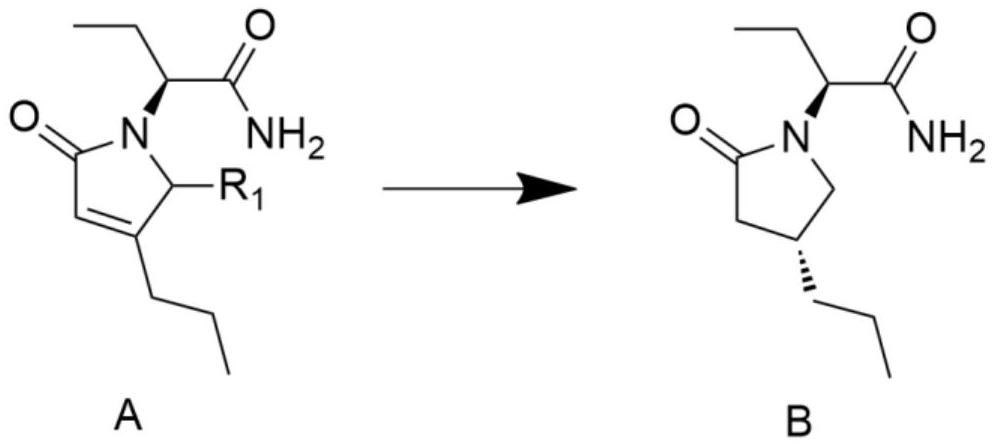
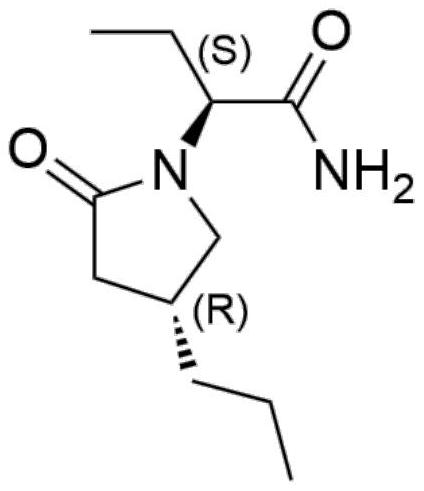
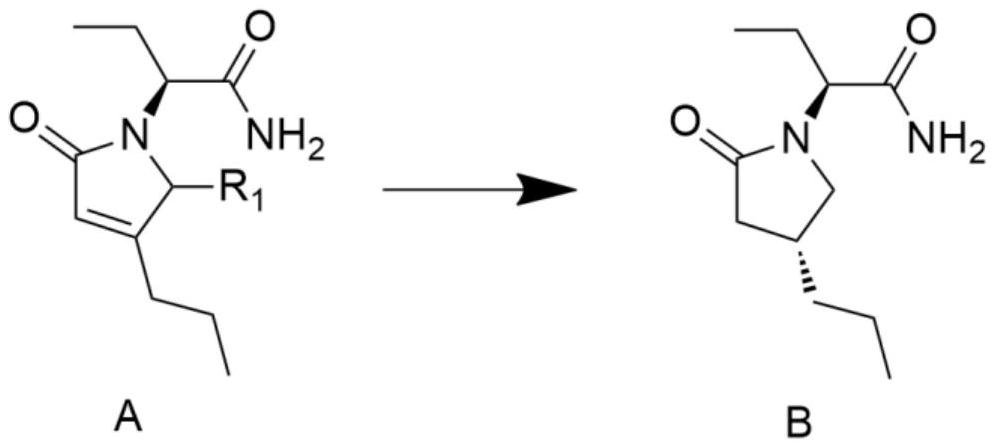
A kind of preparation method of high chiral purity lactam compound
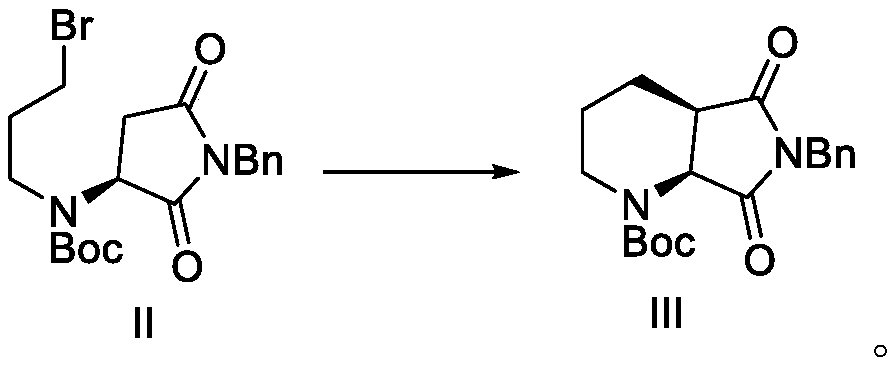
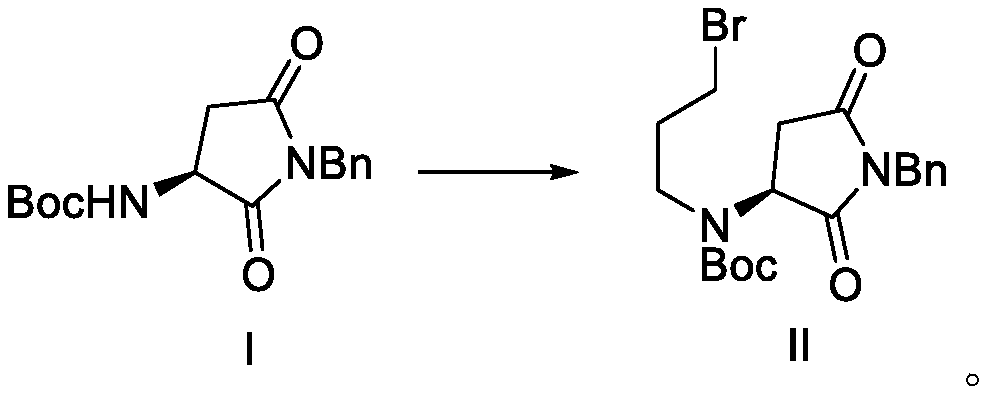
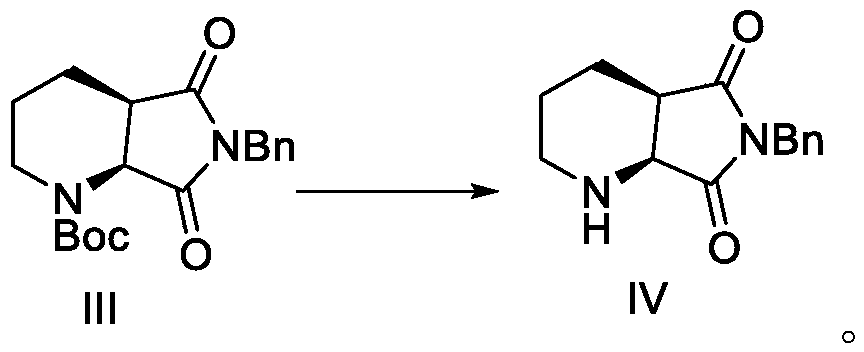
ActiveCN108147988BReduce manufacturing costHigh chiral selectivityOrganic chemistry methodsPtru catalystChiral selectivity
The invention discloses a method for preparing lactam compounds with high chiral purity. The method for preparing the lactam compounds with the high chiral purity includes steps of adding reactive solvents and heavy metal catalysts into a reaction vessel, and stirring the reactive solvents and the heavy metal catalysts for 10-30 minutes; adding reaction raw materials A into the reaction vessel, then adding hydrogen sources into the reaction vessel and carrying out constant-temperature reaction at the temperature ranging from -30 DEG C to 0 DEG C for 20-40 hours; terminating the reaction, filtering reaction products, and rotationally removing organic solvents from the reaction products; adding water and isopropyl acetate into the reaction products and carrying out extraction; carrying out drying, suction filtration and concentration on organic phase to obtain crude products; re-crystallizing the crude products to obtain lactam products B with high chiral purity. The reduction chiral selectivity can be effectively improved without optional added chiral auxiliaries. The method has the advantages of low production cost, convenience in production and operation, safety, atom economy, easiness in industrial production and the like.
Owner:YANGZHOU AORUITE PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com