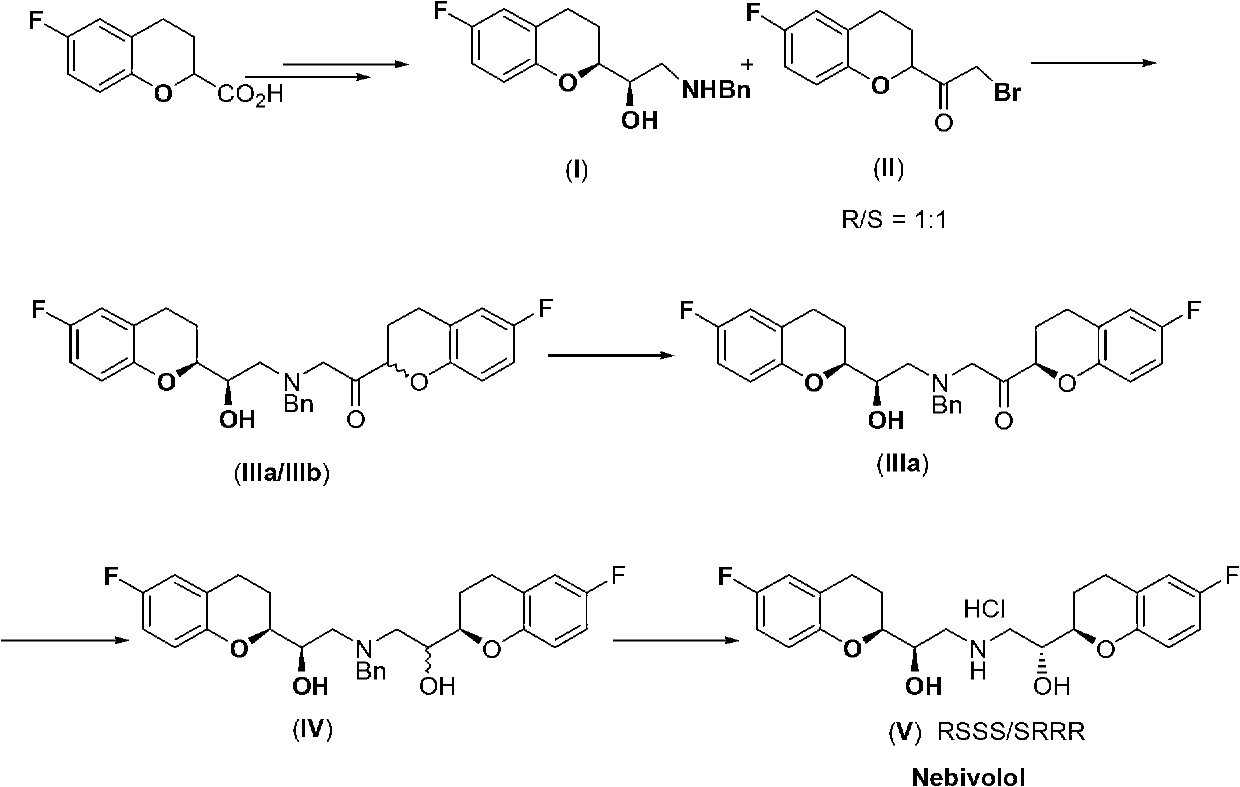
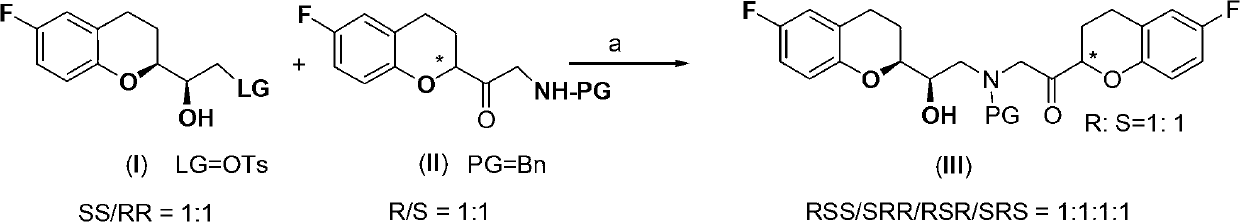
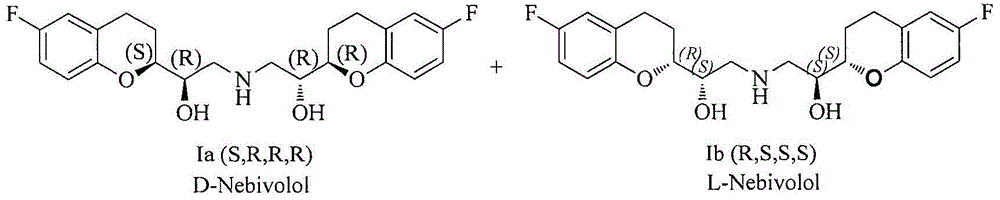
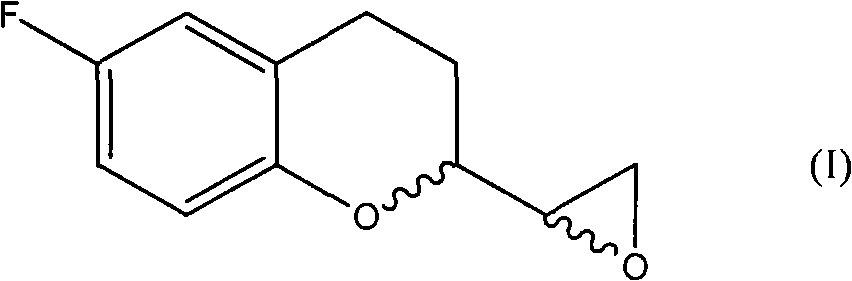
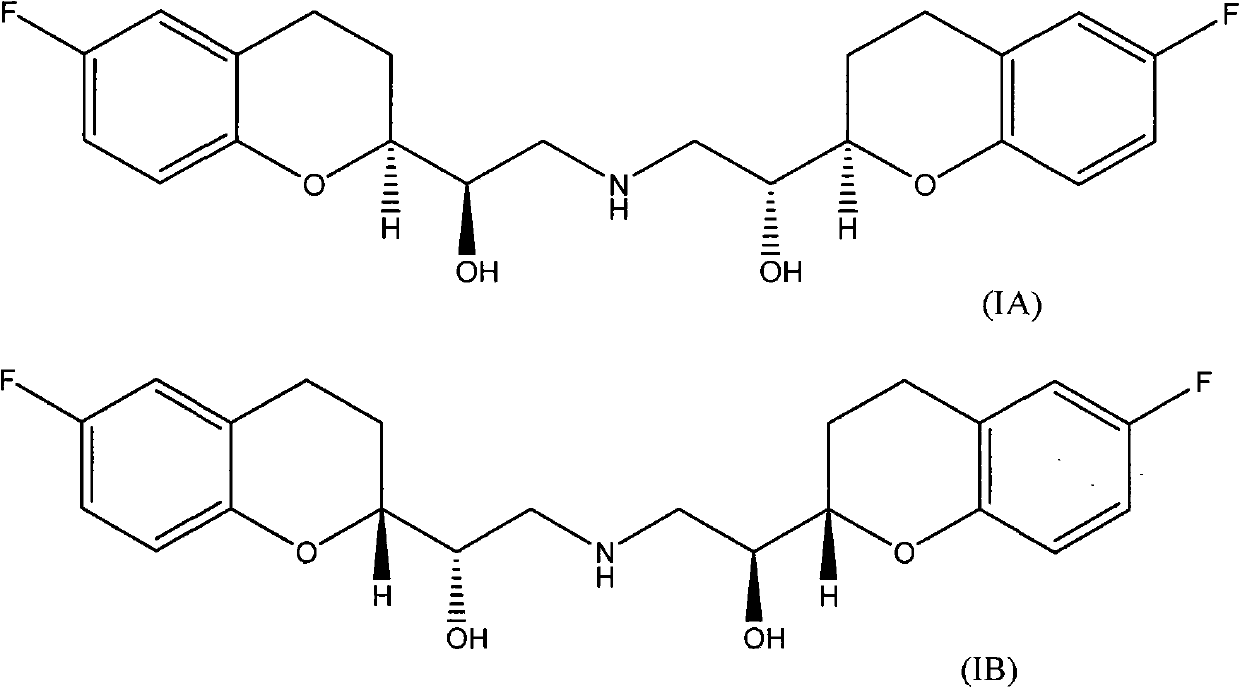
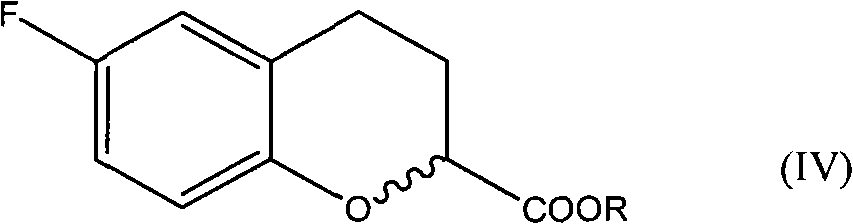
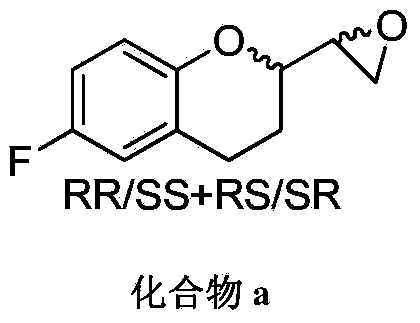
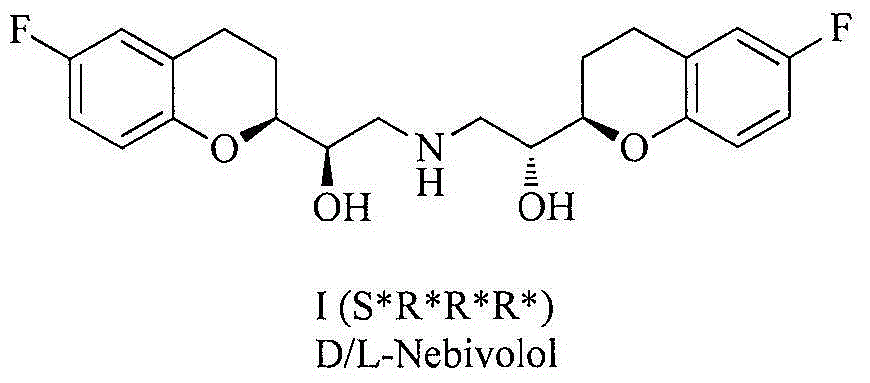Patents
Literature
69 results about "Nebivolol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
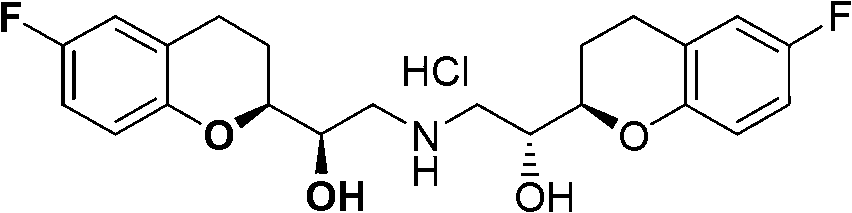
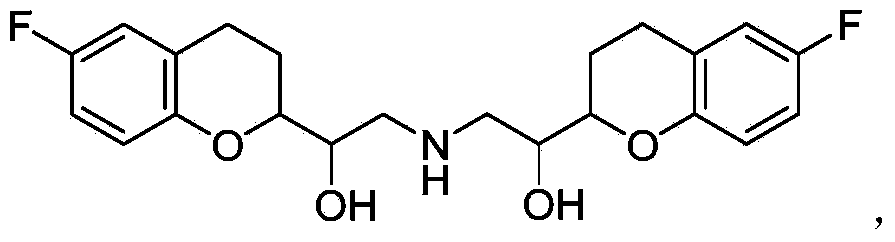
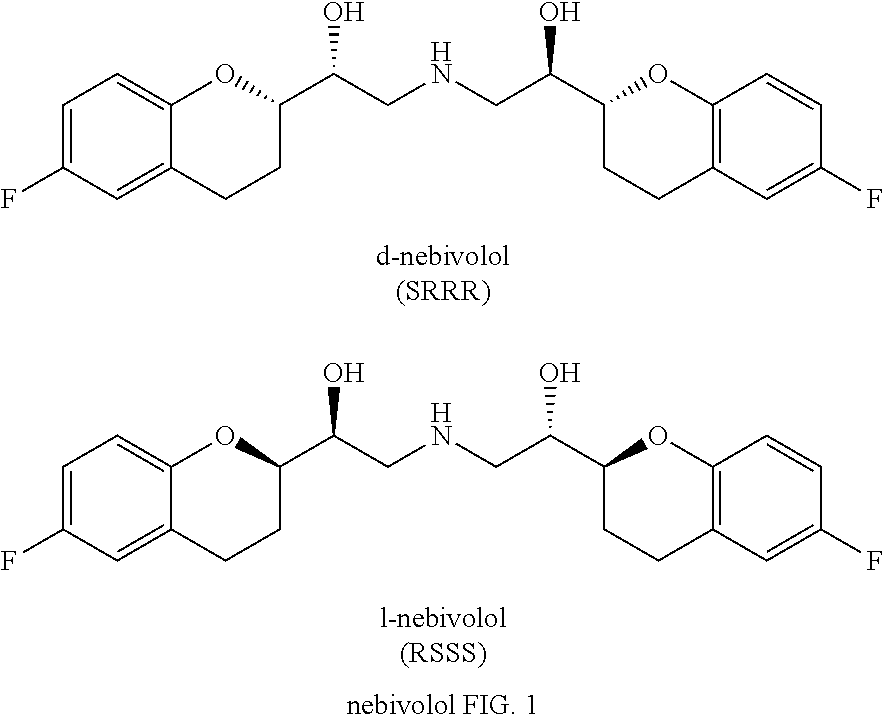
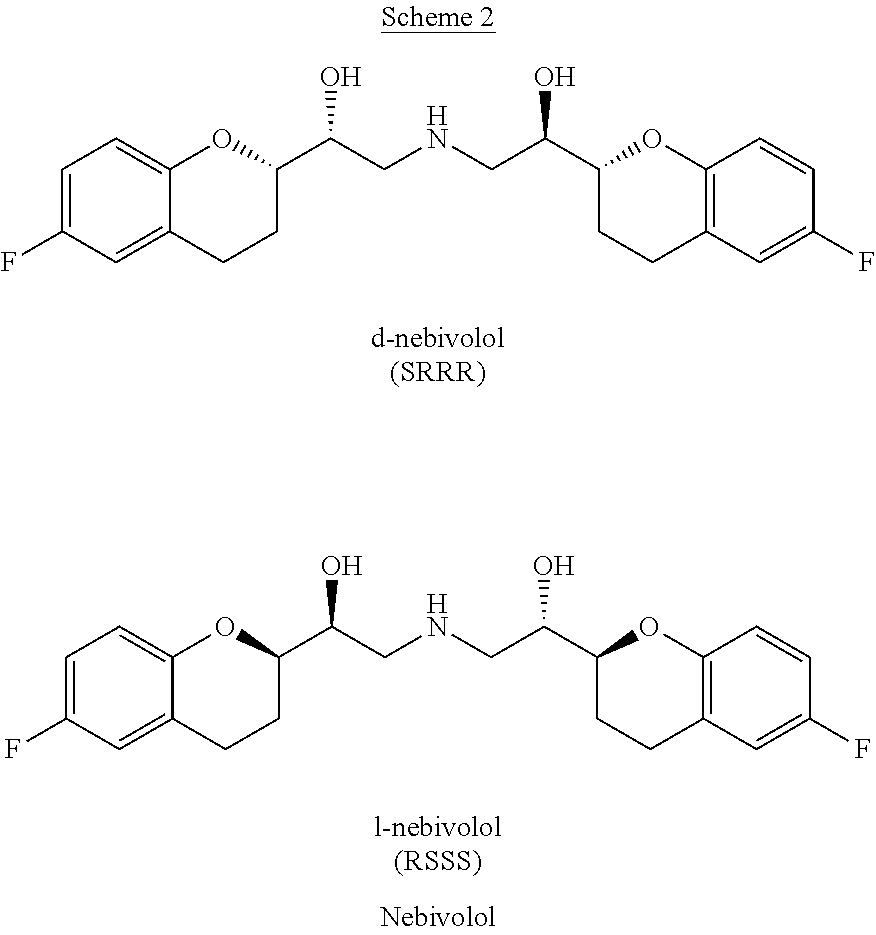
Nebivolol is used to treat high blood pressure.
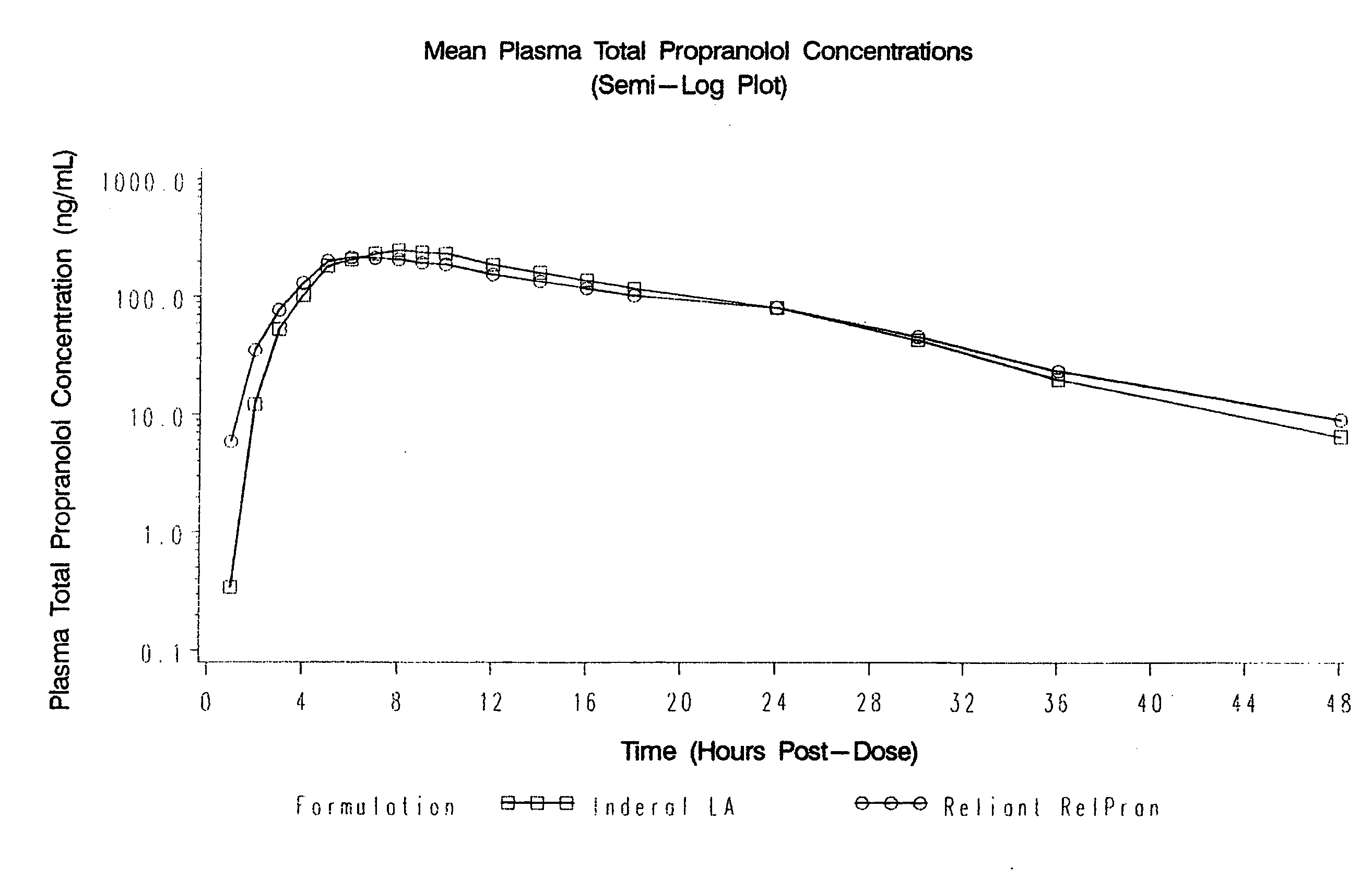
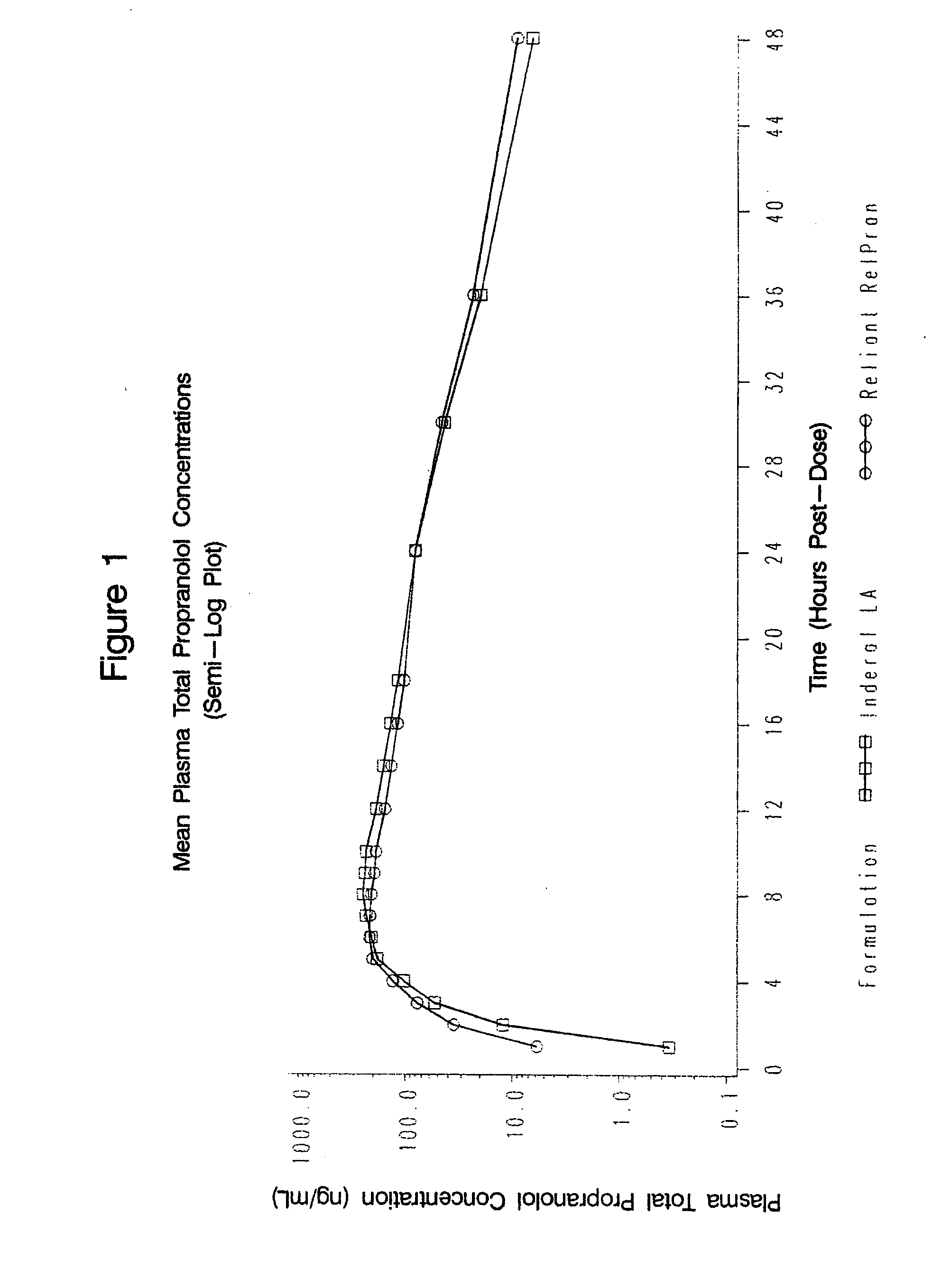
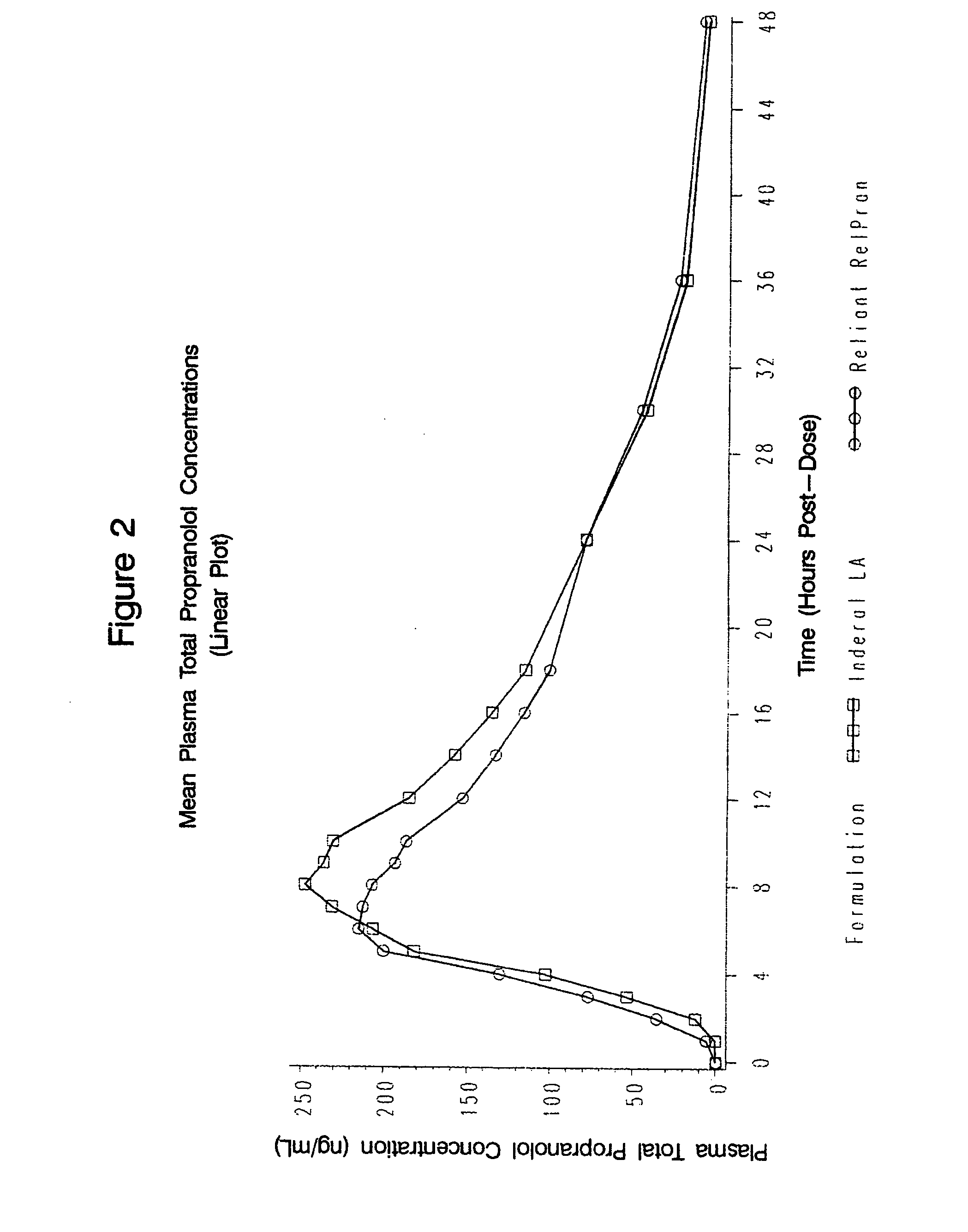
Time-sustained-release formulations comprising a beta-blocker
InactiveUS20080131517A1Providing therapyAvoid problemsPowder deliveryOrganic active ingredientsCarteololBeta blocker
The present invention relates to compositions and methods of treating human subjects with a beta-adrenergic receptor blocking agent (“beta-blocker”) provided in a time-sustained-release delivery system. The time-sustained-release drug delivery systems includes at least three populations of beads, where each population of beads includes a beta-blocker. The beads may be selected from immediate-release beads, enteric-release beads, sustained-release beads, and time-sustained-release beads. The beta-blocker may be selected from acebutolol, atenolol, betaxolol, bisoprolol, esmolol, metoprolol, nebivolol, butoxamine, carteolol, carvedilol, labetalol, nadolol, oxprenolol, penbutolol, propranolol, pindolol, sotalol, and timolol. According to presently preferred embodiments, the beta-blocker is propranolol. The dosage forms of the present invention are useful for treating conditions including hypertension, angina pectoris due to coronary atherosclerosis, hypertrophic subaortic stenosis, congestive heart failure, arrhythmias, angina, anxiety, glaucoma, migraines, esophageal varices, alcohol withdrawal syndrome, irregular heartbeat, tachycardia, tremor, and neuroleptic-induced akathisia. They are also useful in the prophylaxis of migraine headaches.
