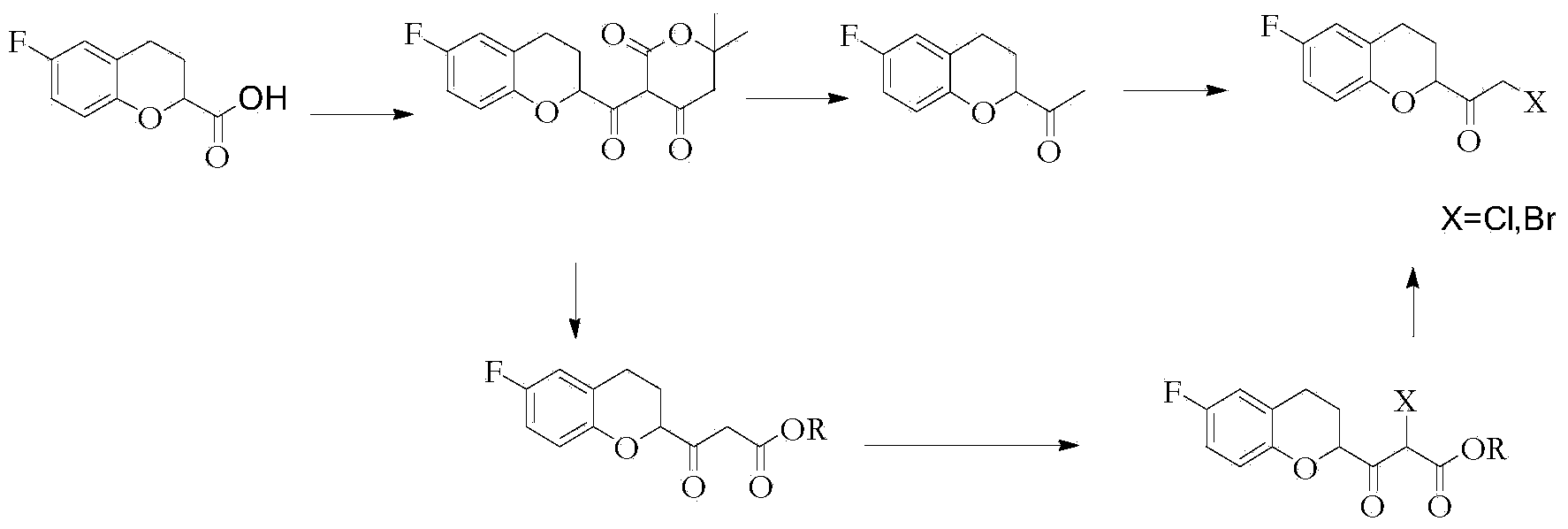
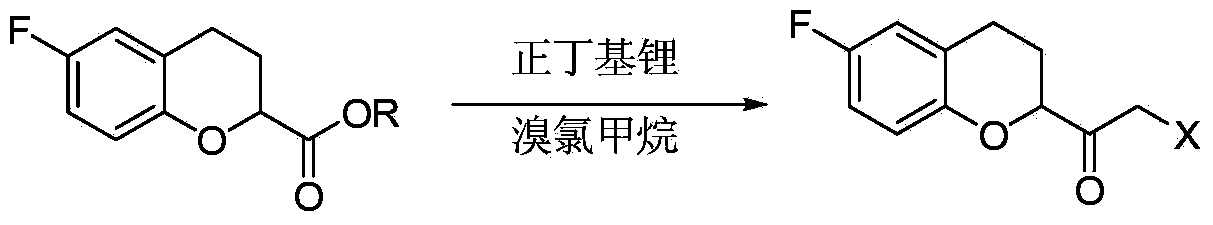
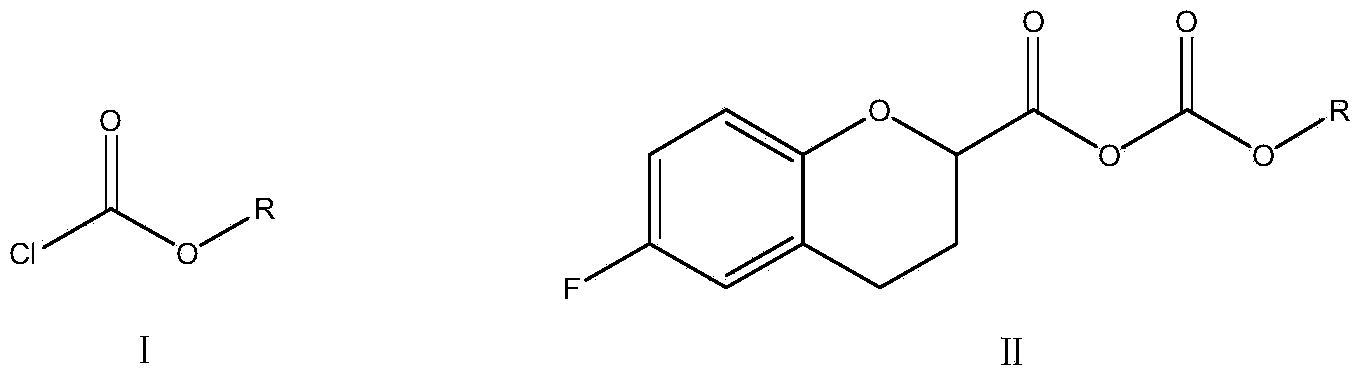
Method for preparing nebivolol midbody
A technology of nebivolol and intermediates, which is applied in the field of drug synthesis, can solve the problems of unfavorable industrial production, high process requirements, and short routes, and achieve the effects of being beneficial to industrial applications, high chiral purity, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 20g of (S)-6-fluorochroman-2-carboxylic acid into the reactor, then add 300mL of organic solvent diethyl ether, then stir and cool down to -10°C, add 20.6g of acid agent triethylamine dropwise, and keep warm for 0.5h , then lower the temperature to -20°C, add 22g of ethyl chloroformate dropwise, after the dropwise addition, control the temperature at -20°C and keep it warm for 1h, after the reaction, directly dropwise add 8.6g of diazomethane to the reaction liquid Diethyl ether solution, control the temperature at -25°C for heat preservation reaction for 2.5 hours, after the reaction, directly pass a sufficient amount of HCl into the reaction solution, and control the temperature at 0°C for halogenation reaction for 3 hours, the halogenation reaction is over Finally, add 60mL of deionized water to the reaction solution and stir until the solid dissolves, then let it stand for stratification, collect the organic layer, concentrate and remove the solvent to obtain 33g...
Embodiment 2
[0044] Take 20g of (R)-6-fluorochroman-2-carboxylic acid and add it into the reactor, then add 300mL of organic solvent diethyl ether, then, stir and cool down to -10°C, add 20.6g of acid agent triethylamine dropwise, keep warm for 0.5h , then lower the temperature to -30°C, add 22g of ethyl chloroformate dropwise, after the dropwise addition, control the temperature at -30°C to keep warm for 1h, after the reaction is over, directly dropwise add 8.6g of diazomethane tetrasodium diazomethane into the reaction solution Carbon chloride solution, keep the temperature at -30°C for 2.5 hours, add a sufficient amount of HBr after the reaction, and control the temperature at 0°C to keep the halogenation reaction for 3 hours. After the halogenation reaction, add Add 60mL of deionized water and stir until the solid dissolves, then let stand to separate layers, collect the organic layer, concentrate and remove the solvent to obtain 39.5g of crude oily product, add 50mL of ethanol, stir an...
Embodiment 3
[0046] Take 20g of (S)-6-fluorochroman-2-carboxylic acid, add 300mL of organic solvent tetrahydrofuran, then stir and cool down to -15°C, add 20.6g of triethylamine dropwise, keep warm for 0.5h, then cool down to -30°C, Add 22g of ethyl chloroformate dropwise. After the dropwise addition, keep the temperature at -30°C for 1 hour. After the reaction, add 8.6g of diazomethane-containing ether solution directly to the reaction solution, and control the temperature at -30°C. ℃ for 2.5 hours, after the reaction, add enough HCl directly to the reaction solution, and control the temperature at -10 ℃ for halogenation reaction for 3 hours, after the halogenation reaction, directly concentrate to remove the organic solvent, and then add dichloro Stir 200mL of methane and 60mL of water until the solid is dissolved, let stand to separate layers, collect the organic layer, and then concentrate to remove the solvent to obtain 34g of crude oil, add 50mL of isopropanol, stir and cool to -5°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com