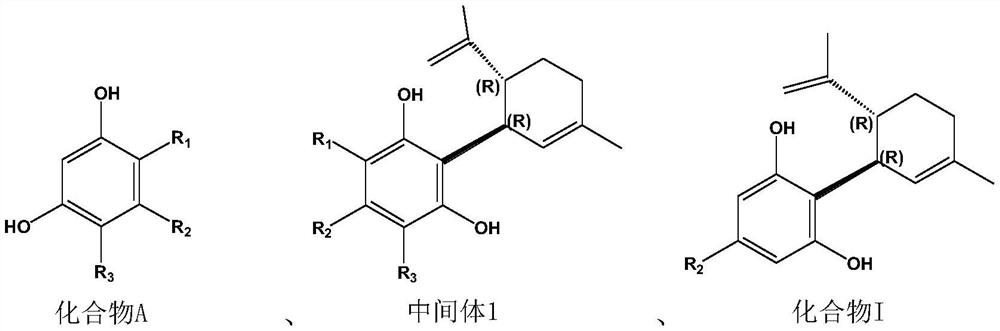
Preparation method of cannabidiol compound
A compound and catalyst technology, applied in the field of preparation of cannabidiol compounds, can solve the problem that the yield is only 46.7%, achieve the effect of improving chiral selectivity, avoiding oxidation impurities and polymerization impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
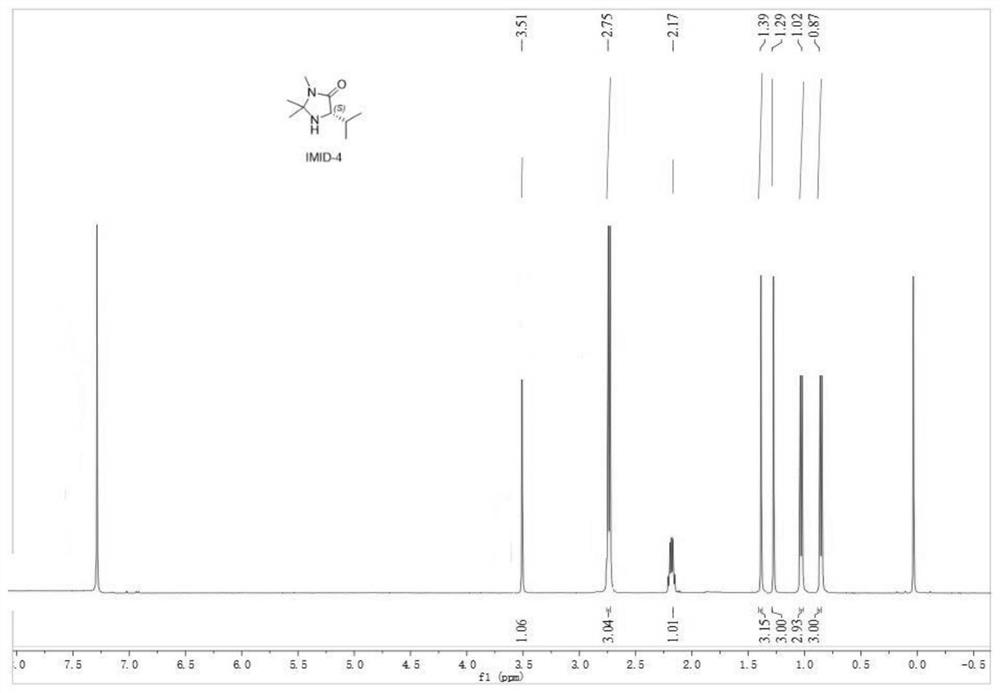
[0061] Preparation Example 1 Synthesis of Chiral Catalyst TfOH-IMID-4
[0062] S1: to MeNH 2 L-valine methyl ester hydrochloride (8.4 g, 100 mmol) was added to the ethanol solution (8.0 M, 50 ml), and the reaction was carried out under stirring at room temperature until the reaction of L-valine methyl ester was completed as monitored by TLC. It was concentrated under reduced pressure to remove ethanol and methylamine to obtain 8.5 g of (S)-2-amino-3-methylbutyrylmethylamide as a colorless oil. The oily substance was dissolved in methanol (100 mL), acetone (59 mL, 800 mmol) and triethylamine (11.1 mL, 80 mmol) were added, the temperature was refluxed for 24 h, and the crude product obtained by concentrating under reduced pressure was purified by column to obtain a colorless oil. IMID-4 (8.3g);
[0063] S2: The obtained IMID-4 was dissolved in EA (50 mL), and TfOH (15 g, 100 mmol) was diluted with EA (20 mL) and slowly added dropwise to the reaction, the above suspension was c...
preparation example 2
[0064] Preparation Example 2 Synthesis of Chiral Catalyst TFA-IMID-4
[0065] The preparation method of IMID-4 is the same as that of Preparation Example 1. The obtained IMID-4 was dissolved in EA (50 mL), and TFA (9.4 g, 100 mmol) was diluted with EA (20 mL) and slowly added dropwise to the reaction, and the above was filtered. The suspension was washed with EA and the filter cake was dried to obtain 12.1 g of white solid TFA-IMID-4 product.
preparation example 3
[0066] Preparation Example 3 Synthesis of Chiral Catalyst TsOH-IMID-4
[0067] The preparation method of IMID-4 was the same as that of Preparation Example 1. The obtained IMID-4 was dissolved in EA (50 mL), and TsOH (18 g, 100 mmol) was diluted with EA (20 mL) and slowly added dropwise to the reaction. The suspension was washed with EA and the filter cake was dried to obtain 15.2 g of white solid TsOH-IMID-4 product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com