Owner:RELIANT PHARMACEUTICALS INC
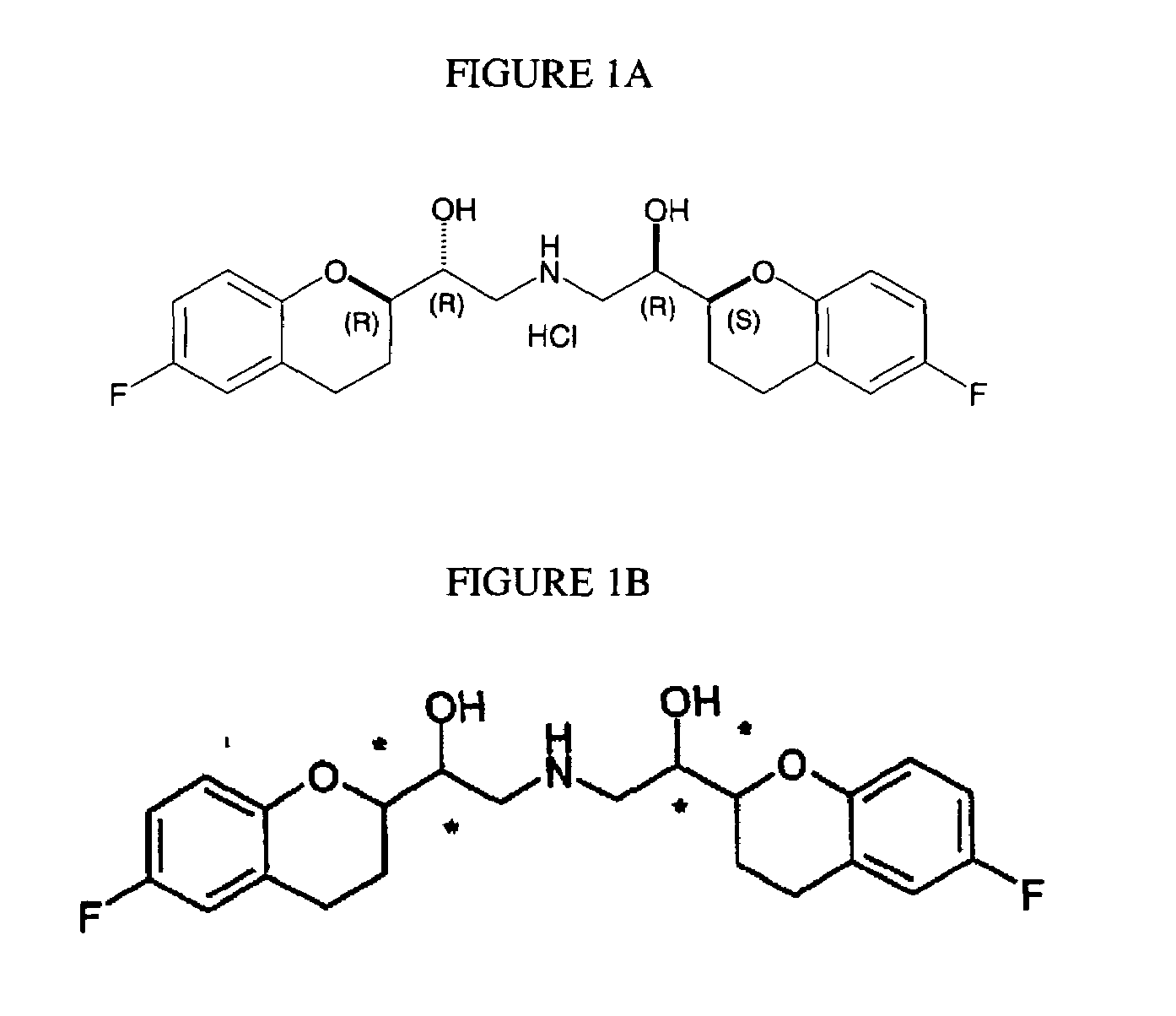
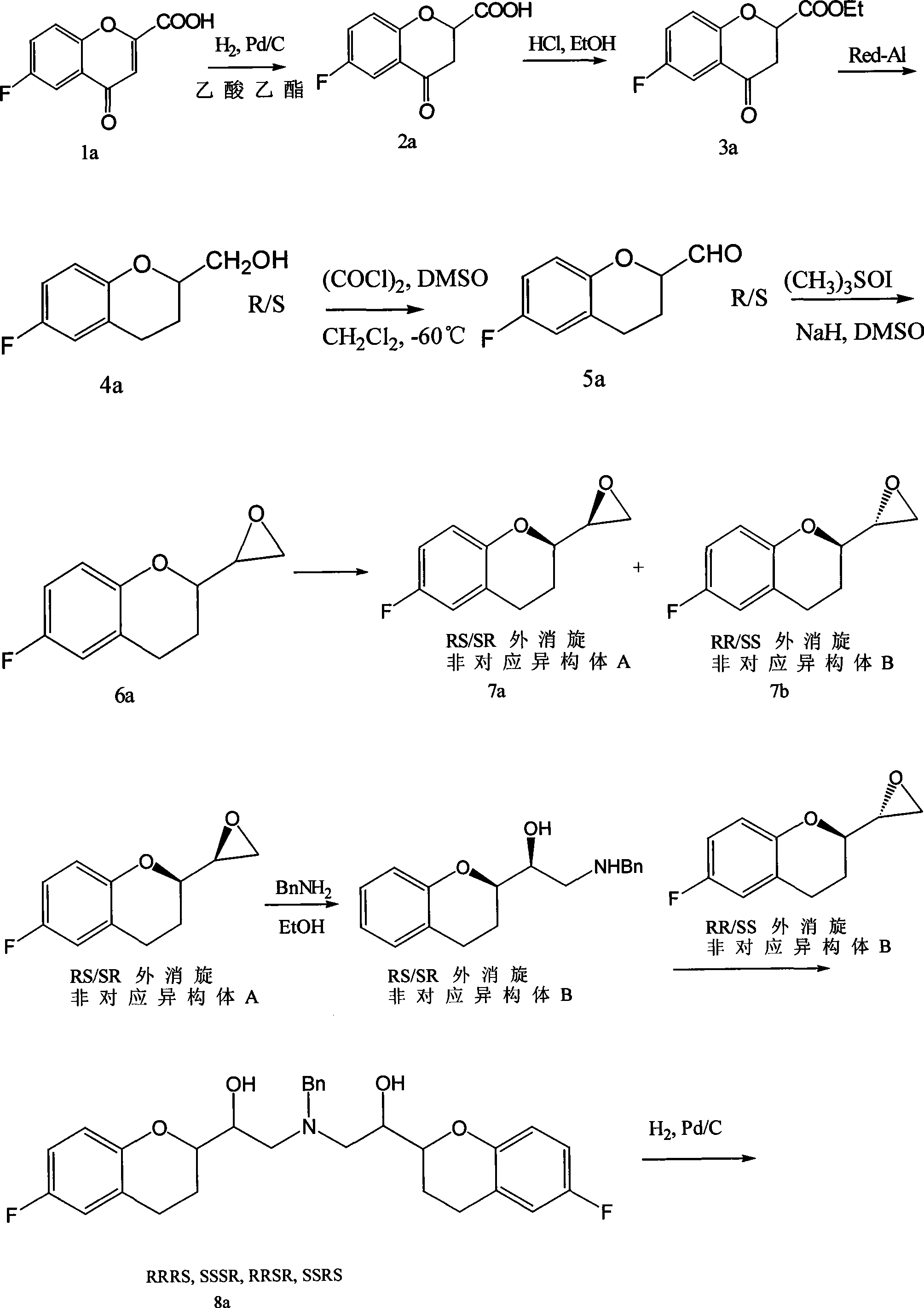
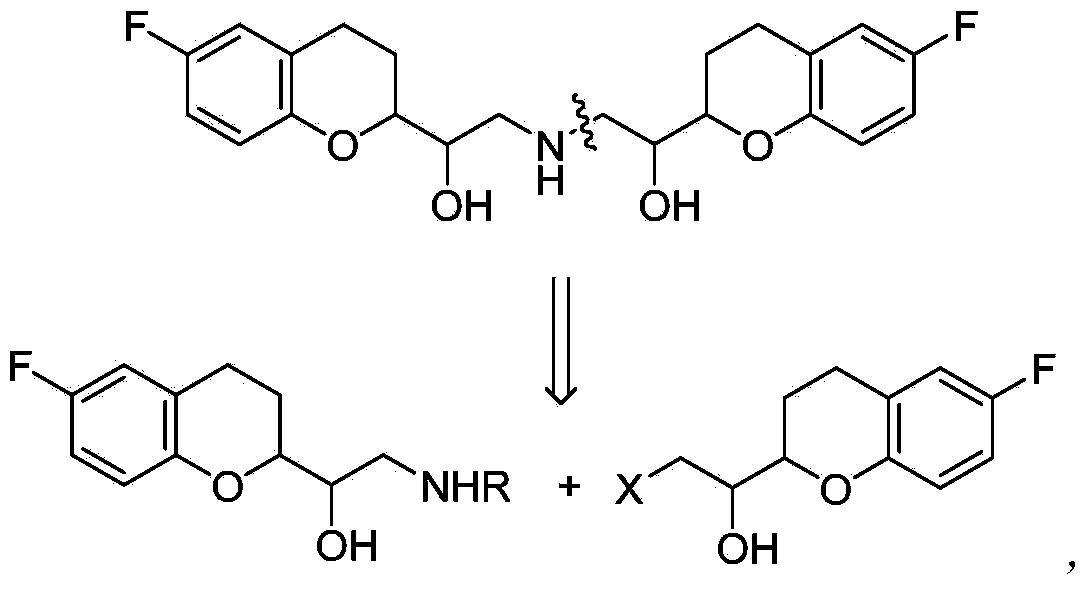
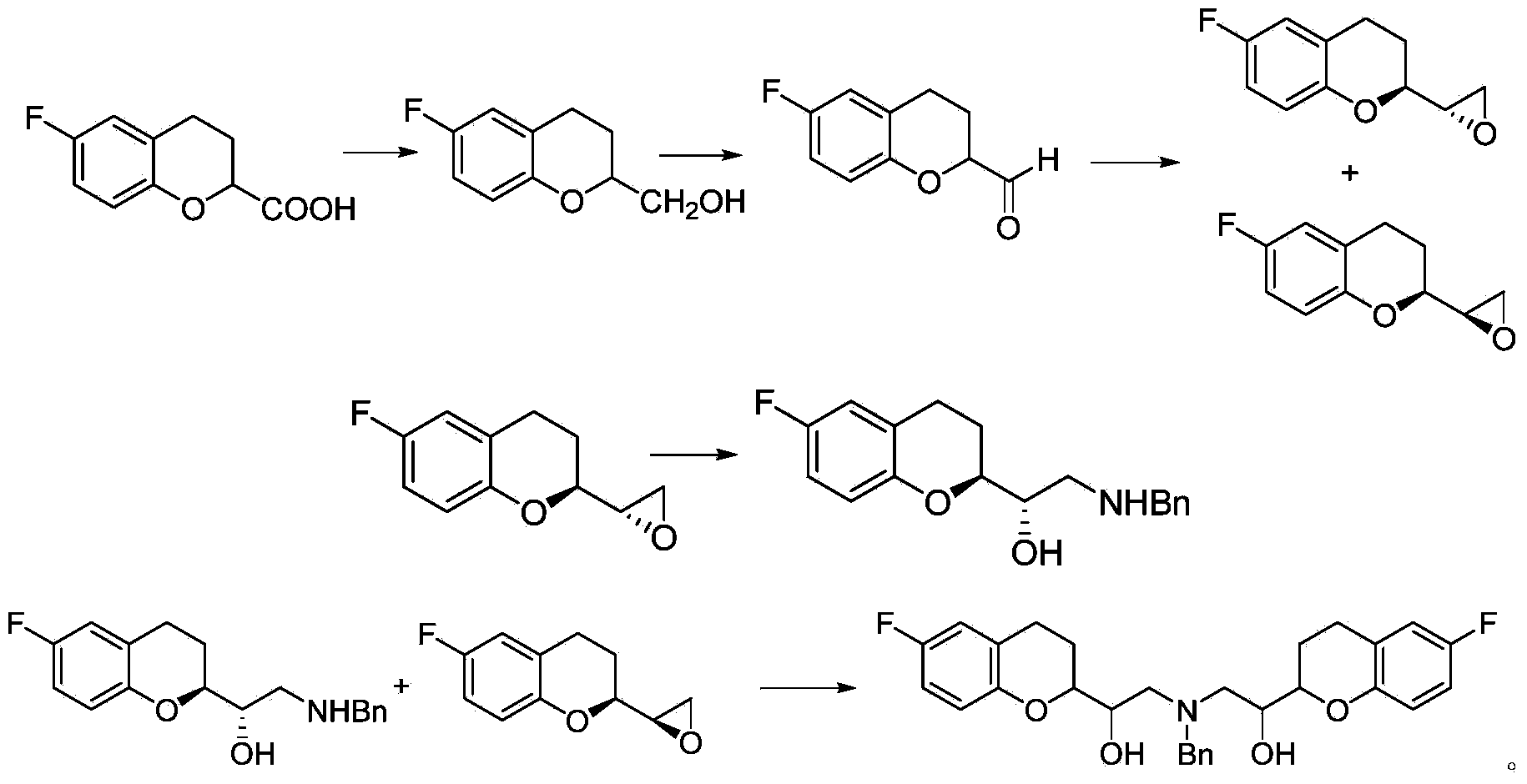
Process for preparation of racemic Nebivolol
Owner:UNIV ZURICH +1
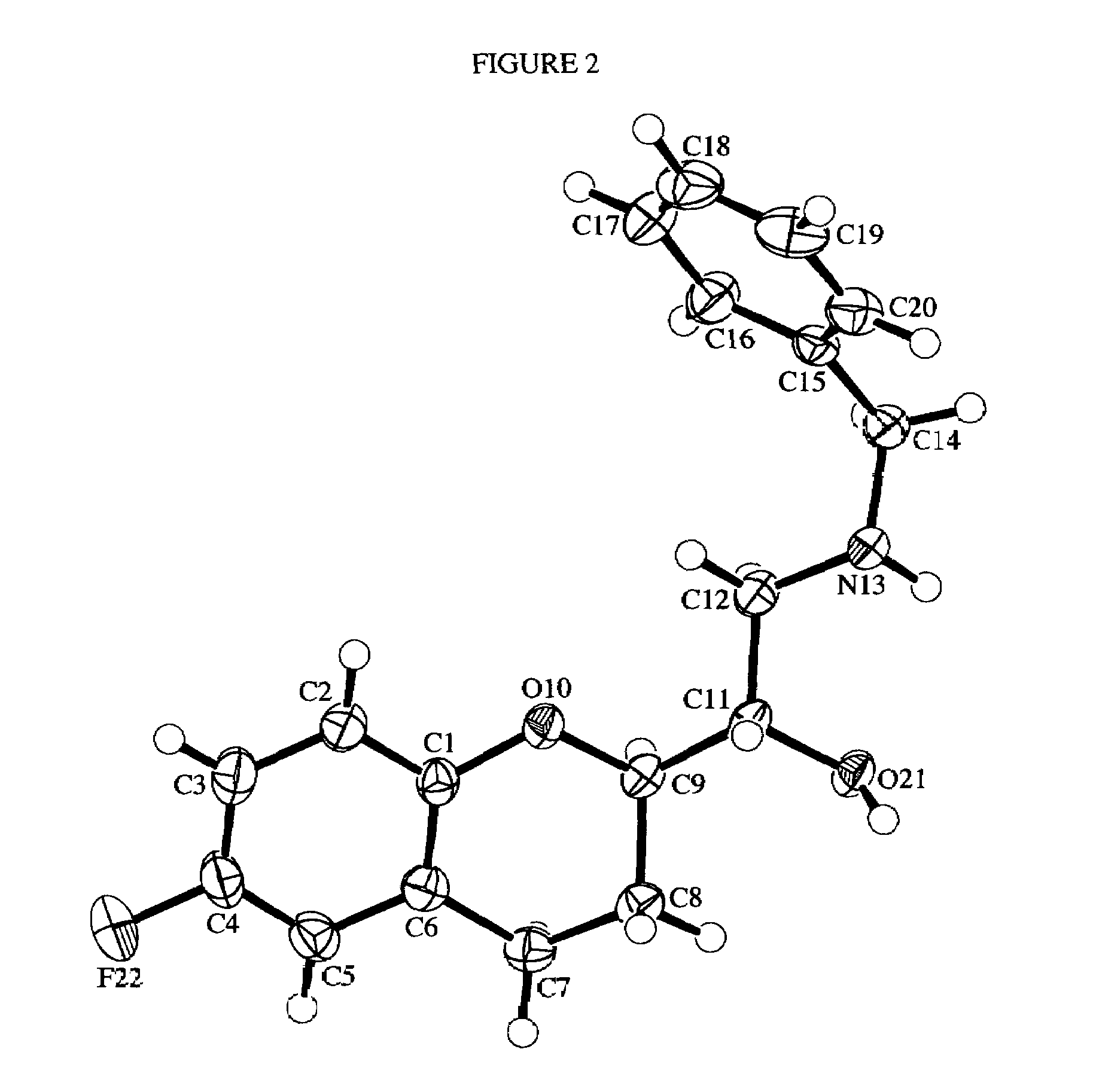
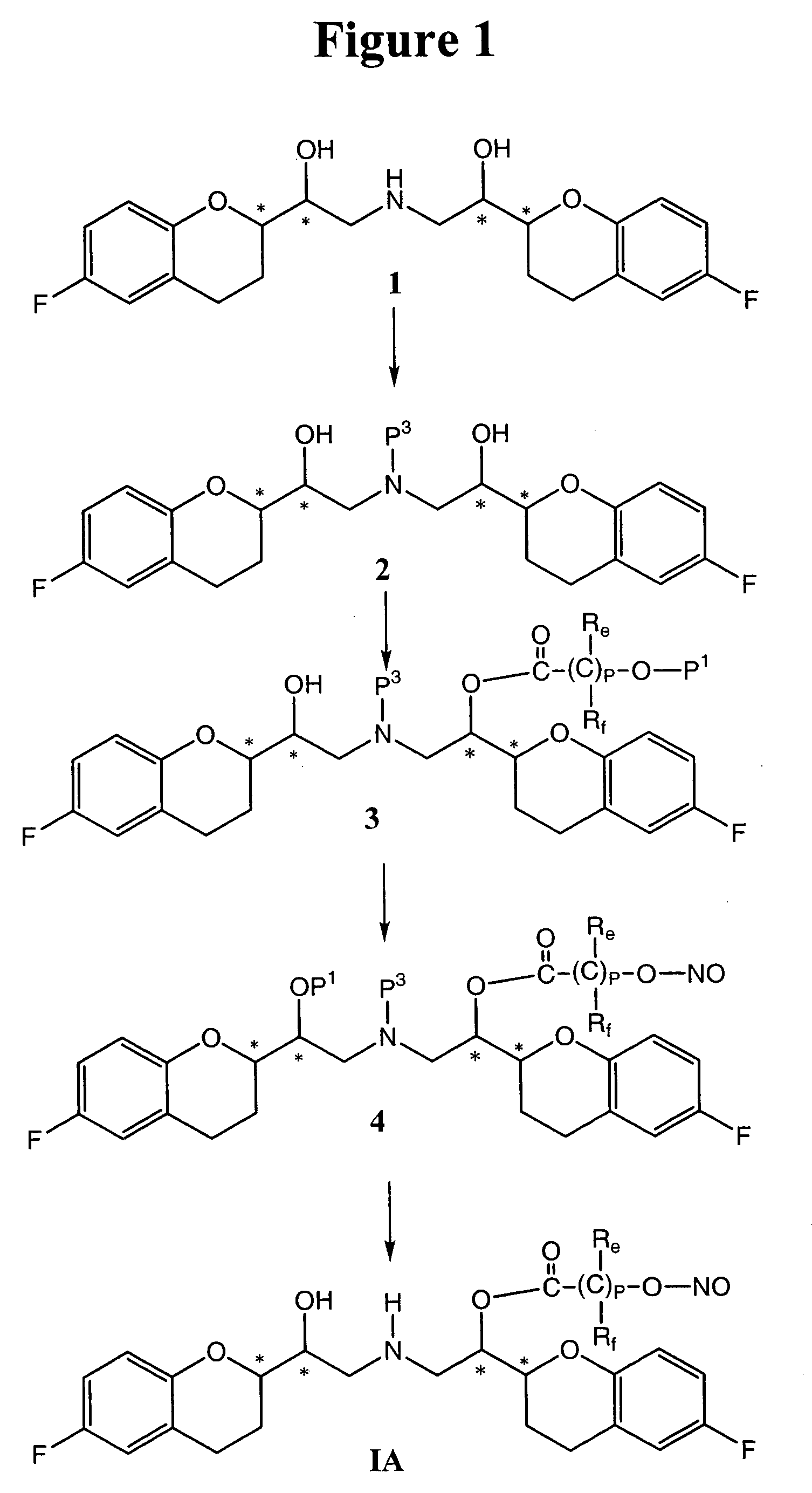
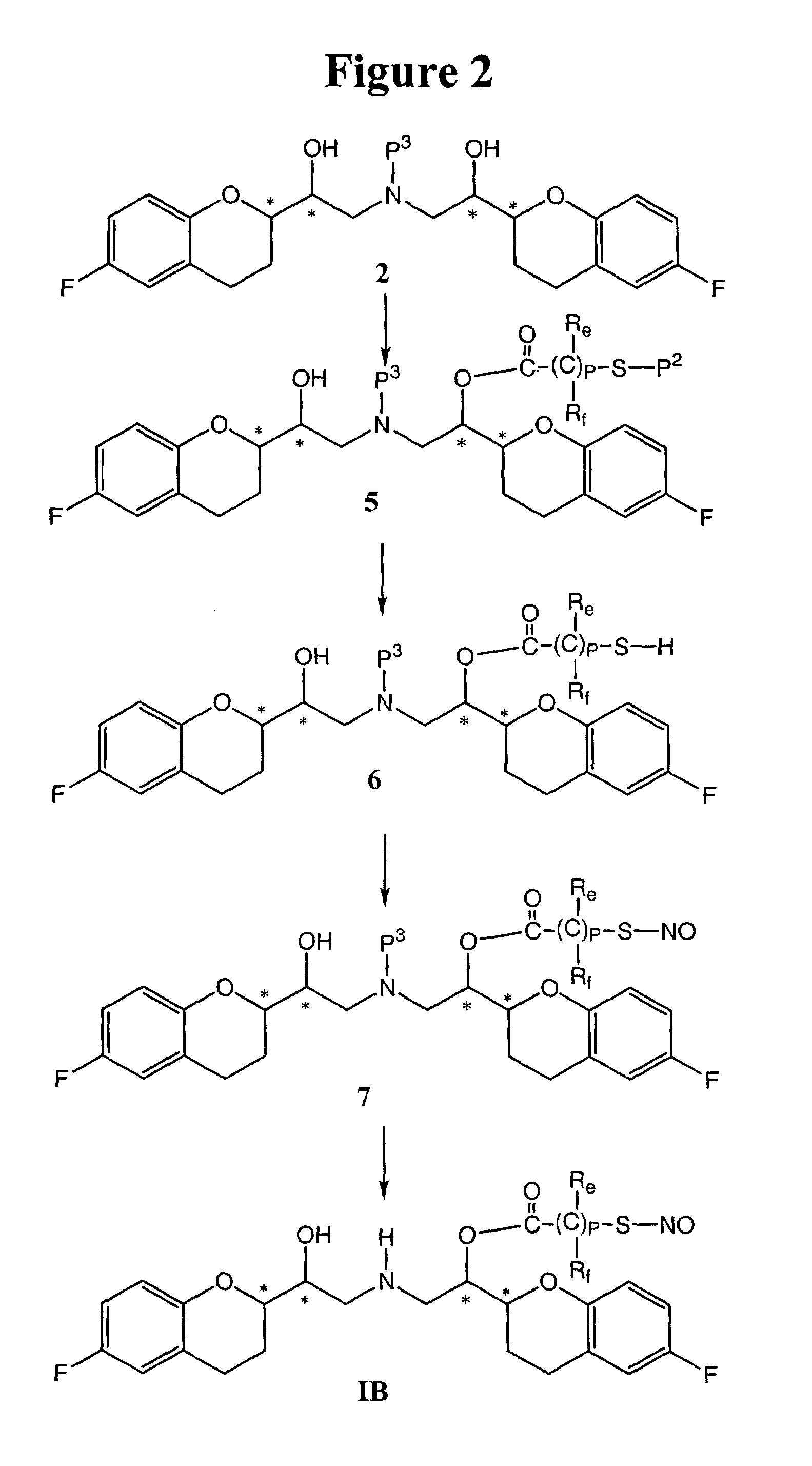
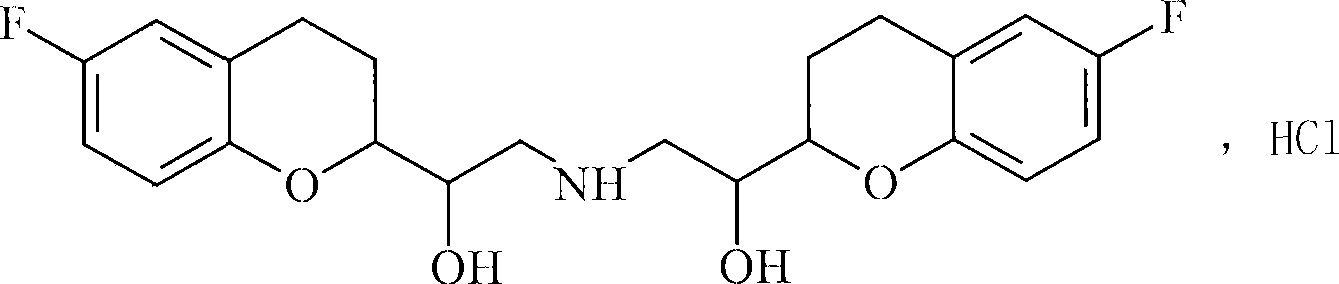
Nitrosated and nitrosylated nebivolol and its metabolites, compositions and methods of use
InactiveUS7138430B2Prevention of platelet aggregation and platelet adhesionAntibacterial agentsBiocideMetaboliteAntioxidant
Owner:NICOX SA
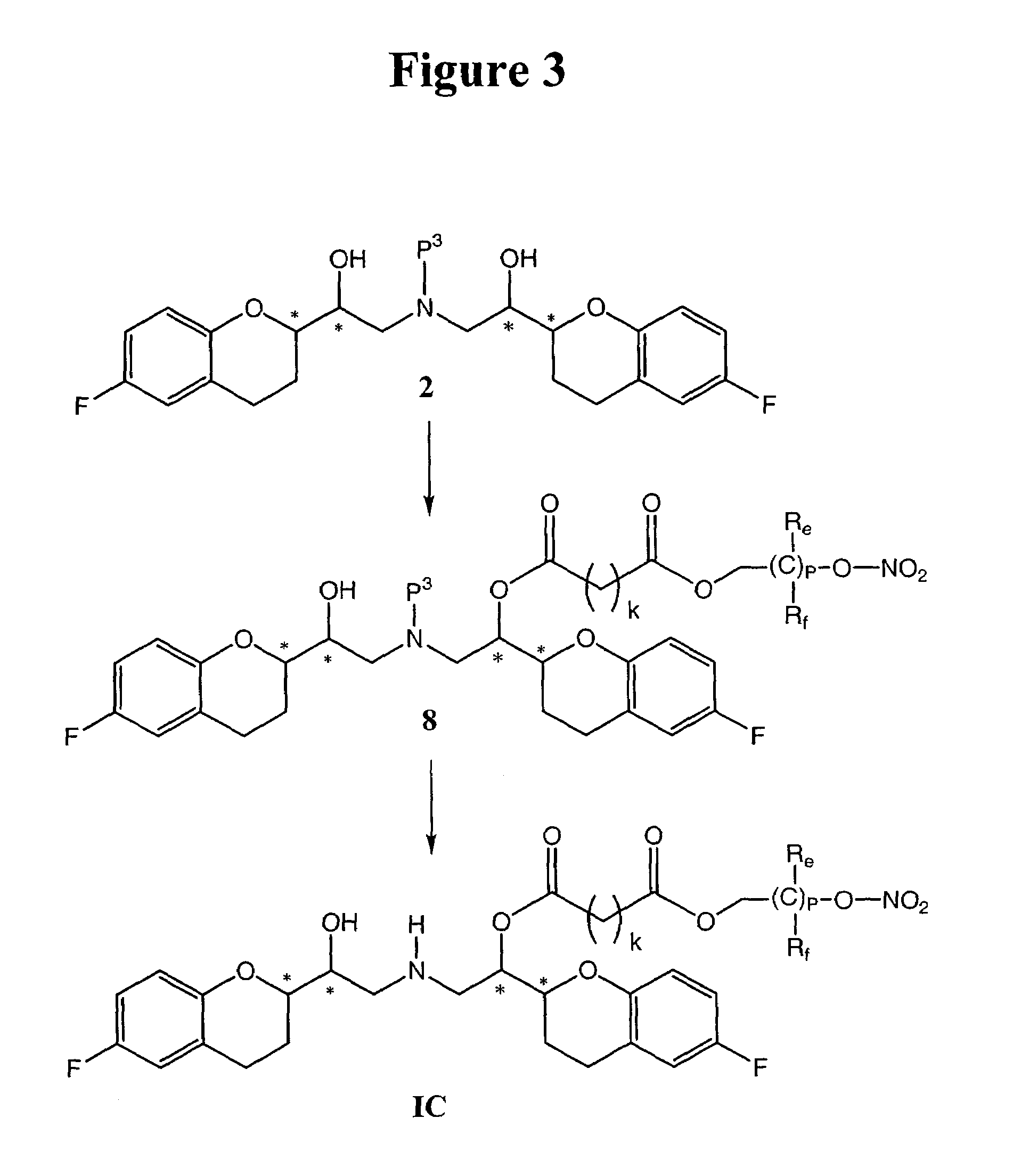
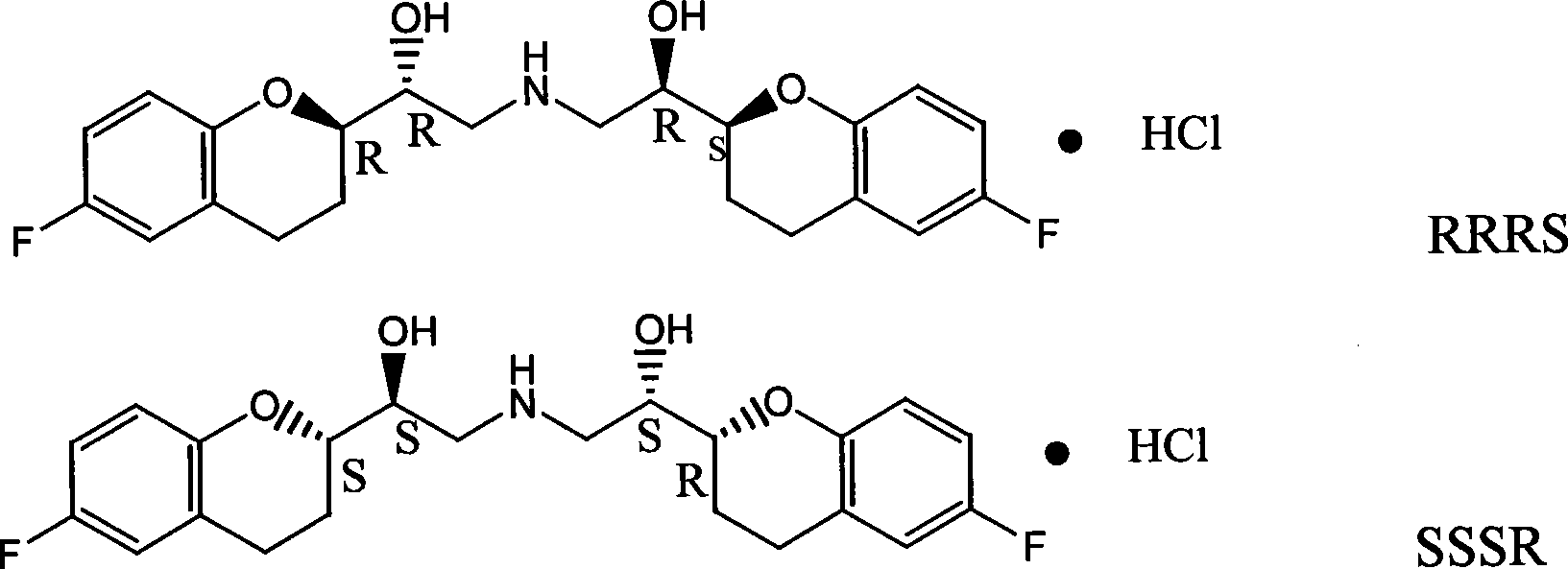
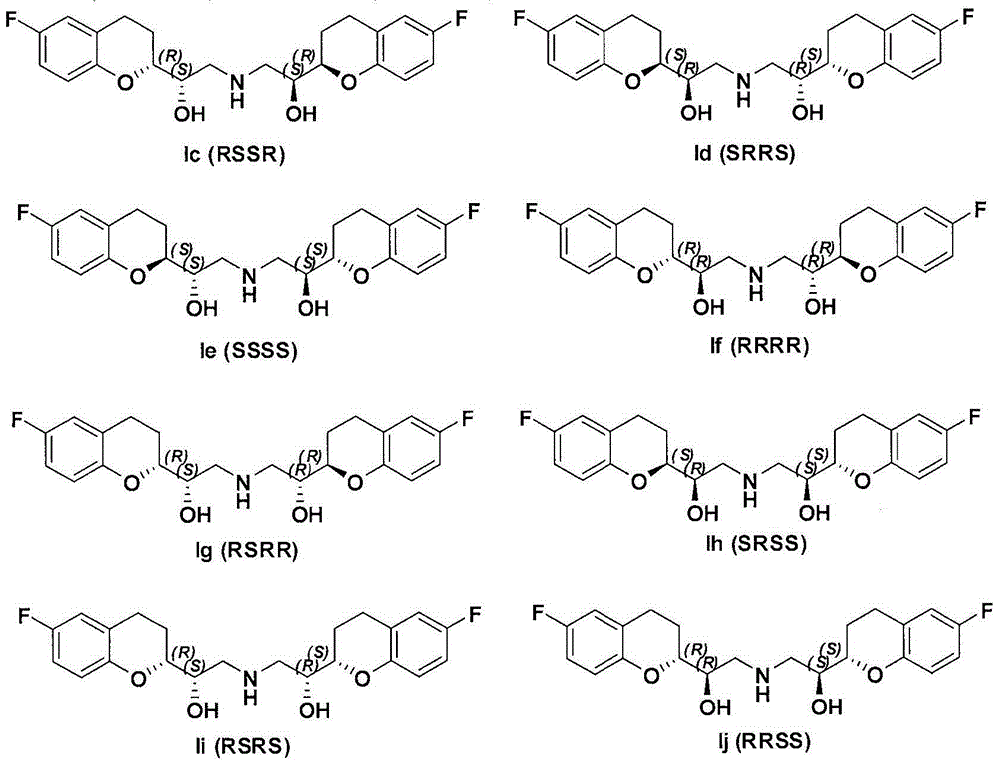
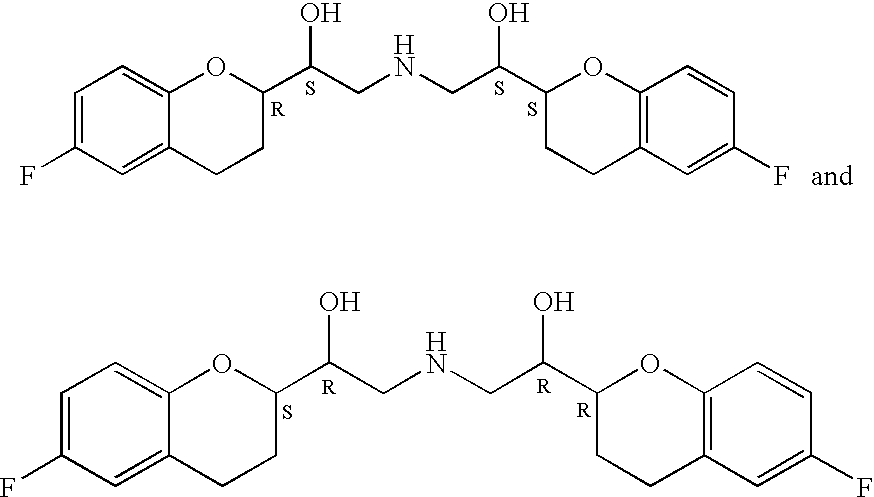
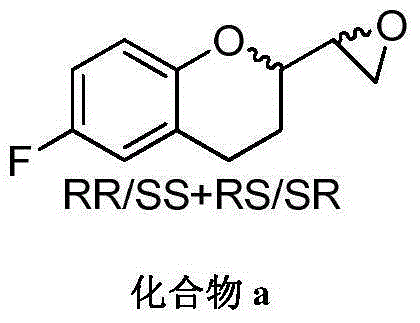
Method for preparing RRRS and SSSR type nebivolol intermediate mixture
ActiveCN101463024ASimple and fast operationImprove separation efficiencyOptically-active compound separationOrganic racemisationAlcoholNebivolol
The invention discloses a method for preparing a mixture of a RRRS nebivolol intermediate and a SSSR nebivolol intermediate respectively shown in formula II and formula III. The method is characterized by optionally adopting one of the following two manners: (1) adding a precipitation solvent to an alcohol solvent of the mixture containing RRRS, SSSR, RRSR and SSRS nebivolol intermediates as shown in formula I, heating, cooling to separate out crystal, and filtering; and (2) adding the mixture of the RRRS, SSSR, RRSR and SSRS nebivolol intermediates of the formula I to a mixed solvent of the alcohol solvent and the precipitation solvent, heating to dissolve, cooling to separate out crystal, and filtering, wherein, X is H, or C1-C6 alkyl or C1-C6 alkoxyl, and n is 1-5. The method has the advantages of simple and convenient operation, and high separation efficiency, and is available for industrialized production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Pharmaceutical composition comprising hydroxylated nebivolol
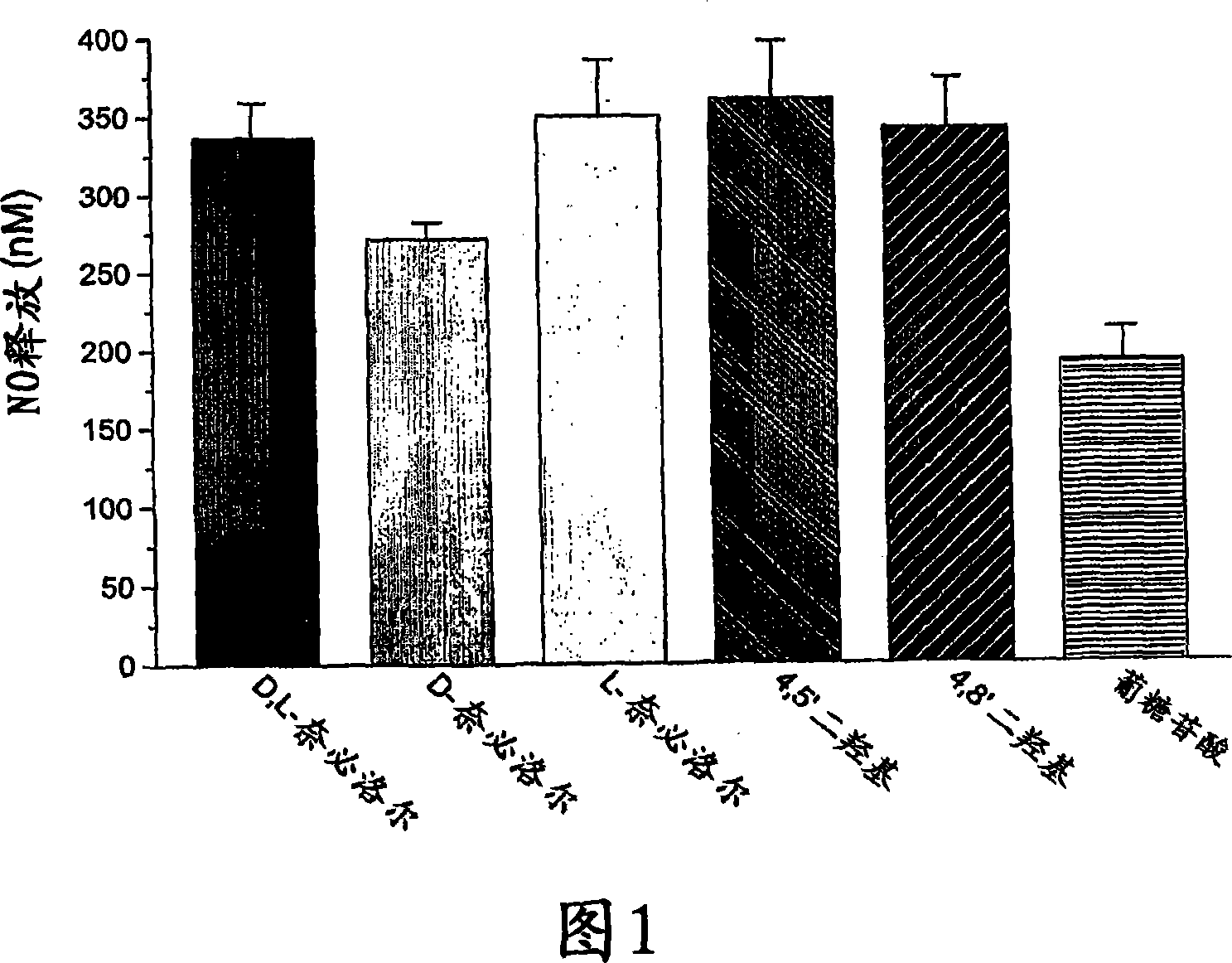
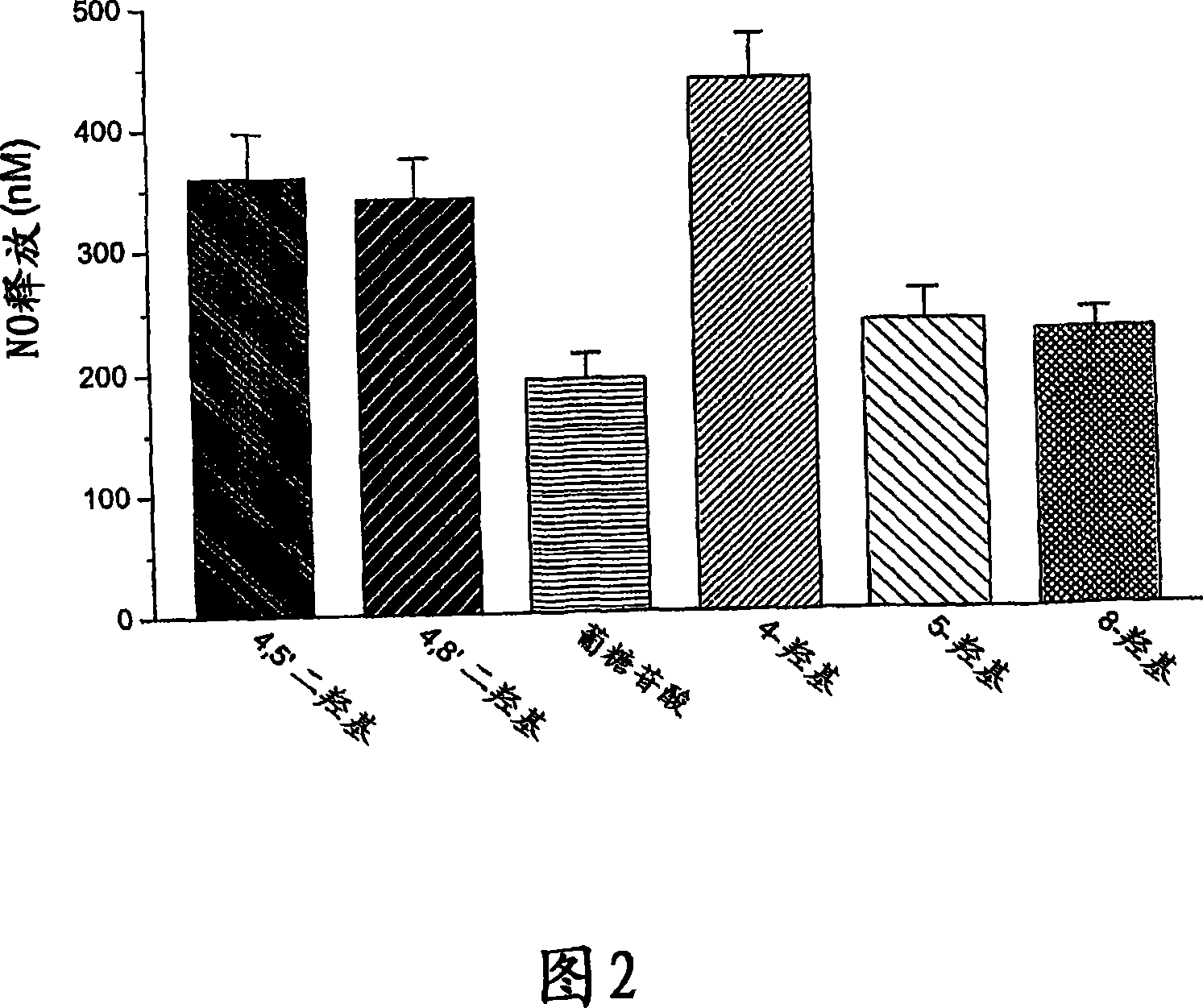
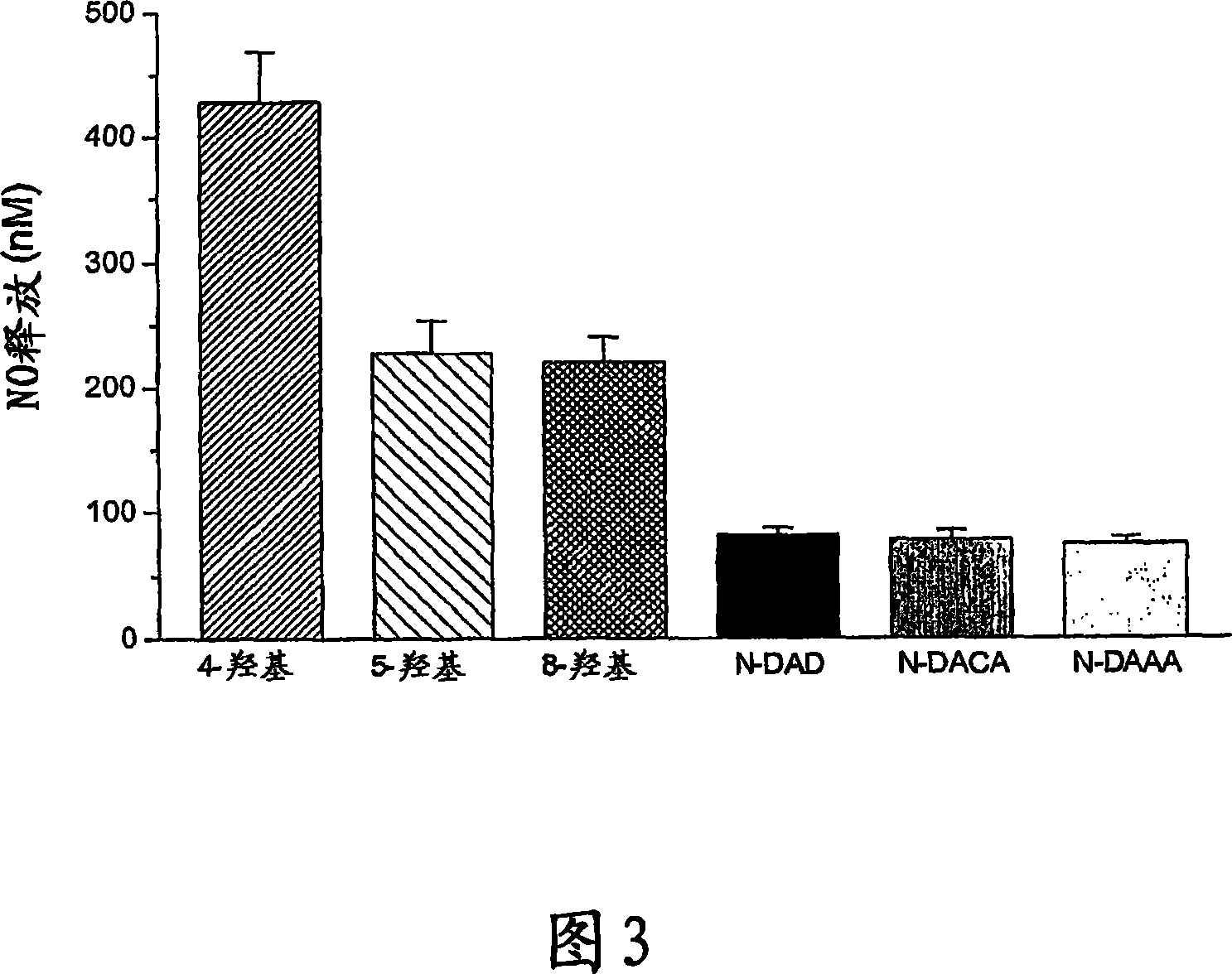
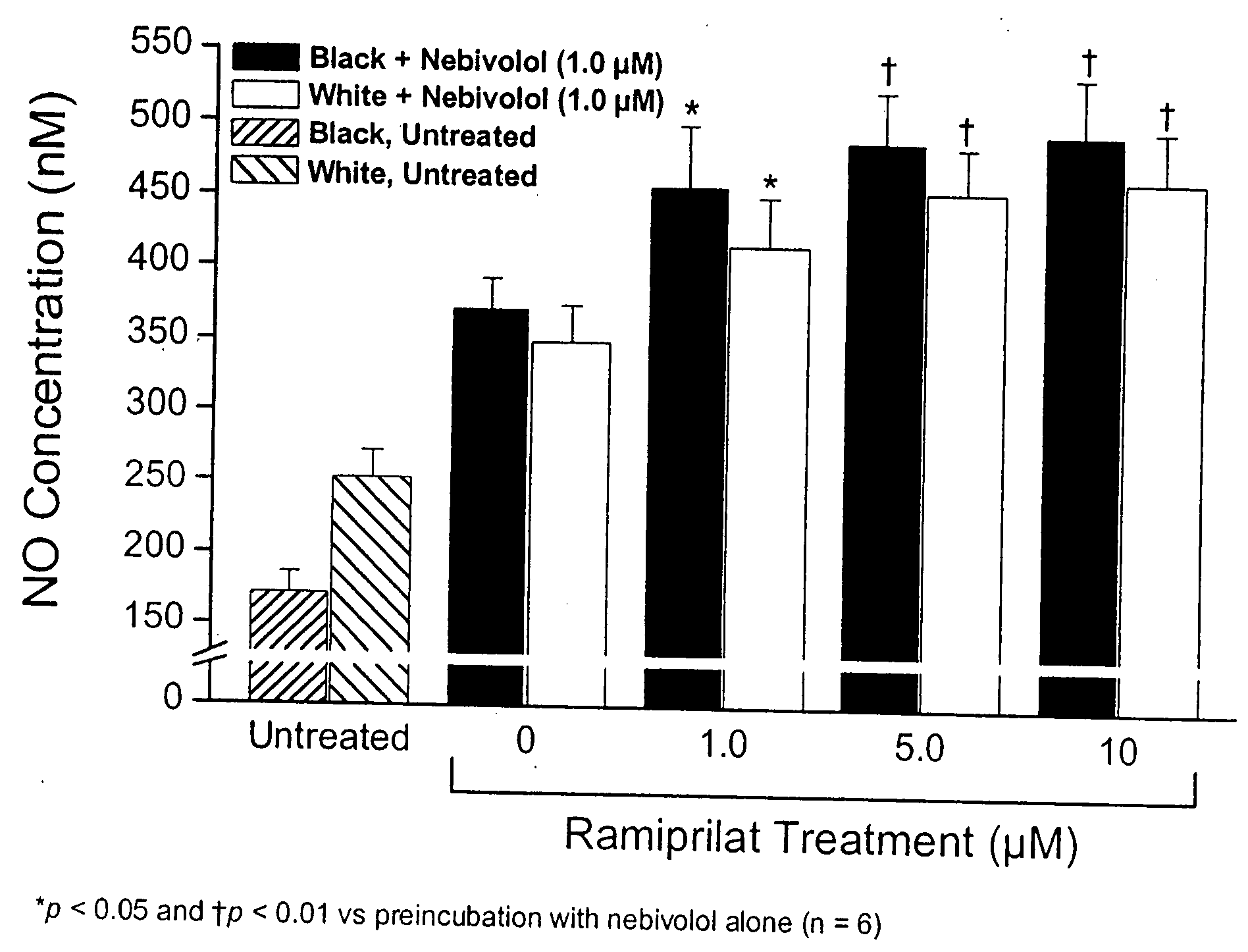
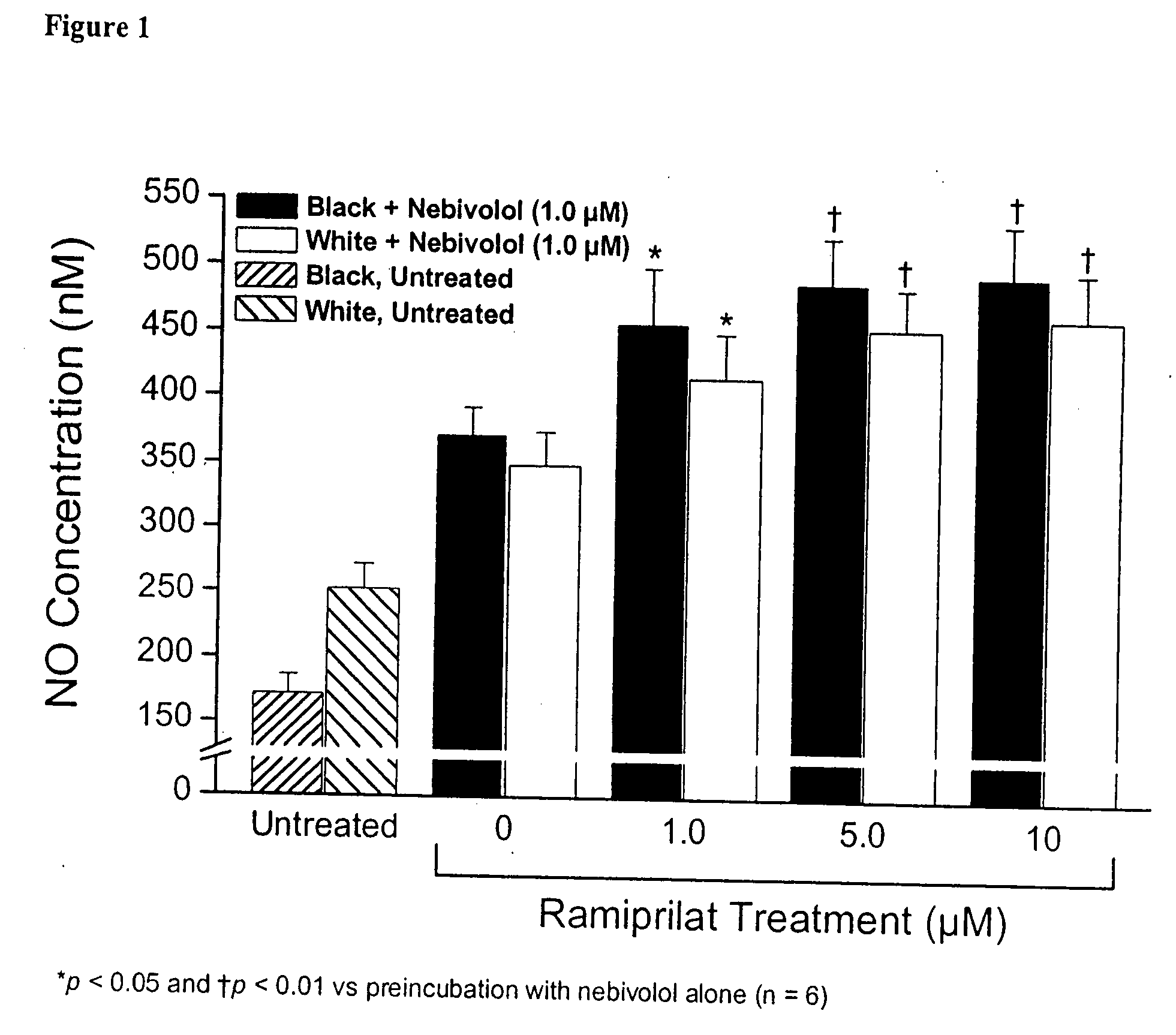
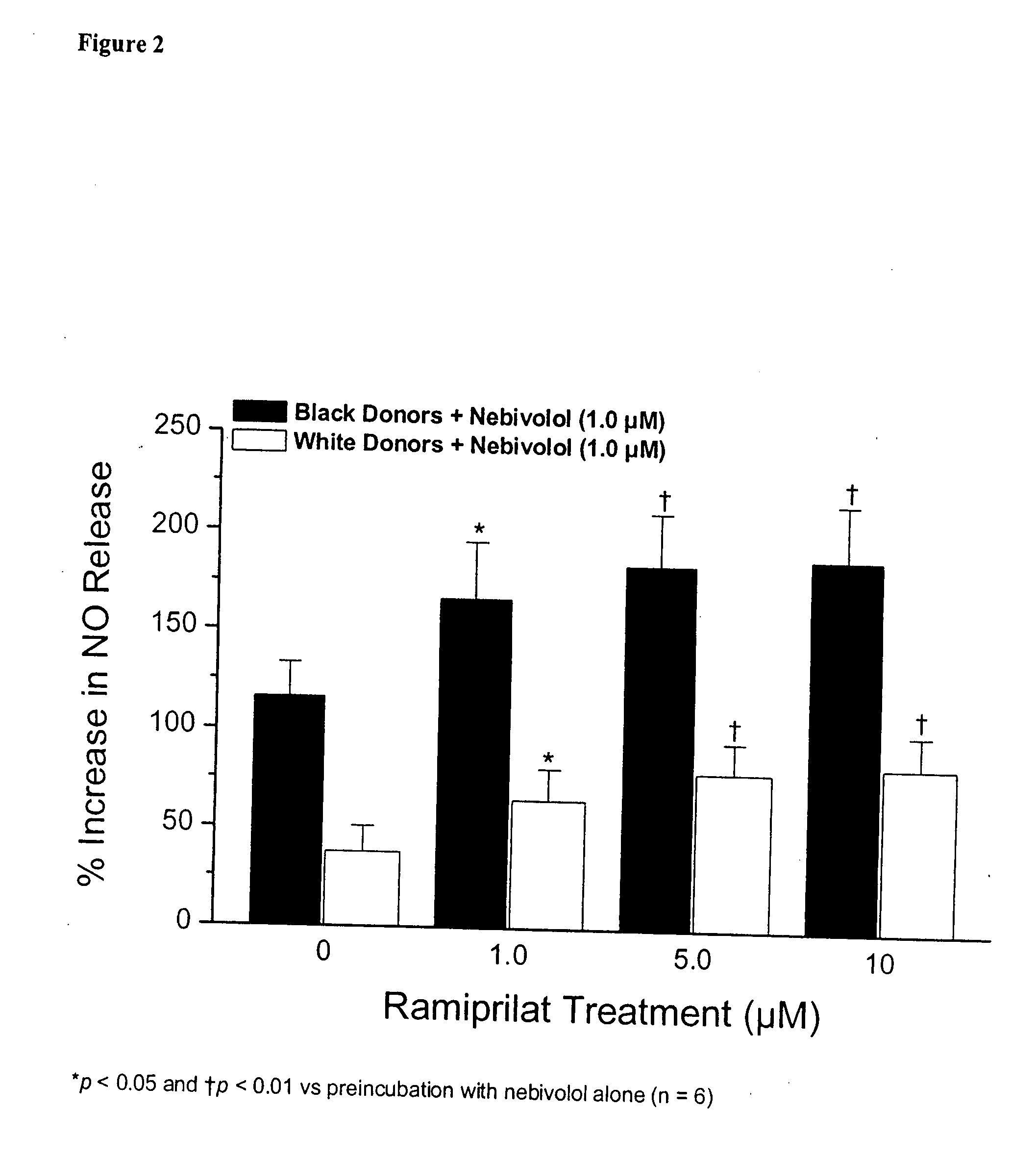
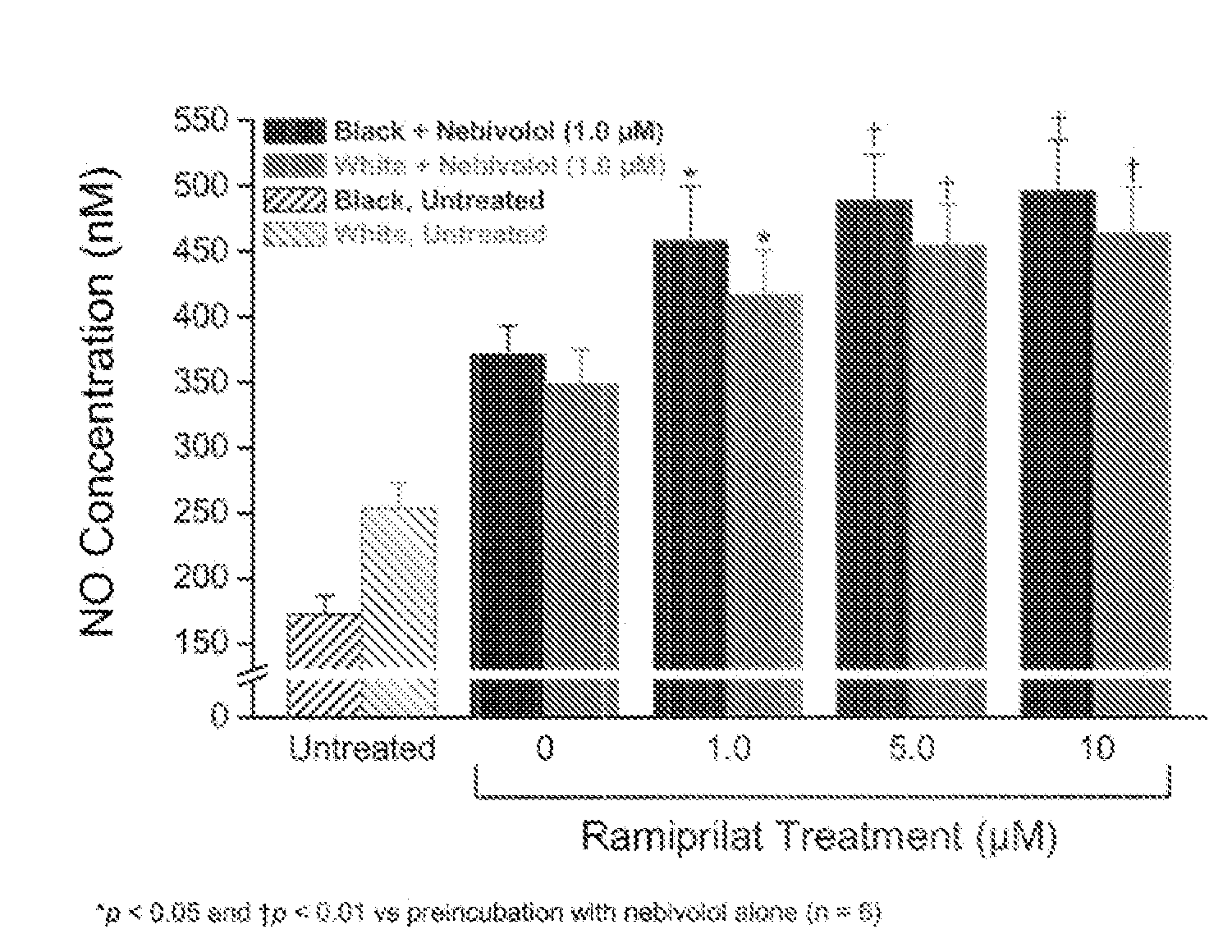
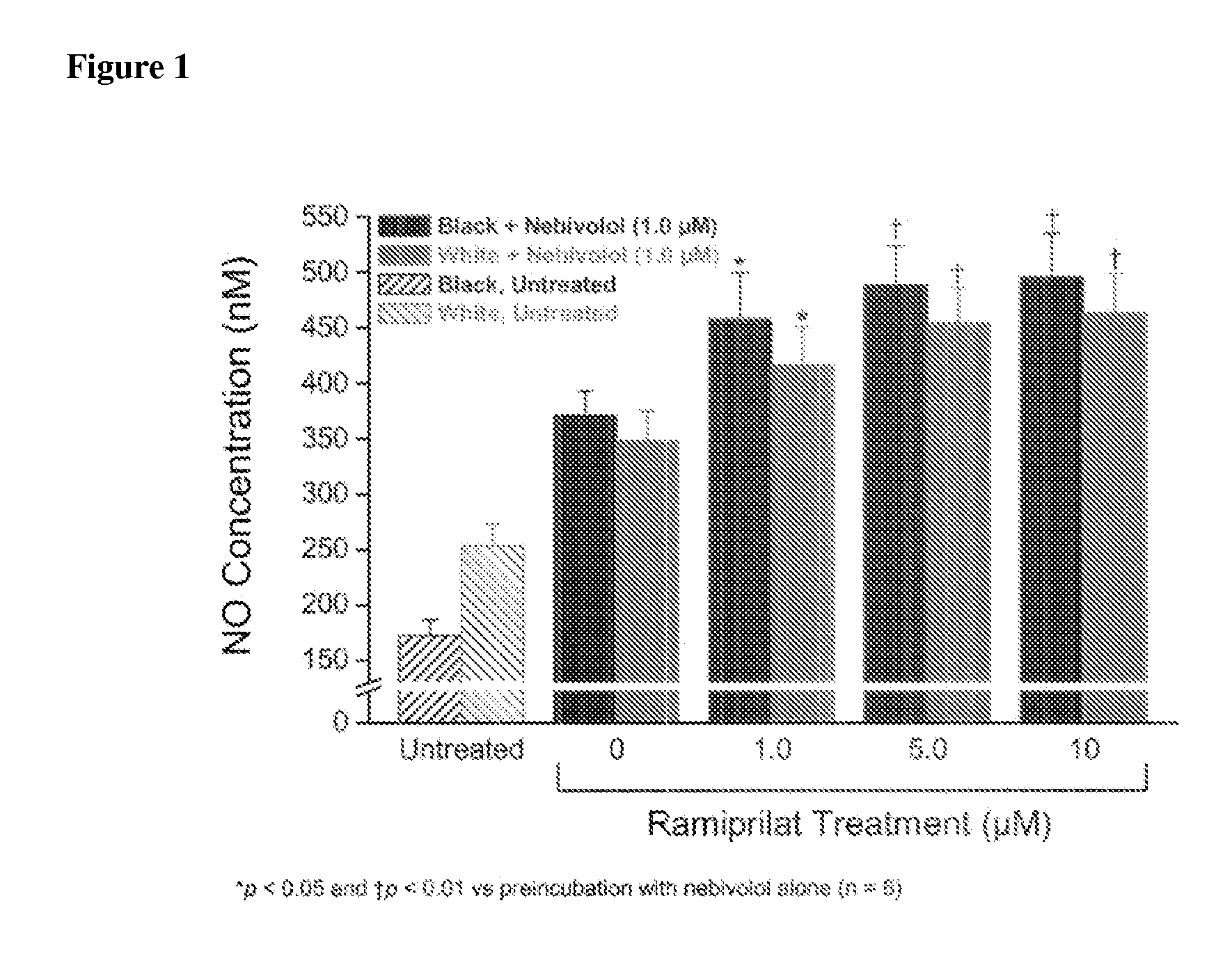
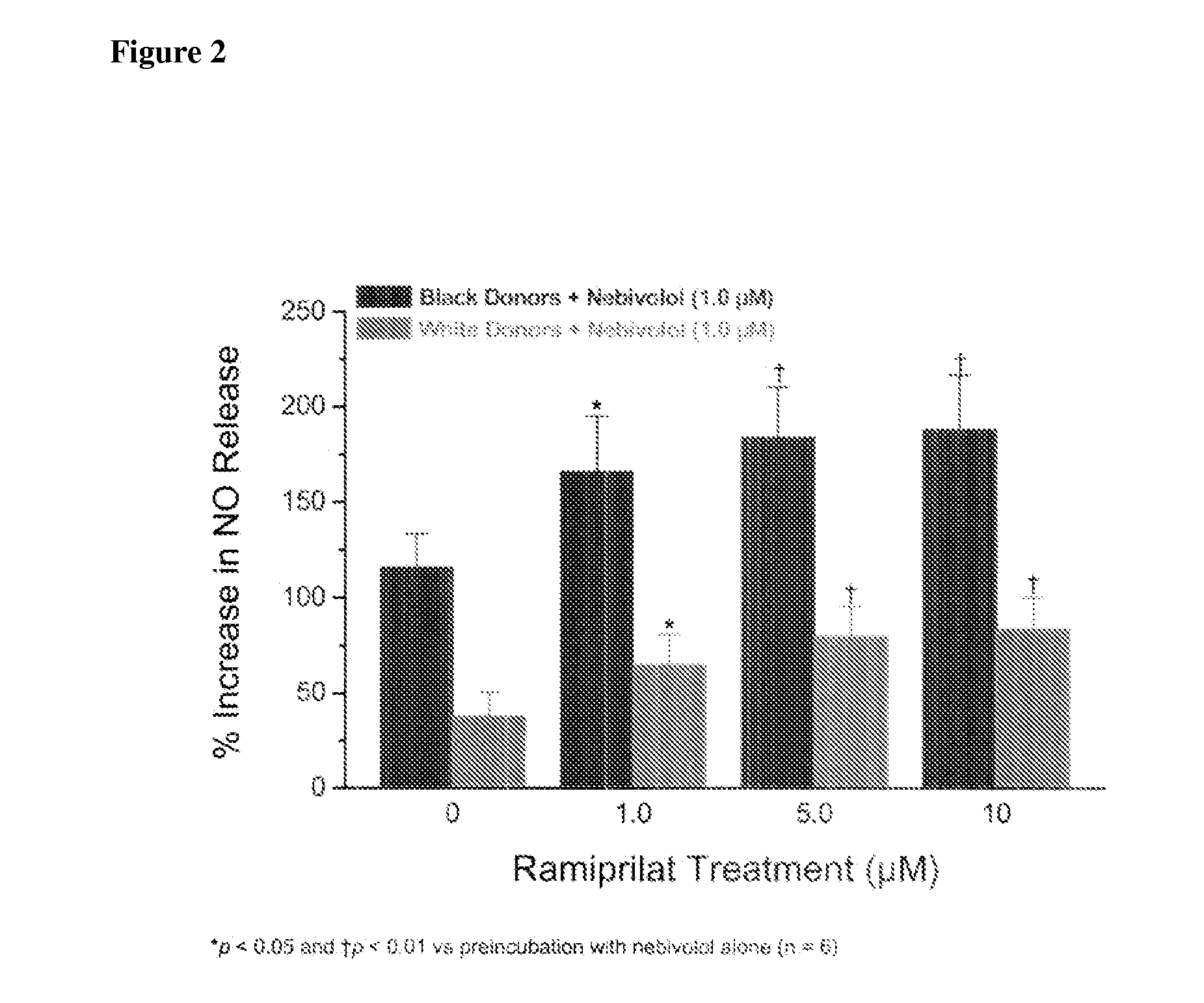
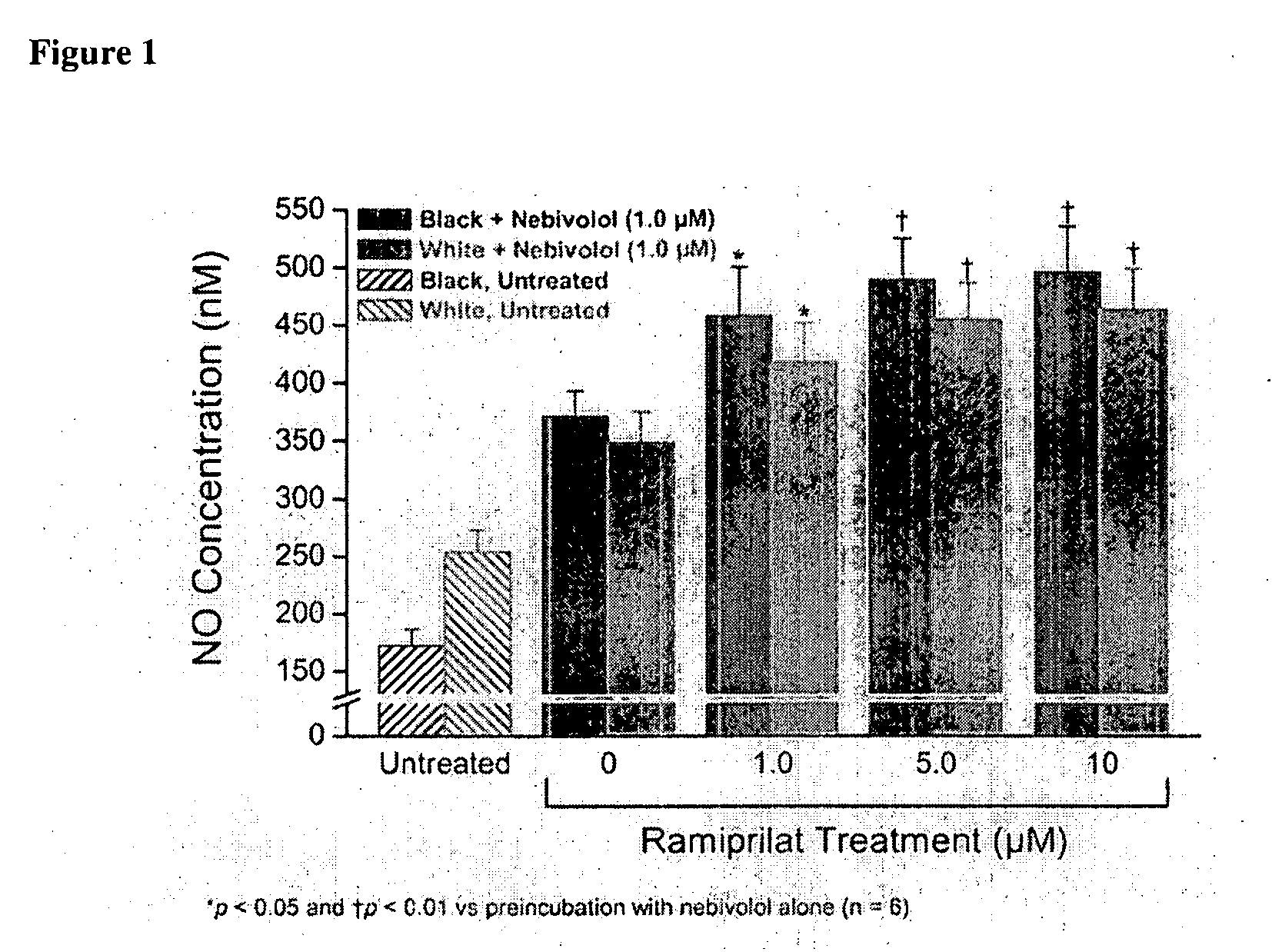
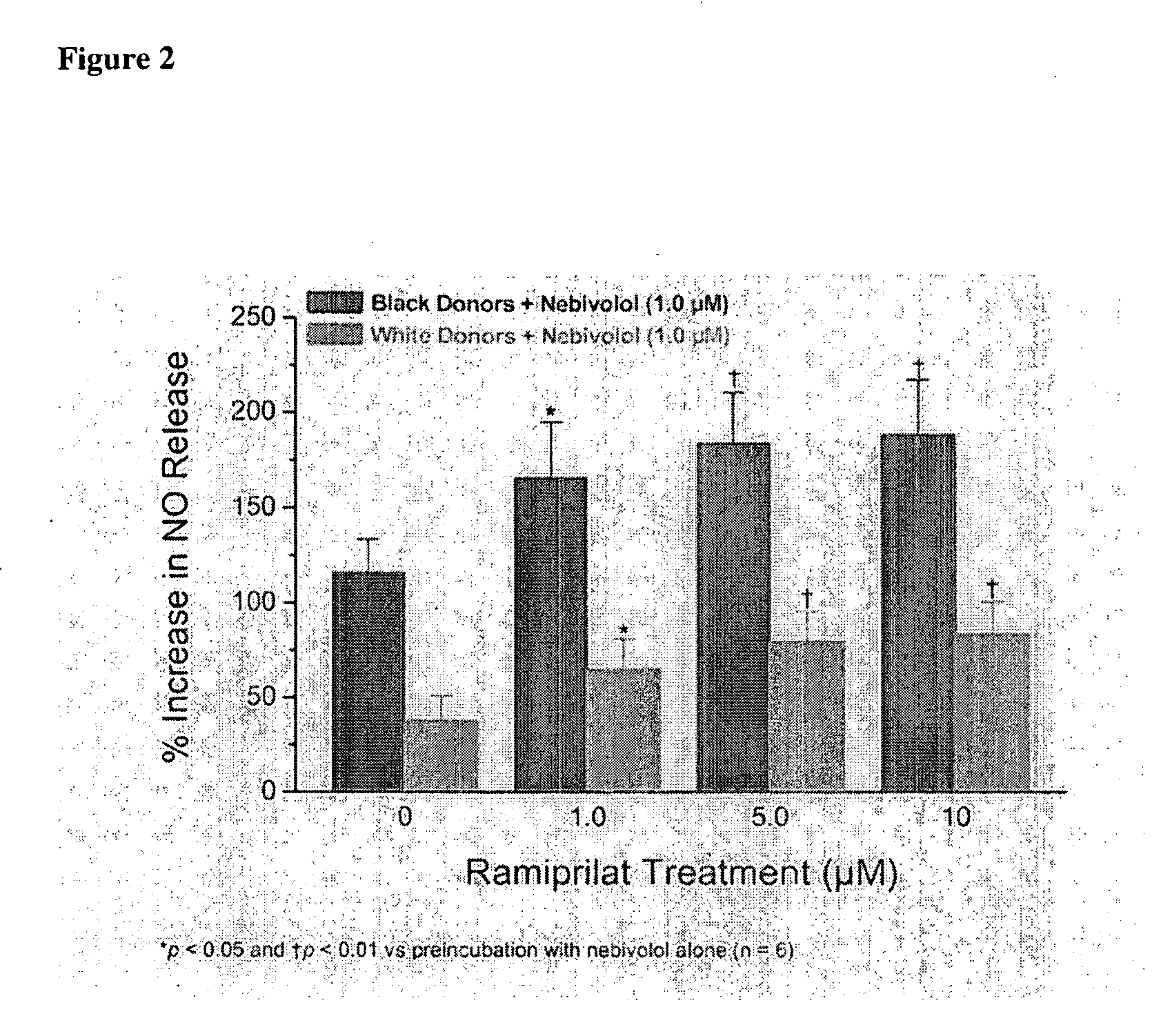
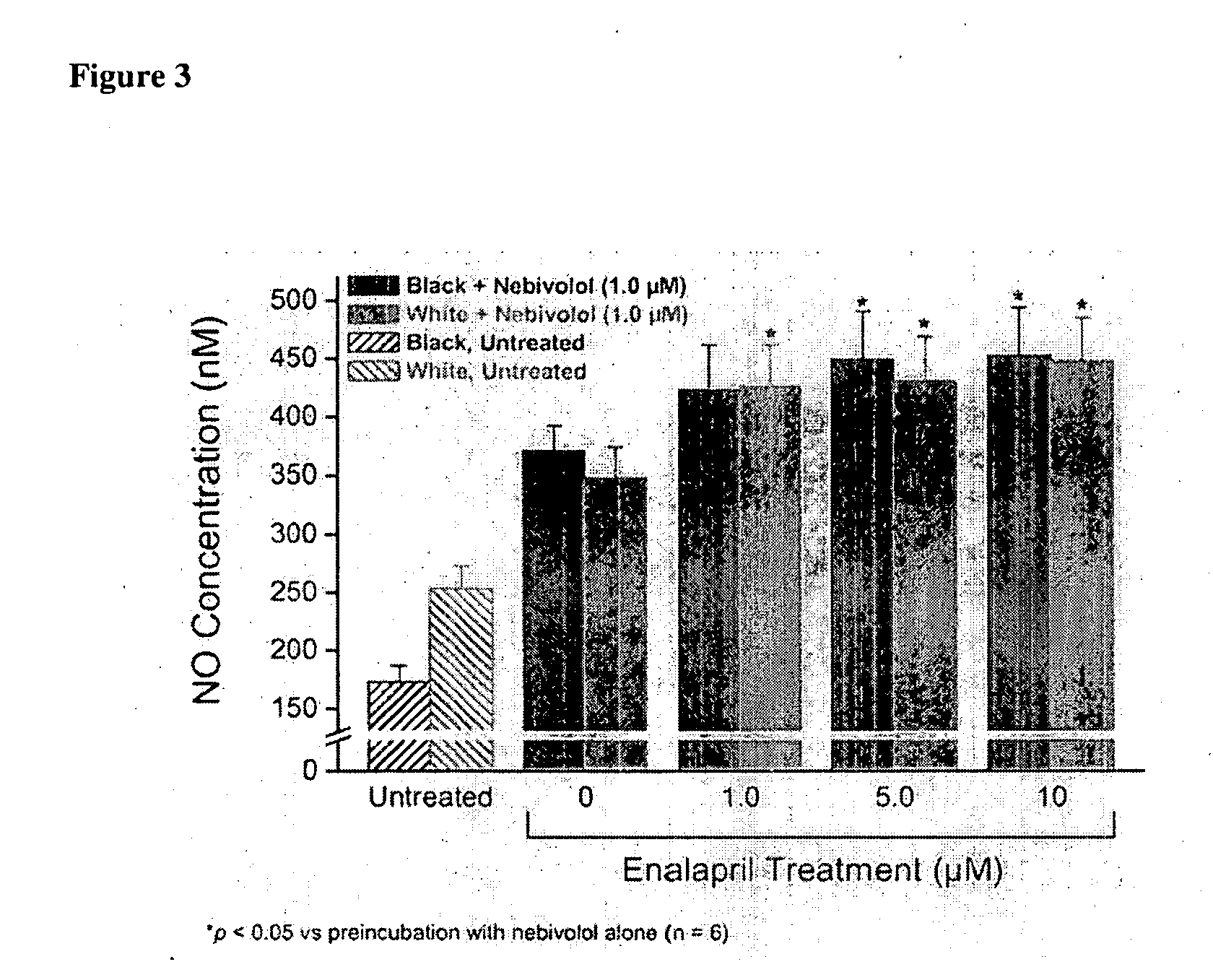
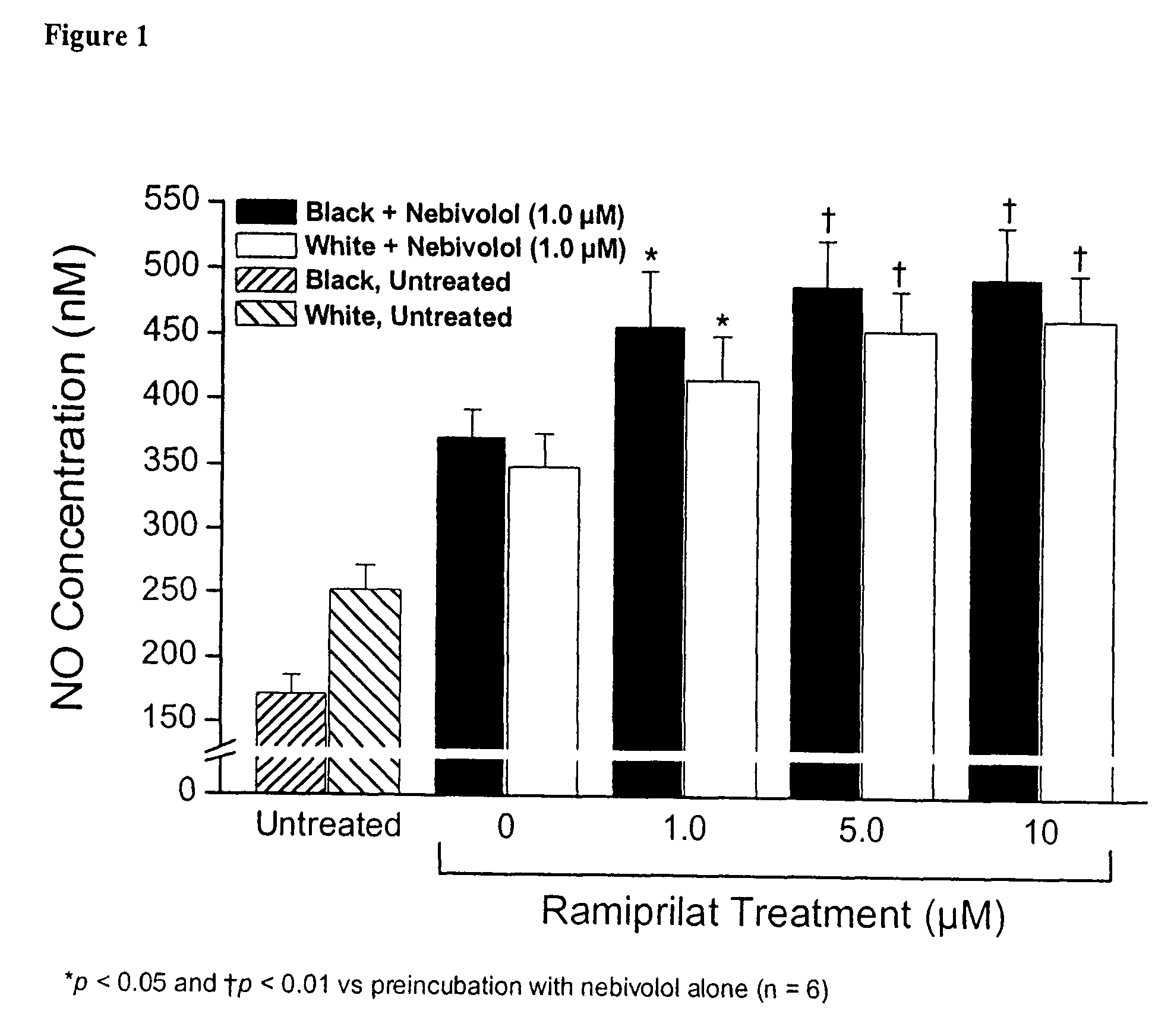
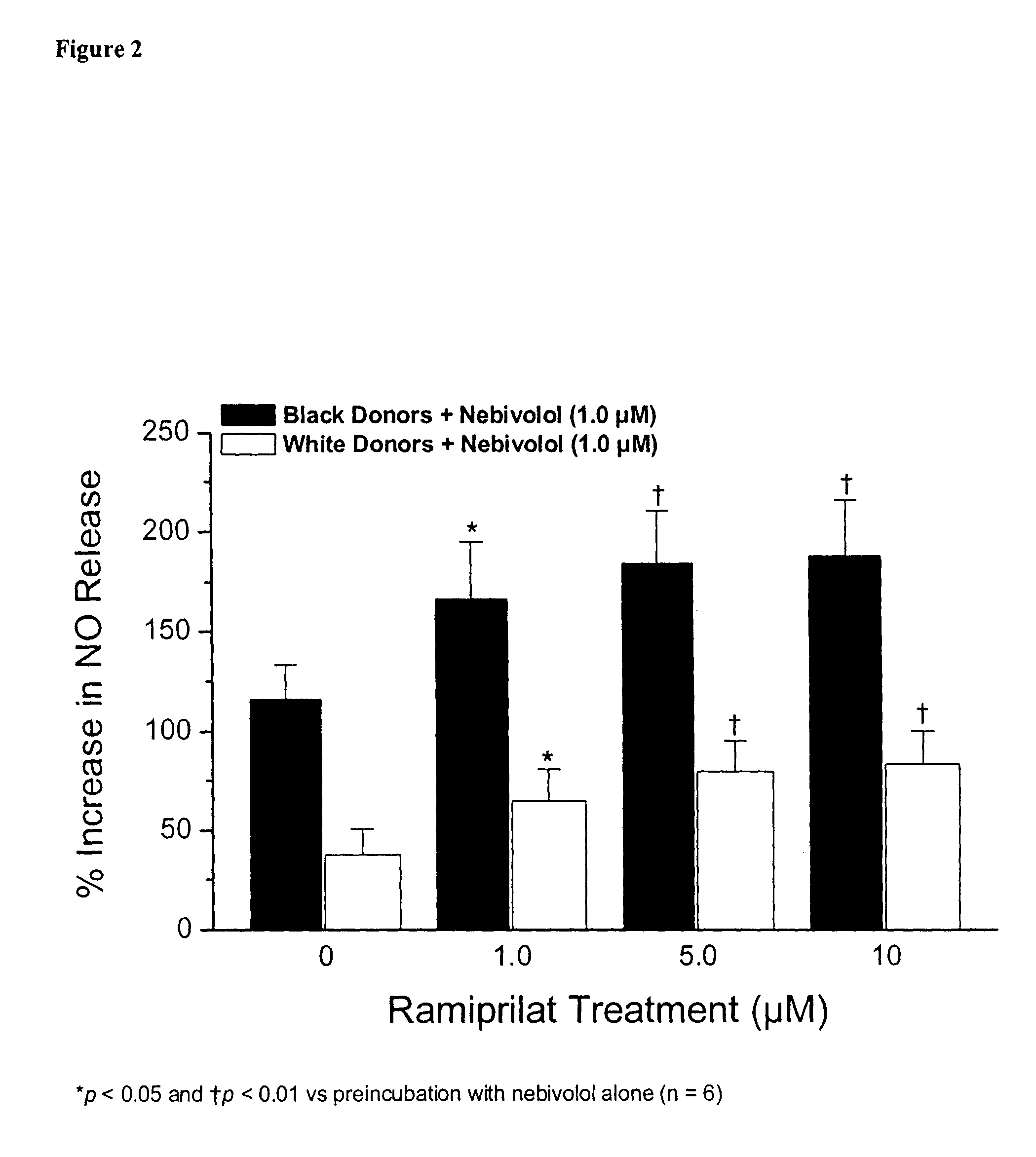
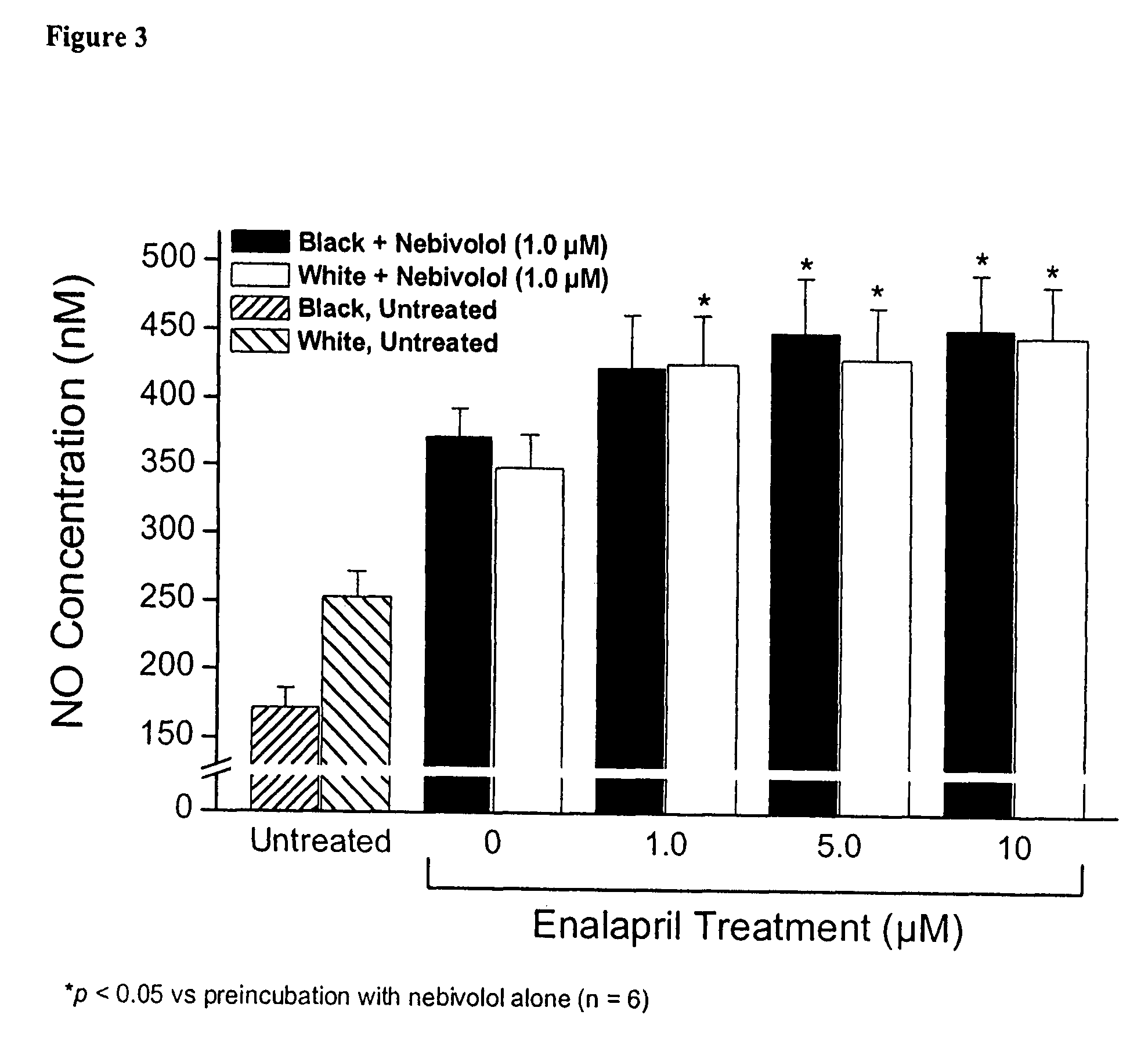
Hydroxylated nebivolol metabolites increase NO release in human endothelial cell preparations in a concentration-dependent manner after acute administration. In addition, hydroxylated nebivolol metabolites (including but not limited to 4-hydroxy-6,6'difluoro) have the ability to increase NO release in human endothelial cells after chronic administration. The present invention provides hydroxylated nebivolol metabolites for use in the treatment of cardiovascular diseases. Furthermore, the present invention provides methods of treating and / or preventing vascular diseases by administering at least one hydroxylated metabolite of nebivolol capable of releasing a therapeutically effective amount of nebivolol to targets affected by vascular diseases. Nitric oxide.
Owner:MYLAN LAB INC (US)
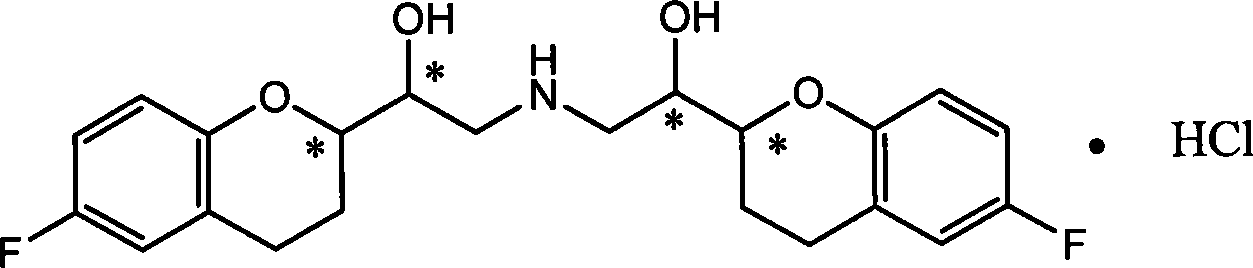
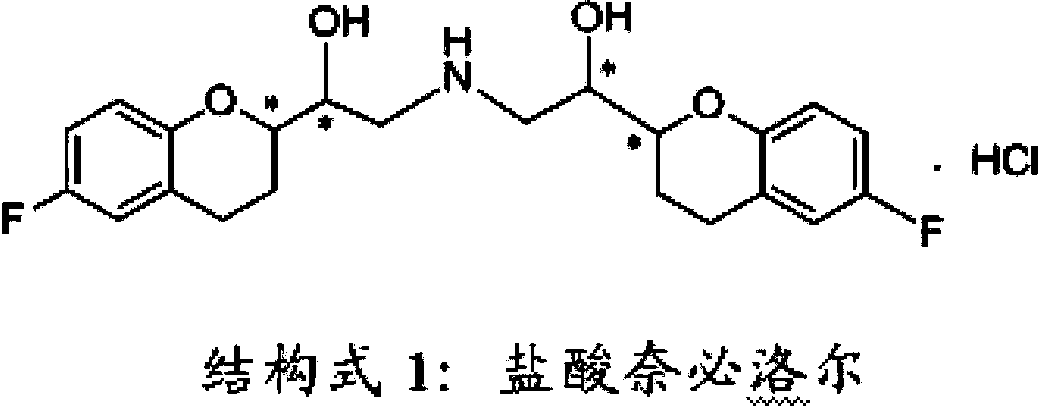
Method for preparing nebivolol racemate hydrochloride
InactiveCN102816141AHigh chemical purityReduce the discharge of three wastesOrganic chemistryBulk chemical productionIsomerizationAcyloin condensation
The invention discloses a method for preparing nebivolol racemate hydrochloride and belongs to the technical field of pharmaceutical synthesis. The method includes that a formula I compound and a formula II compound are used as raw materials to be subjected to an acyloin condensation to prepare a formula III compound, the formula III compound is subjected to an epimerization reaction to increase the ratio between III a and III b from 1:1 to 9:1, the formula III compound is subjected to a recrystallization purification to obtain a high purity III a, the III a is subjected to a carbonyl reduction to generate a formula IV compound, the formula IV compound is directly subjected to a catalytic hydrogenation to remove a benzyl protecting group to obtain a nebivolol racemate crude product, the nebivolol racemate crude product is directly salified by hydrogen chloride, and the recrystallization is performed to obtain the high purity nebivolol racemate hydrochloride. According to the method, the reaction is easy to control, the postprocessing is easy, and the production efficiency is high.
Owner:JINAN ASIA PHARMA TECH
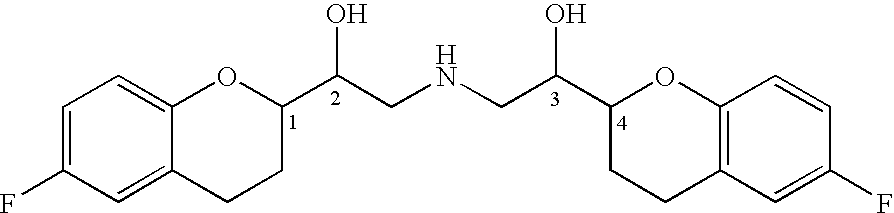
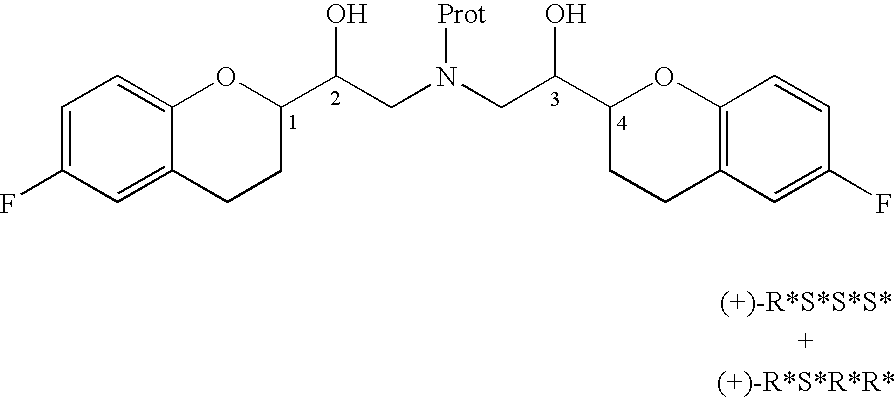
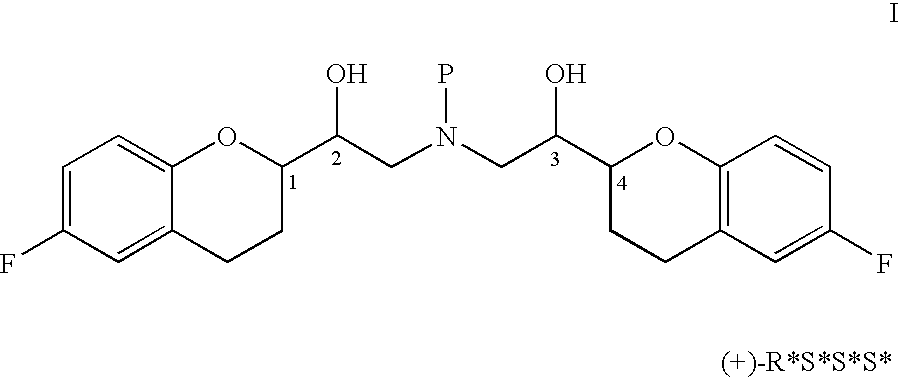
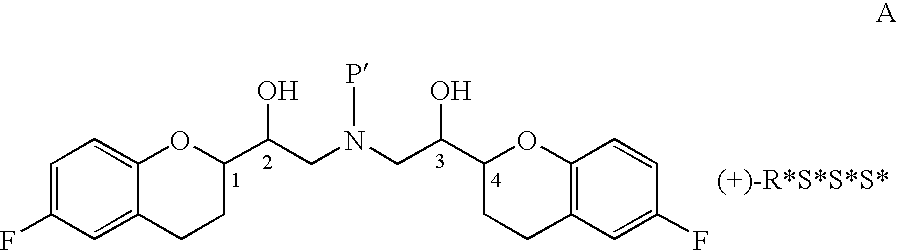
Novel process for preparation of nebivolol intermediates
The present invention relates to a process for separation of desired diastereomeric pair from a mixture of diastereomeric pairs thereby obtaining nebivolol intermediates. Thus, the mixture of (+)-[1S*(R*)]-6-fluoro-3,4-di-hydro-α-[[(phenylmethyl)amino]methyl]-2H-1-benzopyran-2-methanol, (+)-[1S*(S*)]-6-fluoro-3,4-dihydro-2-oxi -ranyl-2H-1-benzopyran and ethanol is heated to reflux temperature and stirred for 8 hours at the same temperature to obtain (±)-[2R*[1S*,5S*(S*)]]+[2R*[1S*,5R*(R*)]]-α,α′-[phenylmethyliminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran ran-2-methanol]. Then the reaction mass is cooled to 10° C., the pH is adjusted to 2 with HCl gas and stirred for 45 minutes at 25° C. to 30° C. Then the separated solid is filtered and dried to give (+)-[2R*[1S*,5S*(S*)]]-α,α′-[phenylmethyliminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol] hydrochloride salt, which can be converted into nebivolol.
Owner:HETERO DRUG
Compositions comprising nebivolol
ActiveUS20080161296A1Improve dysfunctionConvenient treatmentBiocideAnimal repellantsVascular diseaseEndothelial dysfunction
Nebivolol has been shown to be beneficial in the treatment of cardiovascular diseases such hypertension, congestive heart failure, arterial stiffness and endothelial dysfunction. The present invention features a pharmaceutical composition comprising nebivolol and at least one other active agent, wherein the at least one other active agent is a cardiovascular agent.
Owner:MYLAN LAB +1
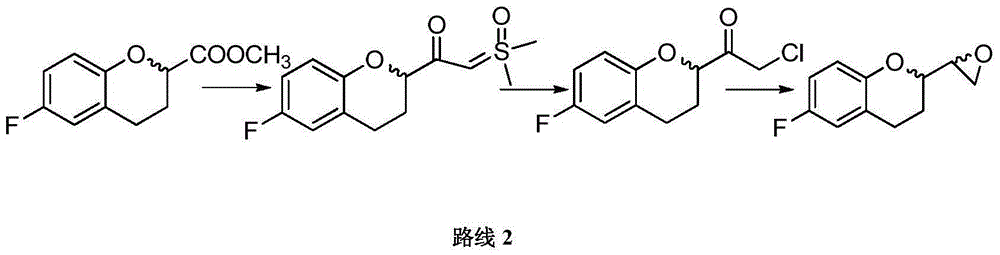
Synthetic method of nebivolol
ActiveCN103833717ASimple and fast operationShort synthetic routeOrganic chemistryNebivololDiastereomer
The invention discloses a synthetic method of nebivolol. The synthetic method comprises the following steps: by taking a 2-amino-1-(-6-fluoro-2-chromanyl) ethanol diastereomer mixture as an initial raw material, firstly, recrystallizing and separating the 2-amino-1-(-6-fluoro-2-chromanyl) ethanol diastereomer mixture to respectively obtain corresponding diastereomers A and B; carrying out reactions such as diazotization, halogenation and cyclization on the diastereomer B to synthesize an epoxy compound; and then, carrying out a reaction on the obtained epoxy compound and the diastereomer A to obtain nebivolol. The method disclosed by the invention is mild in reaction condition, simple and convenient to operate and short in synthetic line, and is suitable for industrialized batch production of nebivolol.
Owner:BEIJING NORMAL UNIVERSITY +1
Compositions Comprising Nebivolol
InactiveUS20090227646A1High selectivityImprove dysfunctionBiocideAnimal repellantsVascular diseaseEndothelial dysfunction
Nebivolol has been shown to be beneficial in the treatment of cardiovascular diseases such hypertension, congestive heart failure, arterial stiffness and endothelial dysfunction. The present invention features a pharmaceutical composition comprising nebivolol and at least one other active agent, wherein the at least one other active agent is a cardiovascular agent.
Owner:MYLAN PHARMA INC +1
Compositions Comprising Nebivolol
InactiveUS20090215844A1High selectivityImprove dysfunctionBiocideAnimal repellantsVascular diseaseEndothelial dysfunction
Nebivolol has been shown to be beneficial in the treatment of cardiovascular diseases such hypertension, congestive heart failure, arterial stiffness and endothelial dysfunction. The present invention features a pharmaceutical composition comprising nebivolol and at least one other active agent, wherein the at least one other active agent is a cardiovascular agent.
Owner:FOREST LAB HLDG LTD +1
Nebivolol hydrochloric acid orally disintegrating tablet and preparation method thereof
InactiveCN101361720APromote dissolutionFast absorptionOrganic active ingredientsPill deliverySolubilityOrally disintegrating tablet
The invention discloses a Nebivolol HCL orally disintegrating tablet, which comprises the following components by weight: 3.7-7.5 percent of Nebivolol HCL, 75.0-85.0 percent of water-solubility bulking agent, 6.0-15.5 percent of disintegrant, 2.0-3.5 percent of disguising odor and 0.7-1.5 percent of lubricant.
Owner:刘全胜
Nebivolol and its metabolites in combination with nitric oxide donors, compositions and methods of use
InactiveUS20060009513A1Prevention of platelet aggregation and platelet adhesionAntibacterial agentsBiocideMetaboliteAntioxidant
The invention describes novel compositions comprising nebivolol and / or at least one metabolite of nebivolol and at least one nitric oxide donor, and, optionally, at least one antioxidant or a pharmaceutically acceptable salt thereof, and / or at least one compound used to treat cardiovascular diseases or a pharmaceutically acceptable salt thereof, and / or at least one nitrosated compound used to treat cardiovascular diseases. The compounds and compositions of the invention can also be bound to a matrix. The nitric oxide donor is a compound that donates, transfers or releases nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor, stimulates endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and may preferably be isosorbide dinitrate and / or isosorbide mononitrate. The antioxidant may preferably be a hydralazine compound or a pharmaceutically acceptable salt thereof. The invention also provides methods for treating and / or preventing vascular diseases characterized by nitric oxide insufficiency; and for treating and / or preventing Raynaud's syndrome; and for treating and / or preventing cardiovascular diseases or disorders.
Owner:NICOX SA
Improvement method for preparing 6-fluorin-3,4-dihydro-2H-1-benzopyranyl-2-epoxy ethane
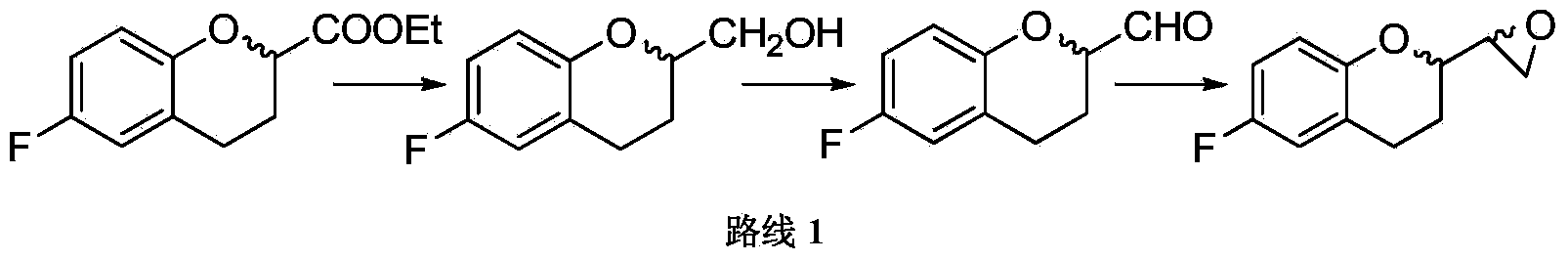
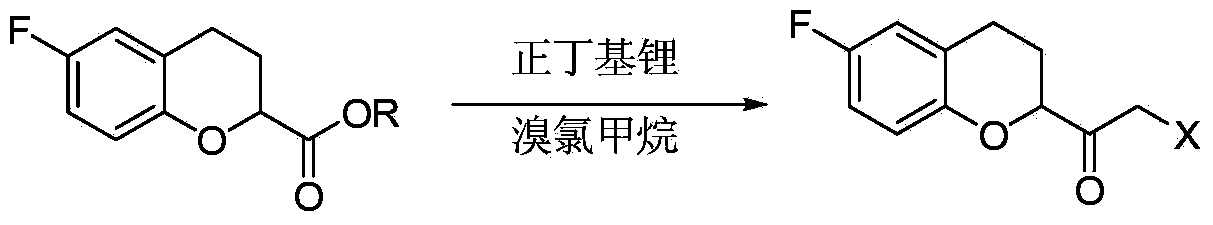
The invention relates to an improvement method for preparing a nebivolol key intermediate, namely 6-fluorin-3,4-dihydro-2H-1-benzopyranyl-2-epoxy ethane. The method comprises the following steps of: (a) condensing a compound (IV) and dihalogenated methane in the presence of an organic metal lithium compound to obtain a compound (II); (b) reducing the compound (II) to obtain a compound (III); and (c) performing cyclization on the compound (III) under alkaline condition to obtain a compound (I). The scheme of the invention has the advantages of readily available raw materials, easiness in operating, greatly increased reaction yield and purity and high contribution to industrial mass production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method for preparing and purifying nebivolol intermediate
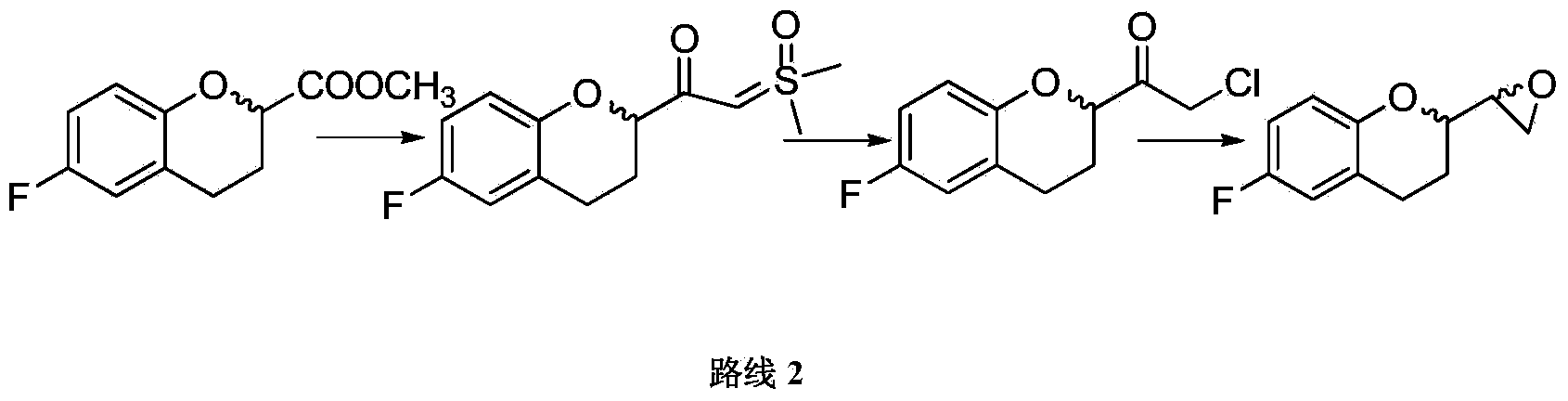
The invention relates to a method for preparing and purifying a nebivolol intermediate and in particular relates to an improved method for preparing and purifying 2-chloro-1-(6-fluro-3,4-dihydro-2H-1-benzopyran-2-yl) acetone shown in a formula (I) as shown in the specification. The 2-chloro-1-(6-fluro-3,4-dihydro-2H-1-benzopyran-2-yl) acetone compound is a key intermediate for synthesizing nebivolol. By adopting the method provided by the invention, a high-purity solid of the key intermediate is obtained with high yield (more than 85%) and high purity (more than 99%), operation is easy, and industrial production can be easily realized.
Owner:PHARMA CHANGZHOU PHARMA FACTORY NO 4
Method for preparing nebivolol midbody
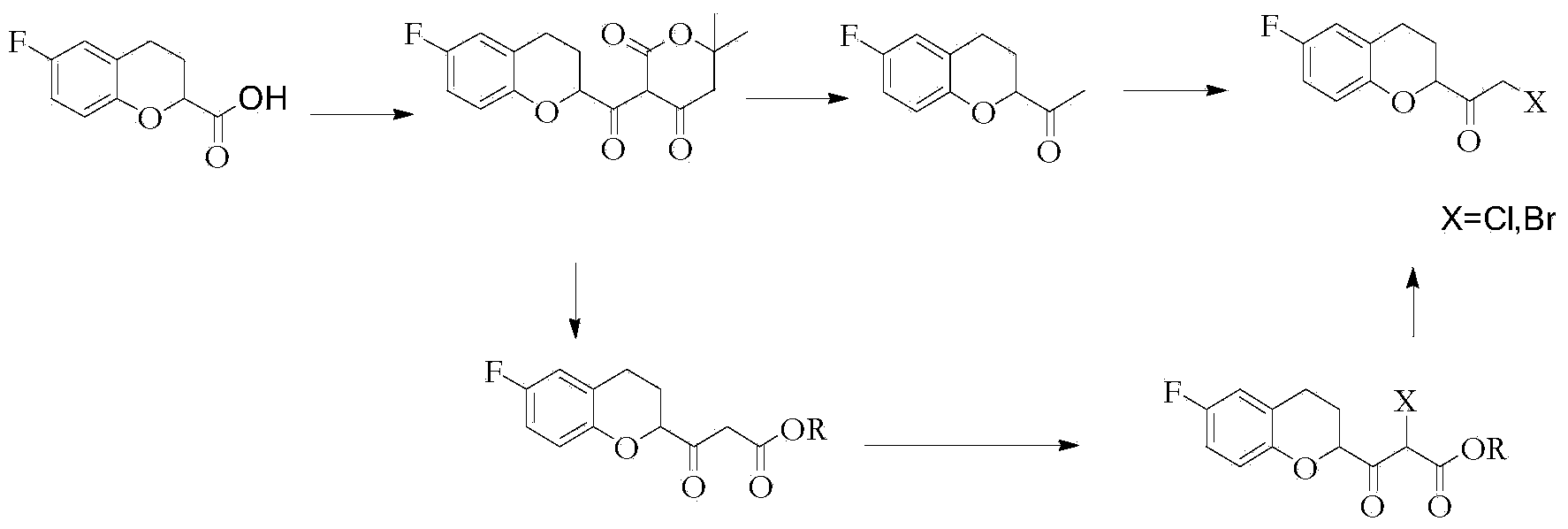
The invention relates to a method for preparing a nebivolol midbody, which belongs to the technical field of medicament synthesis, for solving the problems that the conventional raw material is high in cost, long in route and low in yield. The method comprises the following steps: enabling 6-fluorine chroman-2-formic acid as a raw material to react with chloro-carbonic ester with the presence of a deacid reagent, then adding diazomethane after the reaction is accomplished, so as to enable the midbody to react with the diazomethane to generate a reaction liquid of an intermediate product, and then adding a hydrogen halide gas or a hydrogen halide solution into the reaction liquid to perform halogenating reaction so as to obtain a compound of formula IV, namely, (6-(fluorine-3,4-dihydro-2H-benzopyran-2-methanol-2-yl) ethanone halogenate. The method provided by the invention has the advantages that the reaction route is short, the yield is high, and the used material is low in price and easy to purchase.
Owner:江苏八巨药业有限公司
Pharmaceutical compositions comprising nebivolol
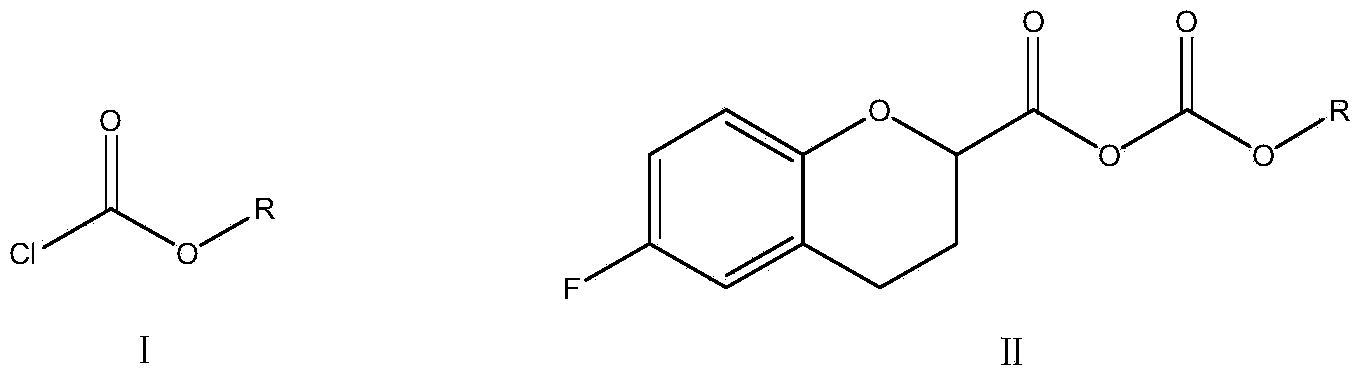
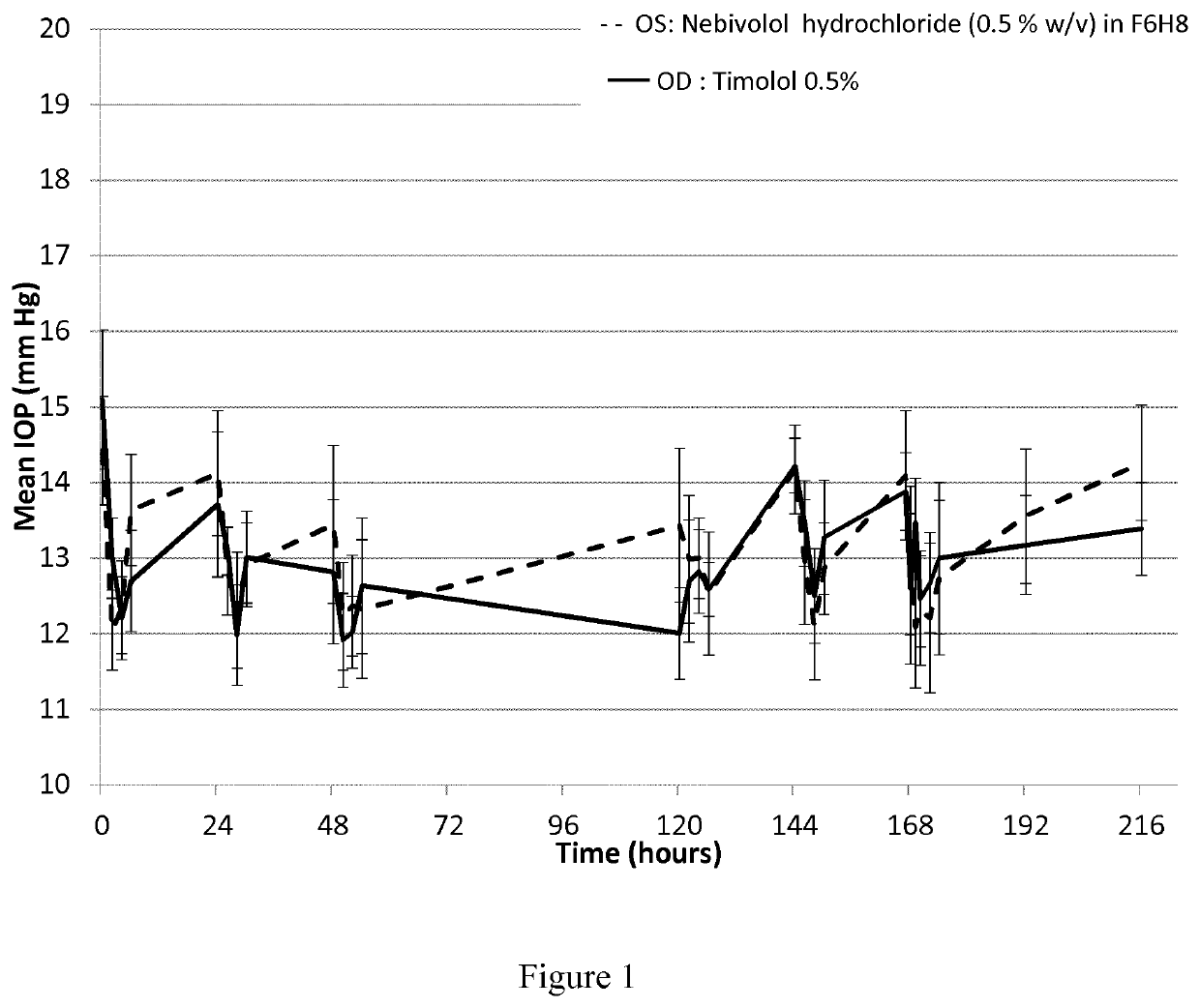
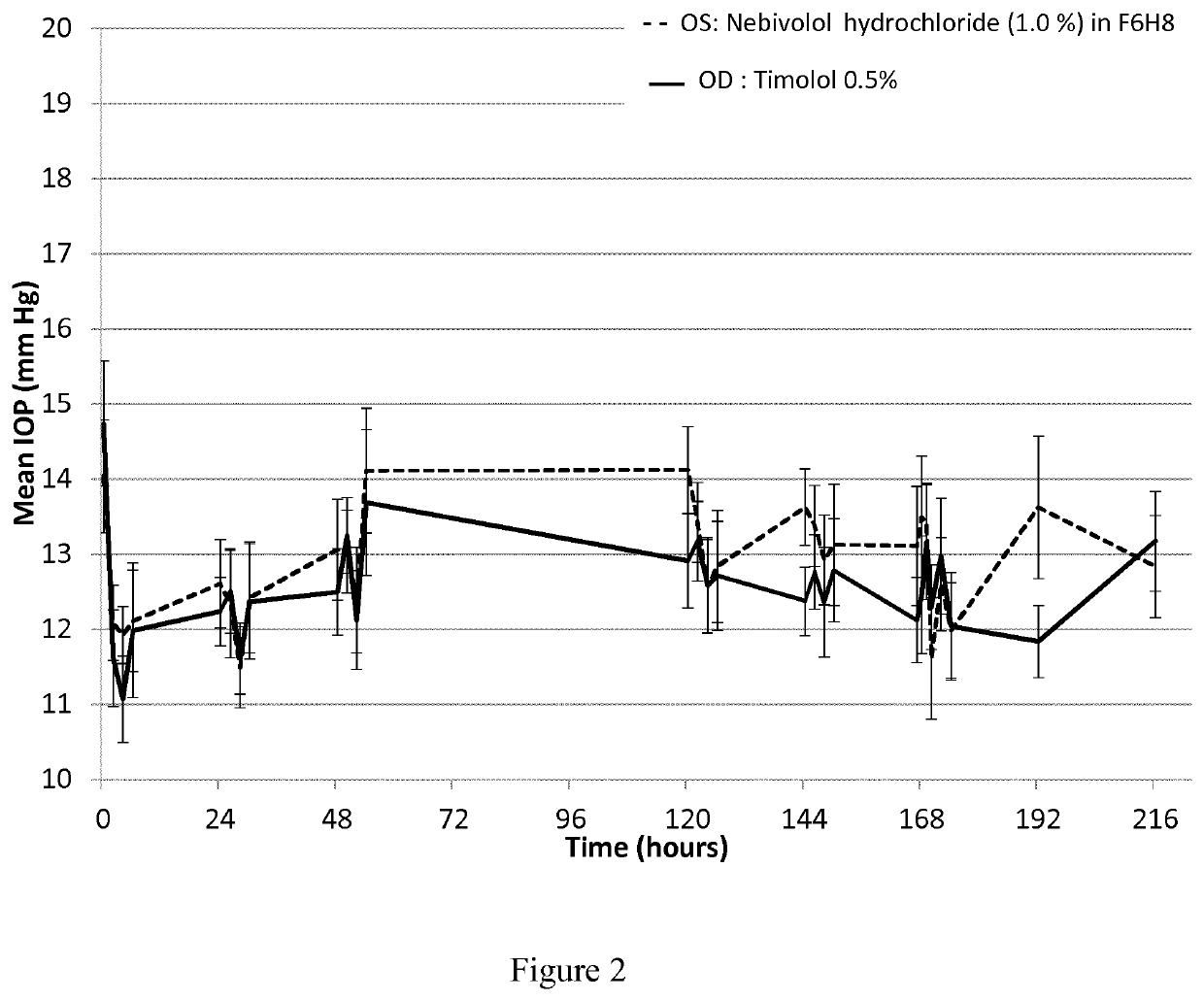
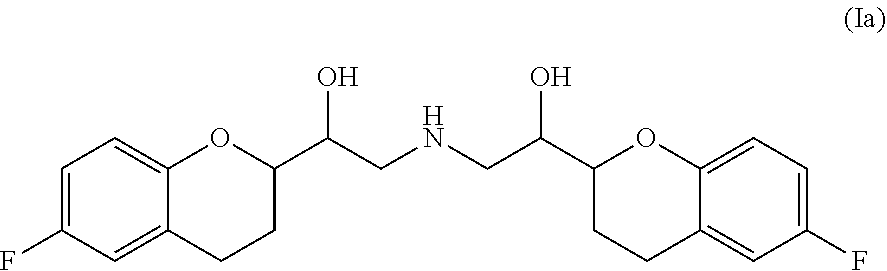
ActiveUS20210106558A1Easily not evaporateEfficient deliveryOrganic active ingredientsPowder deliveryIntra ocular pressureAlkane
The present invention relates to pharmaceutical compositions comprising the selective beta 1 (B1)-receptor blocker nebivolol and / or a pharmaceutically acceptable salt thereof and a liquid vehicle comprising a semifluorinated alkane. The pharmaceutical composition of the present invention is useful for topical administration, for example ophthalmic topical administration and for use in the treatment of glaucoma, increased intraocular pressure, ocular hypertension and / or a symptom associated therewith.
Owner:NOVALIQ GMBH
Compositions comprising nebivolol
ActiveUS7838552B2High selectivityImprove dysfunctionBiocideOrganic chemistryEndothelial dysfunctionActive agent
Nebivolol has been shown to be beneficial in the treatment of cardiovascular diseases such hypertension, congestive heart failure, arterial stiffness and endothelial dysfunction. The present invention features a pharmaceutical composition comprising nebivolol and at least one other active agent, wherein the at least one other active agent is a cardiovascular agent.
Owner:MYLAN LAB +1
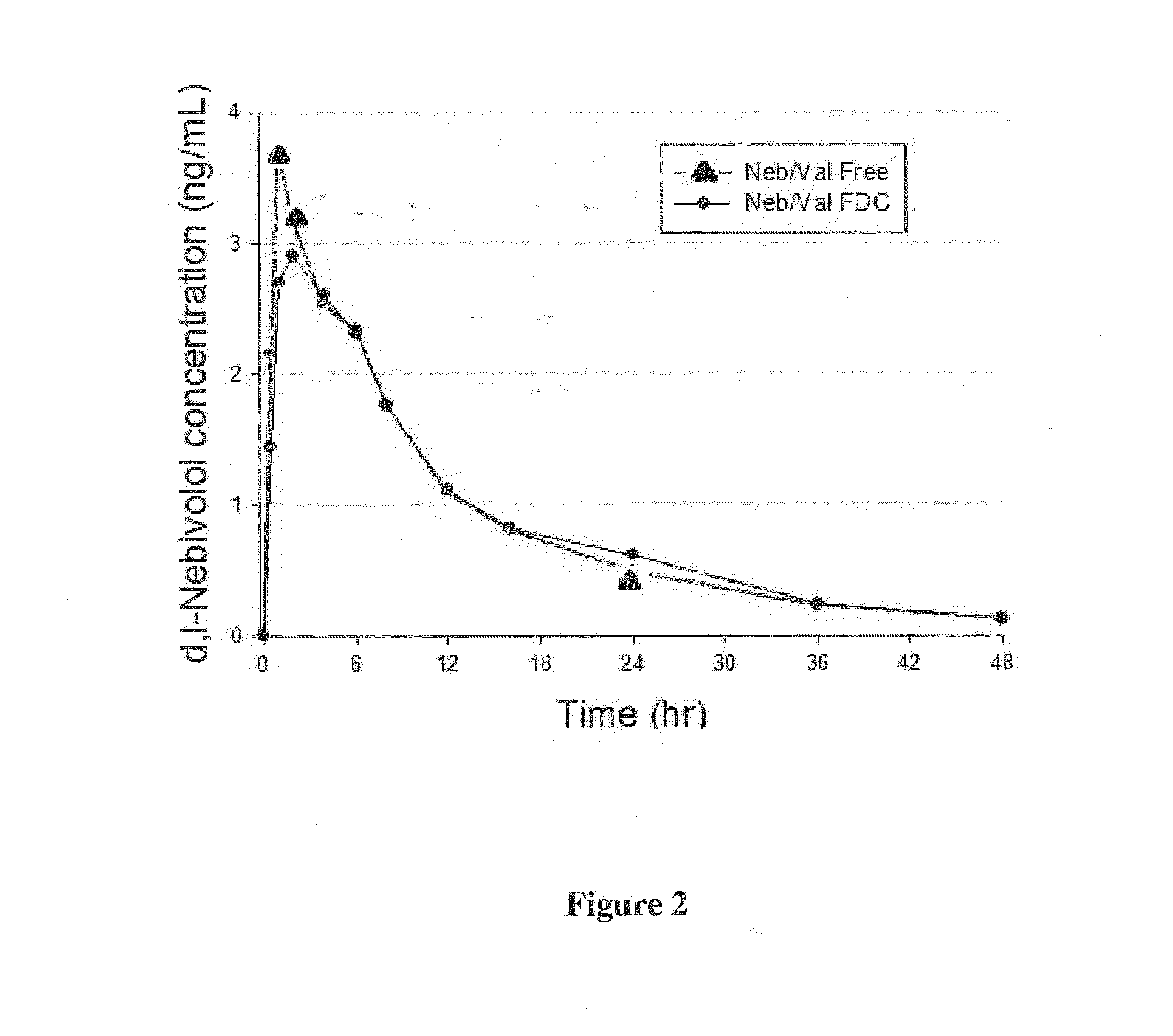
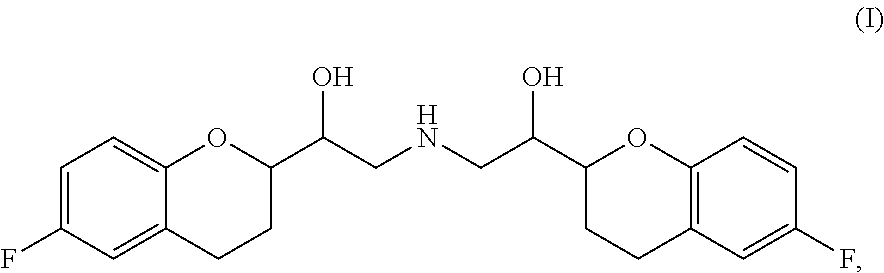
Chemical composition
The present invention is directed to stable chemical compositions and dosage forms that comprise nebivolol and valsartan and which achieve therapeutically effective plasma levels of both actives in hypertensive patients following administration, as well as to methods of lowering blood pressure and treating hypertension using such compositions and dosage forms.
Owner:FOREST LAB HLDG LTD
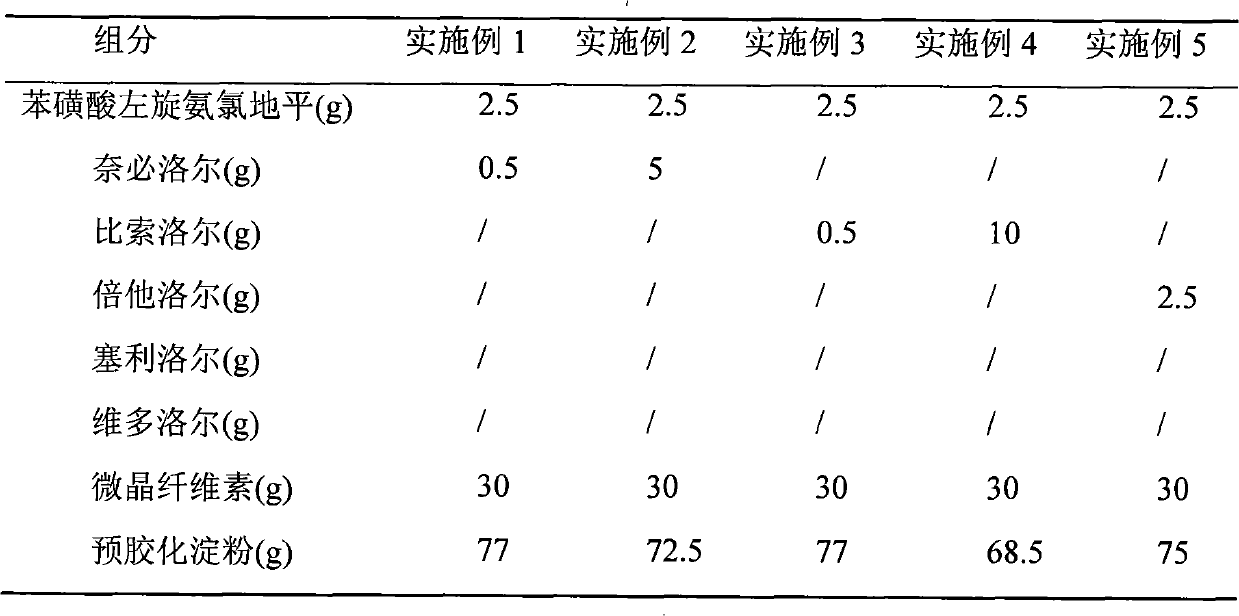
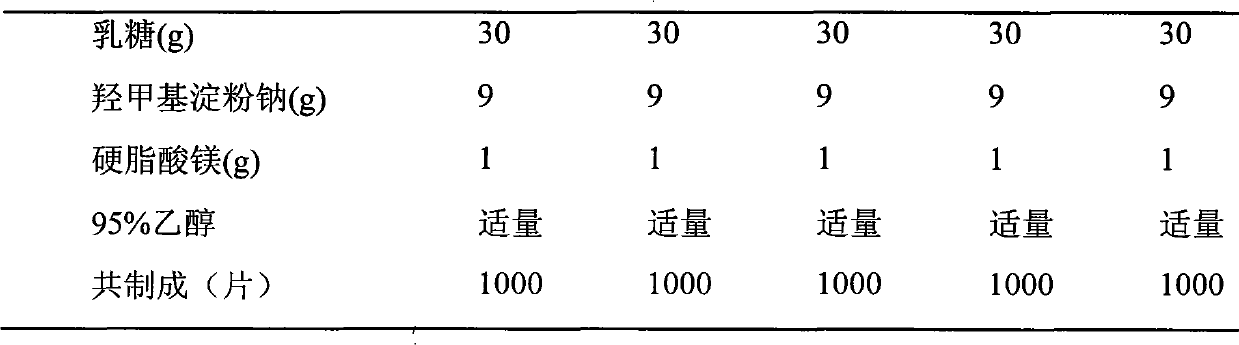
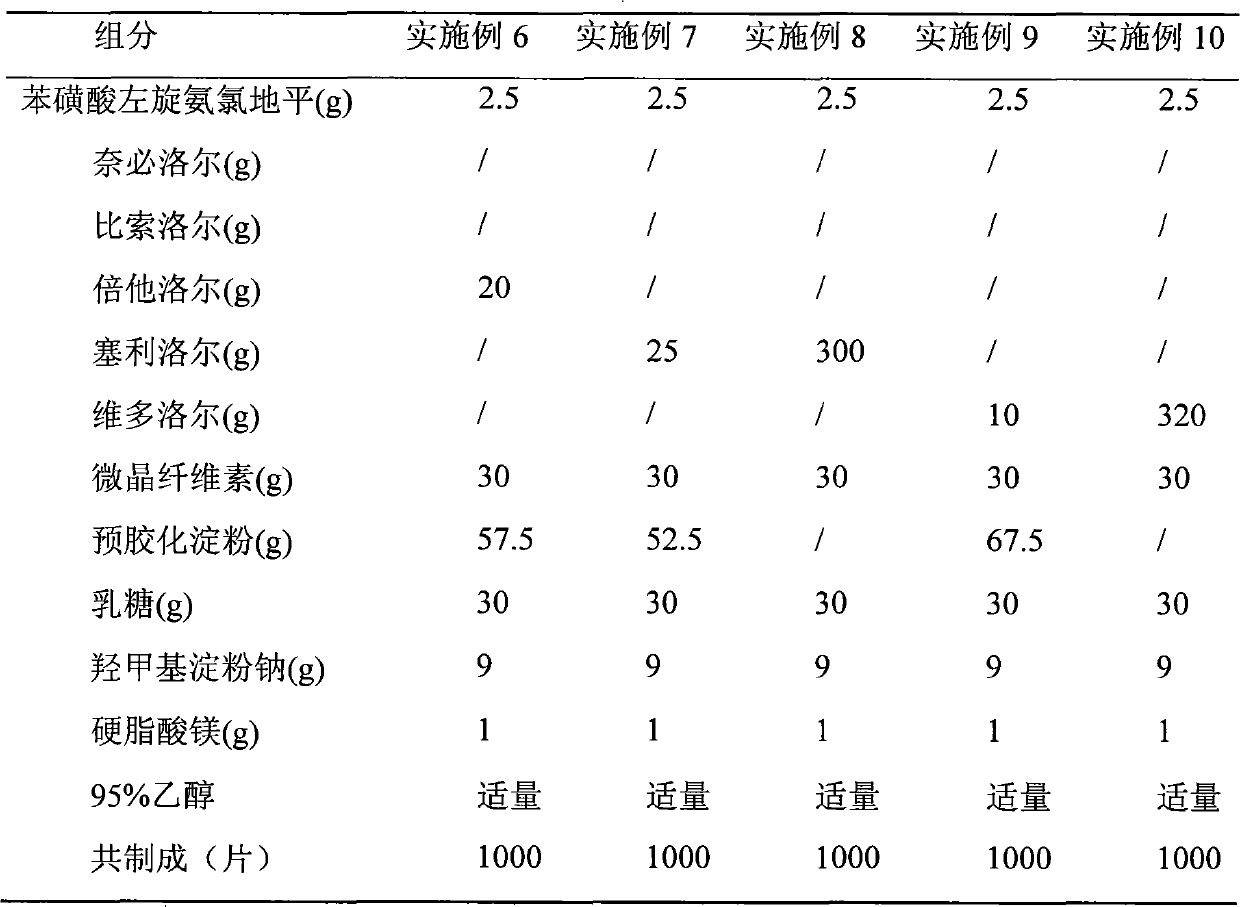
Medical composition of levamlodipine or pharmaceutically acceptable salt thereof and beta-blocker and application thereof
ActiveCN101766611ALess single drug dosageReduce incidenceAmide active ingredientsCardiovascular disorderBeta blockerLevamlodipine
The present invention relates to a medical composition of levamlodipine or a pharmaceutically acceptable salt thereof and a beta-blocker and an application thereof. The active ingredients of the medical composition contain levamlodipine or a pharmaceutically acceptable salt thereof and a beta-blocker. The beta-blocker is selected from one of nebivolol, bisoprolol, betaxolol, celiprolol and nadolol. The present invention also discloses an application of the medical composition to the preparation of a medicament for treating hypertension. The medical composition has the characteristics of evident therapeutic effect and convenient administration.
Owner:SHIHUIDA PHARMA GRP (JILIN) LTD
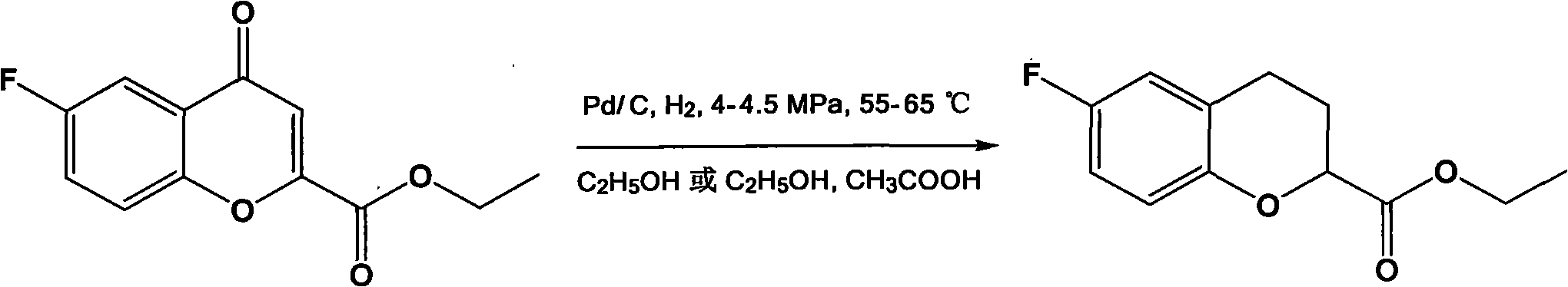
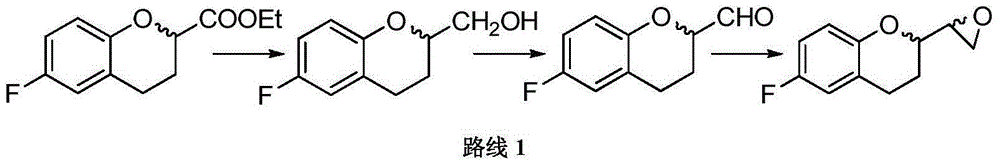
Preparation method for 6-fluorine-3,4-dihydro-2H-1-benzopyran-2-ethyl formate
The invention discloses a preparation method for a Nebivolol intermediate of (6-fluorine-3,4-dihydro-2H-1-benzopyran-2-ethyl formate). In the preparation method, the Nebivolol intermediate is prepared from a Nebivolol intermediate of (6-fluorine-4-oxo-4H-1-benzopyran-2-ethyl formate) serving as a raw material in the presence of a palladium carbon catalyst (Pd / C) and a dehydrating agent in an alcohol solvent or a mixed solvent of alcohol and acid under the conditions of hydrogen pressure of 4 to 4.5 MPa and the temperature of between 55 and 65 DEG C. In the preparation method, a starting material is easy to prepare; the yield is high; a treatment method after the reaction is simple and efficient; and waste liquid treatment burden and environmental pollution are light, so the preparation method is a simple and green process method for preparing the Nebivolol intermediate and is suitable for large-scale production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Process for isolation of desired isomers of nebivolol intermediates
The present invention relates to a simple and commercially viable process for separation of desired isomers of nebivolol intermediates from a mixture containing undesired isomers of nebivolol intermediates. Thus, (+)-[2R*[1S*,5S*(S*)]]+[2R*[1S*,5R*(R*)]]-α,α′-[phenylmethyliminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol] is dissolved in diisopropyl ether at reflux temperature and cooled to below about 30° C. to obtain the desired (+)-[2R*[1S*,5S*(S*)]]-α,α′-[phenylmethyliminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol].
Owner:HETERO DRUGS LTD
Use of a composition comprising at least one beta-blocker for the treatment of sleep disorders
A composition comprising specific beta-blockers such as bisoprolol and nebivolol for the treatment of insomnia and / or another sleep disorder. The composition should be given in such an amount that it causes a less than 40% decrease in the amount of aMT6s in complete nocturnal urin. The composition can be a combination treatment comprising a specific beta-blocker in combination with another known drug e.g., melatonin with similar effect for treatment of insomnia.
Owner:ZLEEPAX EURO
Process for the preparation of nebivolol
ActiveUS8487122B2Efficient processingEasy to separateOrganic chemistryBlood disorderChromatographic separationNebivolol
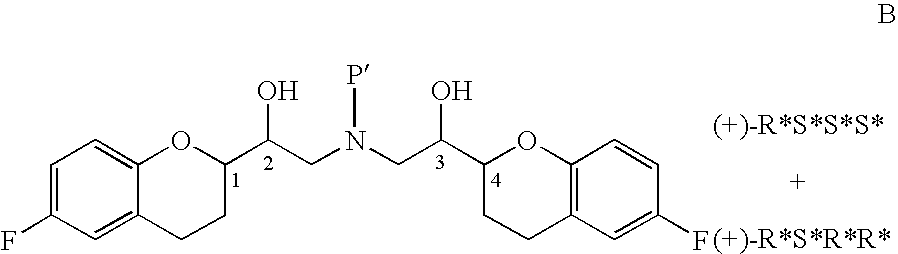
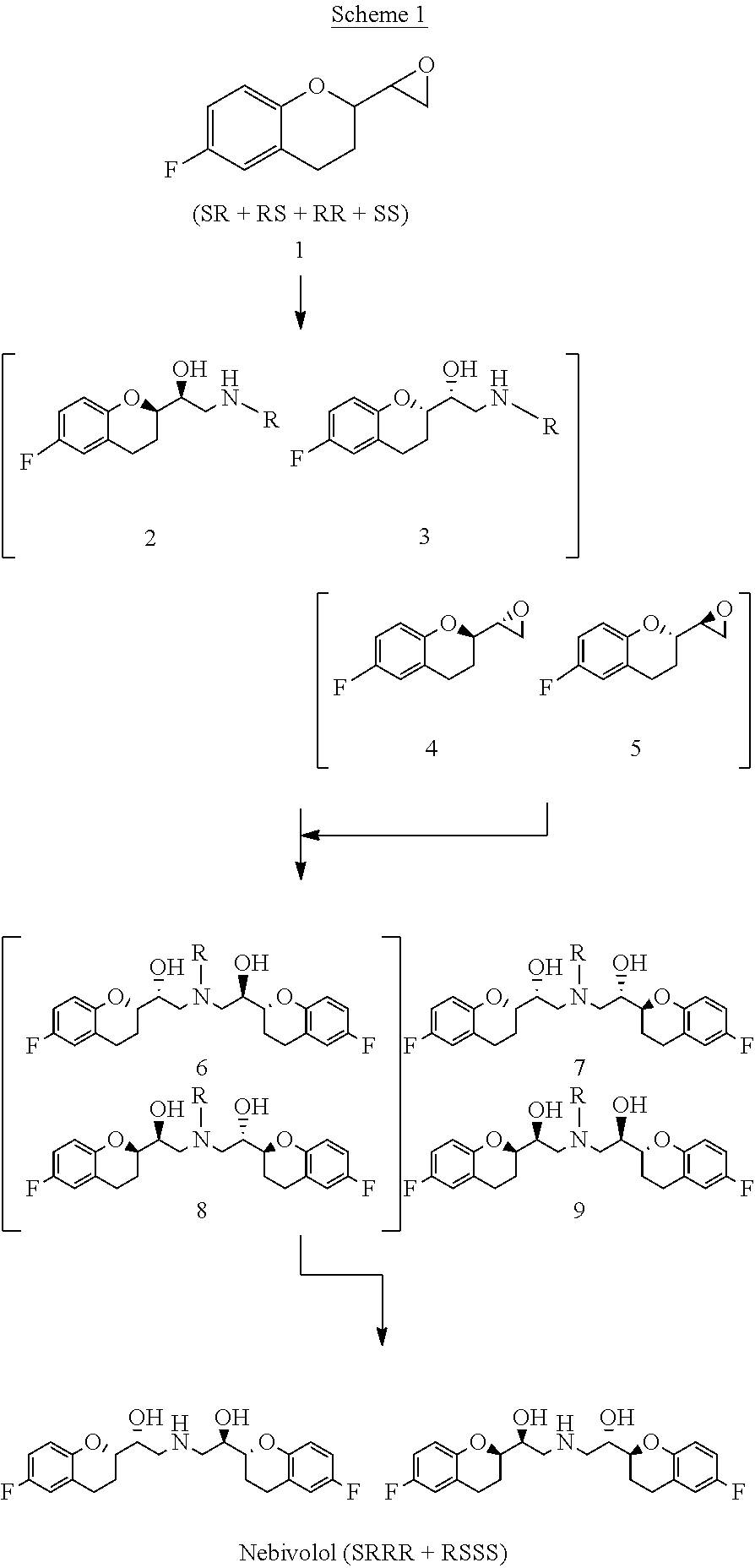
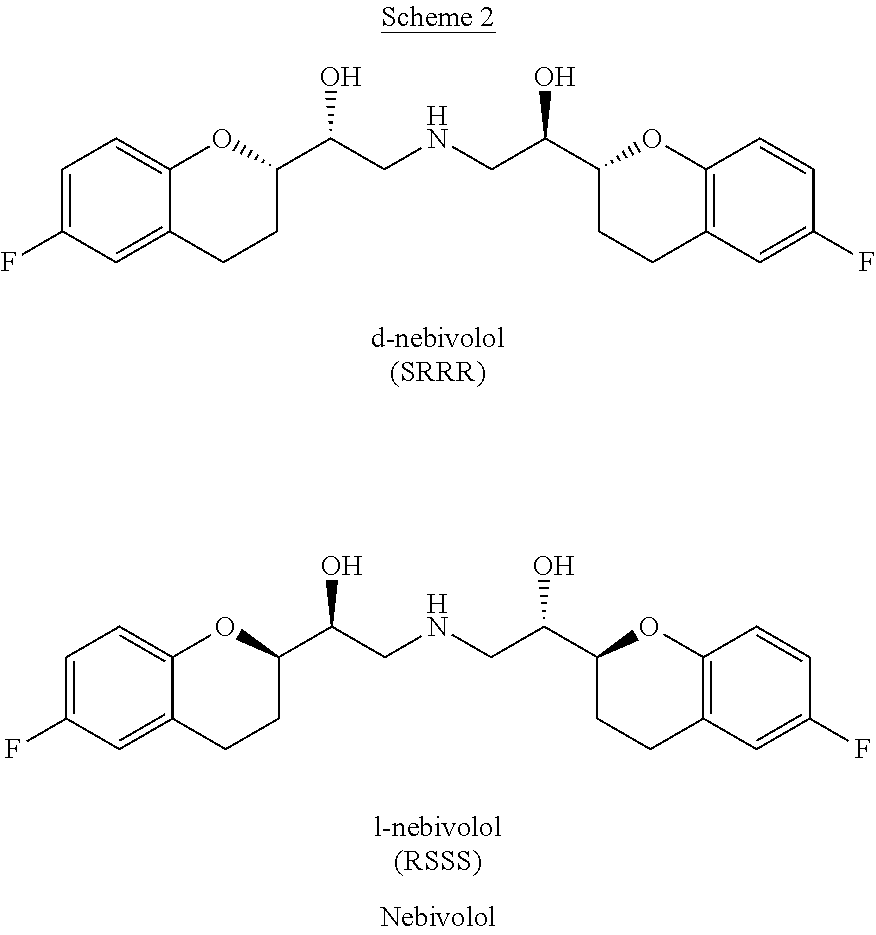
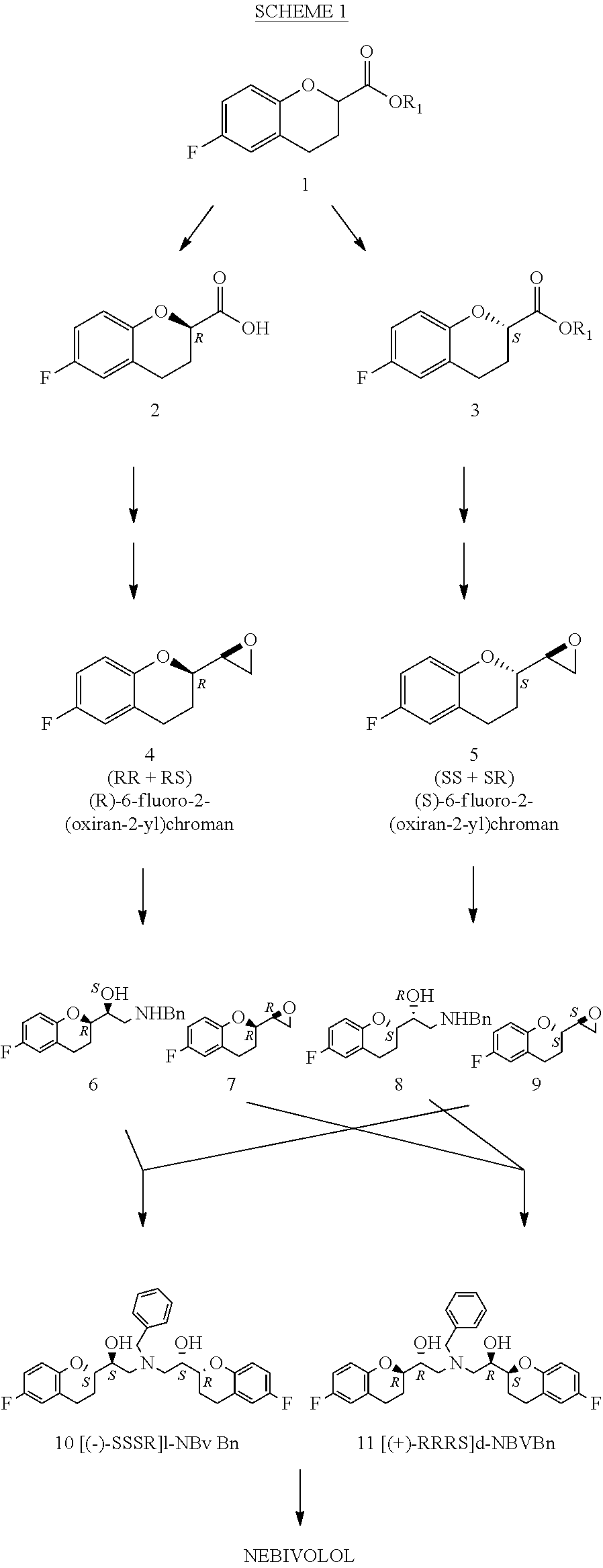
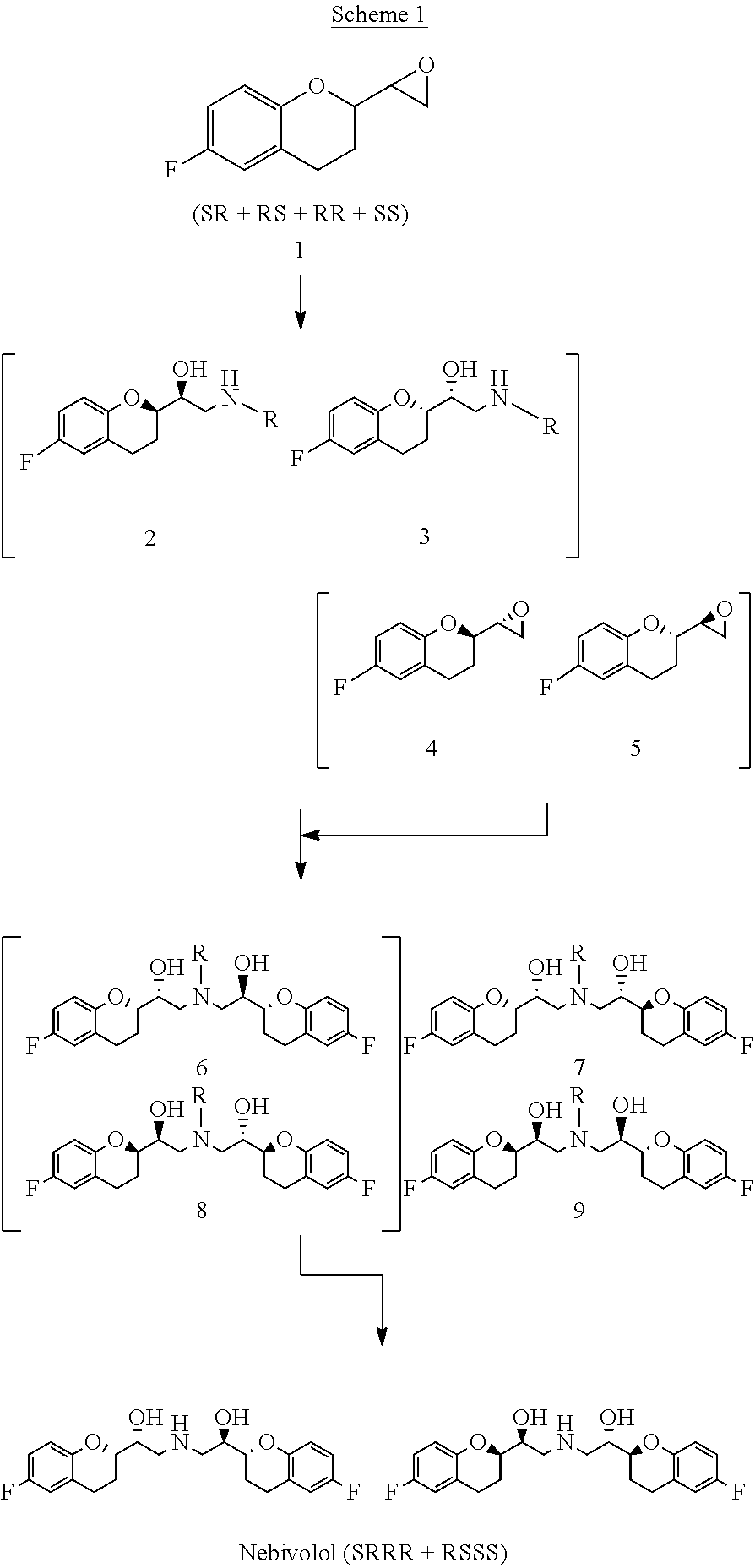
The present invention relates to a novel process for the synthesis of Nebivolol product represented in Scheme (1), comprised of a reduced number of high-yield steps, and characterized by the kinetic resolution of the two epoxide pairs diastereoisomeric therebetween (mixture 1), allowing to avoid complex chromatographic separations.
Owner:MENARINI INT OPERATIN S LUXEMBORG SA
Process for the preparation of nebivolol
ActiveUS20130295622A1Drawback can be obviatedReduce lossesOrganic chemistryFermentationNebivololEngineering
The present invention relates to a novel process for the synthesis of the Nebivolol product depicted in Scheme 1, comprised of a reduced number of high-yield steps, and characterized by the enzymatic resolution of the chroman ester precursor.
Owner:MENARINI INT OPERATIN S LUXEMBORG SA
Process for the preparation of nebivolol
ActiveUS20120316351A1Avoid separationSimilar reaction velocityOrganic chemistryBlood disorderChromatographic separationKinetic resolution
The present invention relates to a novel process for the synthesis of Nebivolol product represented in Scheme (1), comprised of a reduced number of high-yield steps, and characterized by the kinetic resolution of the two epoxide pairs diastereoisomeric therebetween (mixture 1), allowing to avoid complex chromatographic separations.
Owner:MENARINI INT OPERATIN S LUXEMBORG SA
Preparation and purification method of nebivolol intermediate
The present invention relates to a method for preparing and purifying a nebivolol intermediate, in particular to a method for preparing and purifying 2-chloro-1-(6-fluoro-3,4-dihydro- An improved method for 2H-1-benzopyran-2-yl)ethanone, which is a key intermediate for the synthesis of nebivolol. The high-purity solid of the key intermediate is obtained through the method of the present application with high yield (over 85%) and high purity (over 99%), and is easy to operate and suitable for industrial production.
Owner:PHARMA CHANGZHOU PHARMA FACTORY NO 4
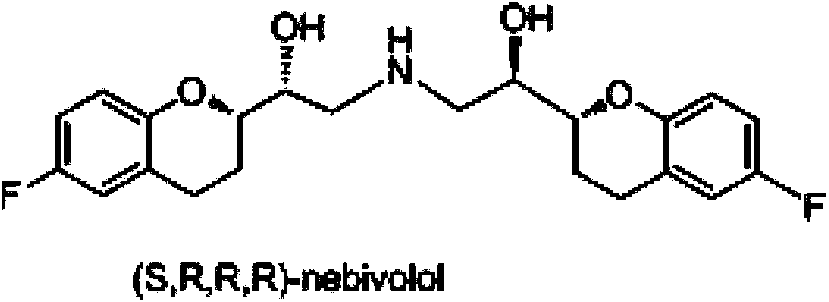
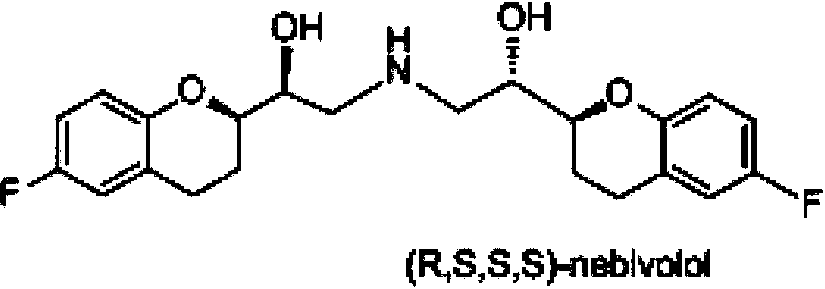
Preparation method of nebivolol and its intermediate compound
The invention discloses a preparation method of nebivolol used for preparing medicines for treating hypertension of slight or medium degrees, and an intermediate compound. The preparation method comprises the following steps: taking 6-fluoro-2-(1-hydroxy-2-paratoluensulfonyl oxygroup-ethyl)-3,4-dihydrobenzopyrans as an initial raw material, introducing amino, then coupling with 6- fluoro-3,4-dihydro-2-epoxy ethyl-2H-1-benzopyran, and preparing (S,R,R,R) and (R,S,S,S)-nebivolol. Compared with a prior art, the preparation method has the advantages of novel design, simple operation and high yield, the usage of hazardous reagent such as ssodium azide and sodium hydride can be avoided, a column chromatography purifying method is avoided, so that the preparation method conforms to industrial production.
Owner:福安药业集团重庆博圣制药有限公司 +2
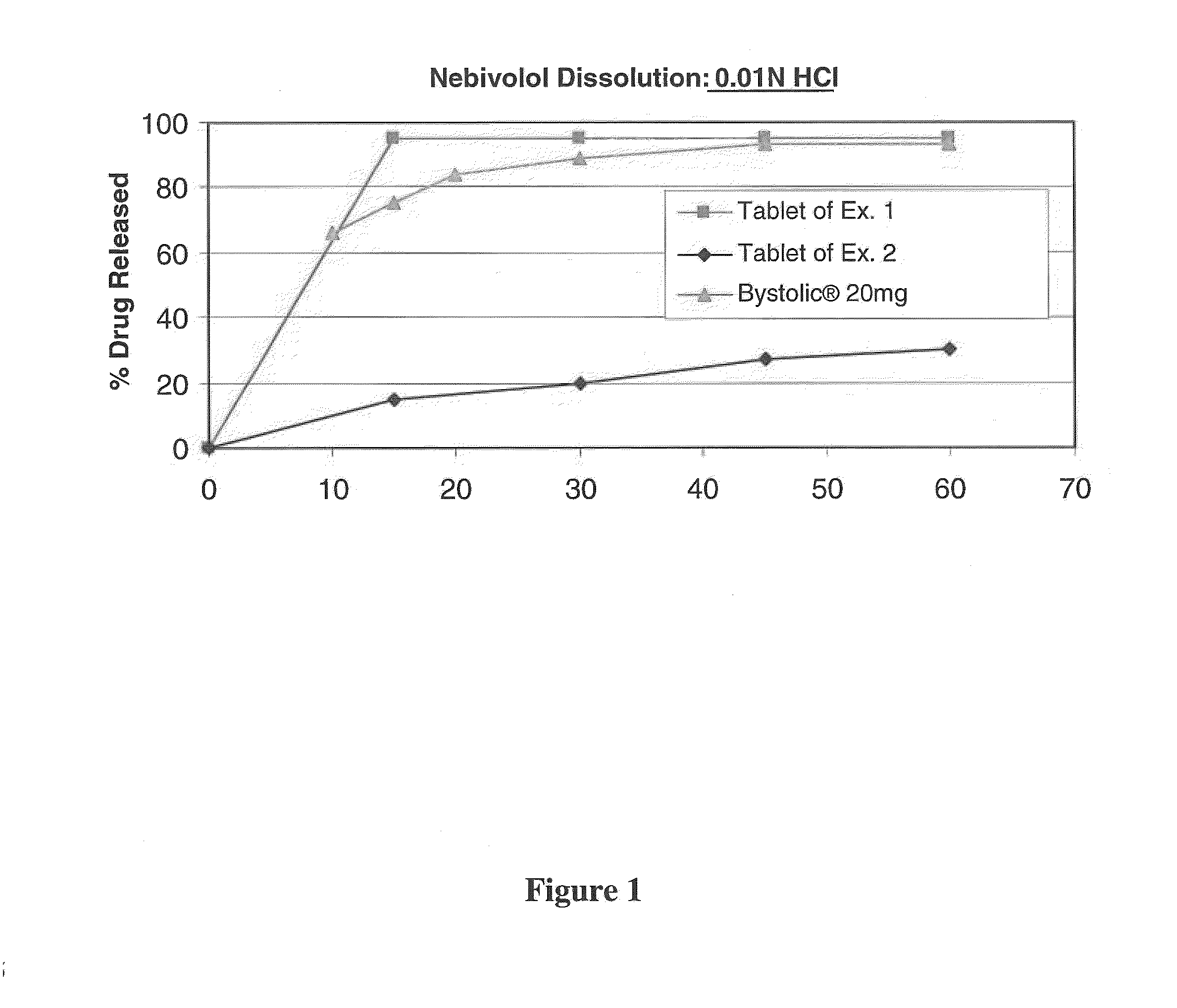
Pharmaceutical composition comprising nebivolol with improved dissolution rate
ActiveCN108463250AReduce manufacturing costDissolution rate deviation is smallOrganic active ingredientsInorganic non-active ingredientsNebivololDissolution
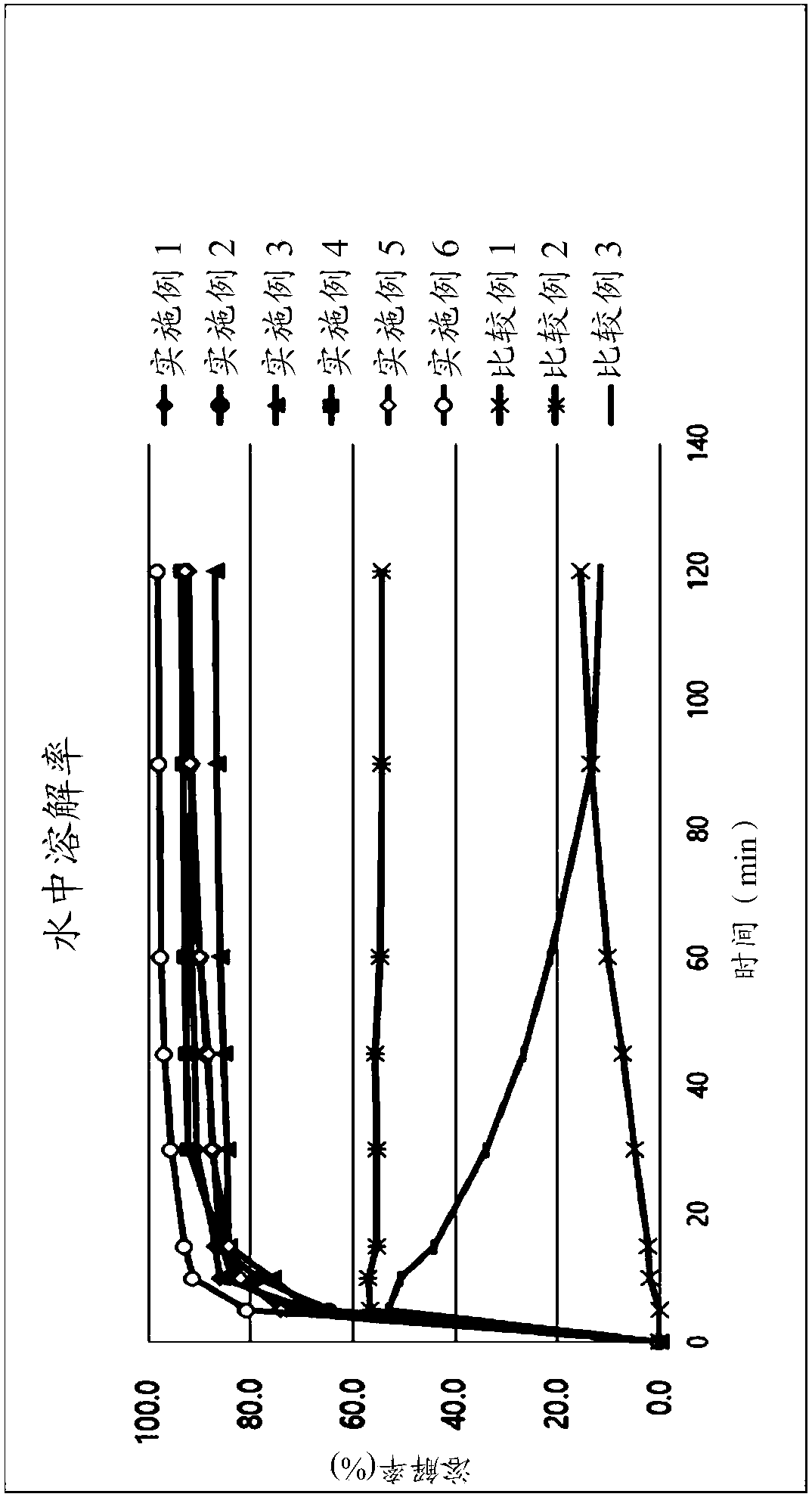
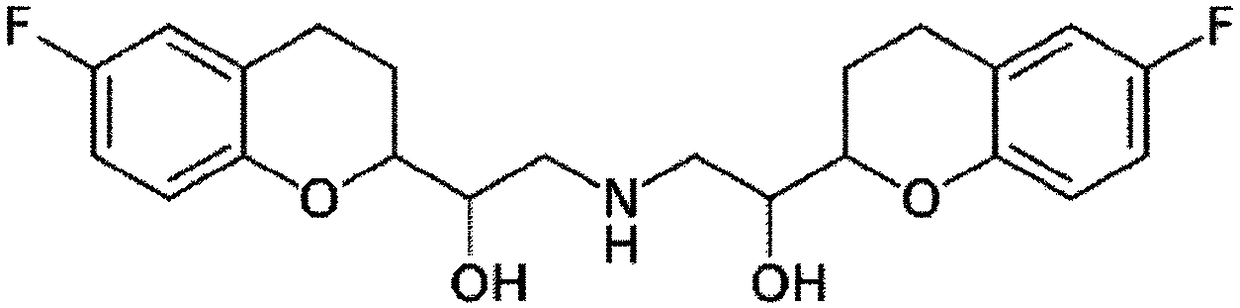
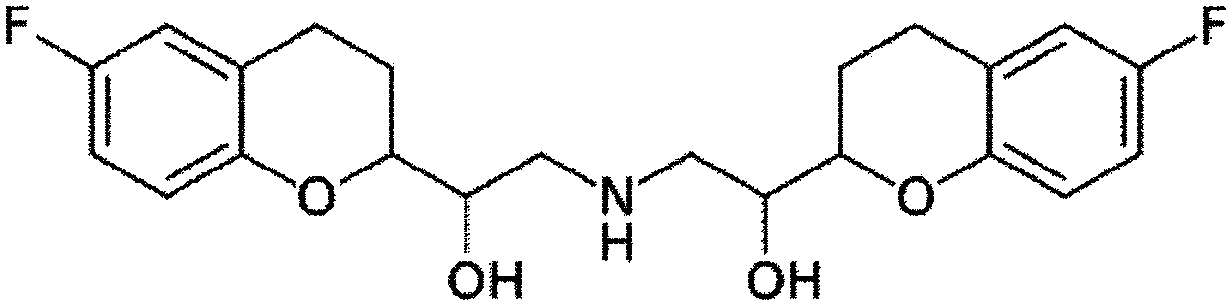
The present invention relates to a pharmaceutical composition comprising nebivolol with an improved dissolution rate and a preparation method thereof. The pharmaceutical composition, containing nebivolol or a pharmaceutically acceptable salt thereof, an alkalizing agent, and a pharmaceutically acceptable additive, for prevention or treatment of cardiovascular diseases, does not cause a decrease inthe dissolution rate of nebivolol and can significantly improve the dissolution rate, not only at low pH, such as pH 1.2, but also at a relatively high pH of 5 to 7, so that an improved therapeutic effect can be expected. In addition, the pharmaceutical composition can reduce the preparation cost since the micronization of nebivolol or the addition of a wetting agent is not required, and can minimize the occurrence of a deviation of bioavailability due to pre- or post-meal intake since there is little deviation of the dissolution rate due to the pH change, thereby maintaining a constant pharmaceutical action, and thus, the pharmaceutical composition is very useful in the preparation of a composite preparation containing a nebivolol preparation, or another active ingredient, such as rosuvastatin calcium.
Owner:ELYSON PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com