Patents
Literature
33 results about "Dihydrobenzopyrans" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
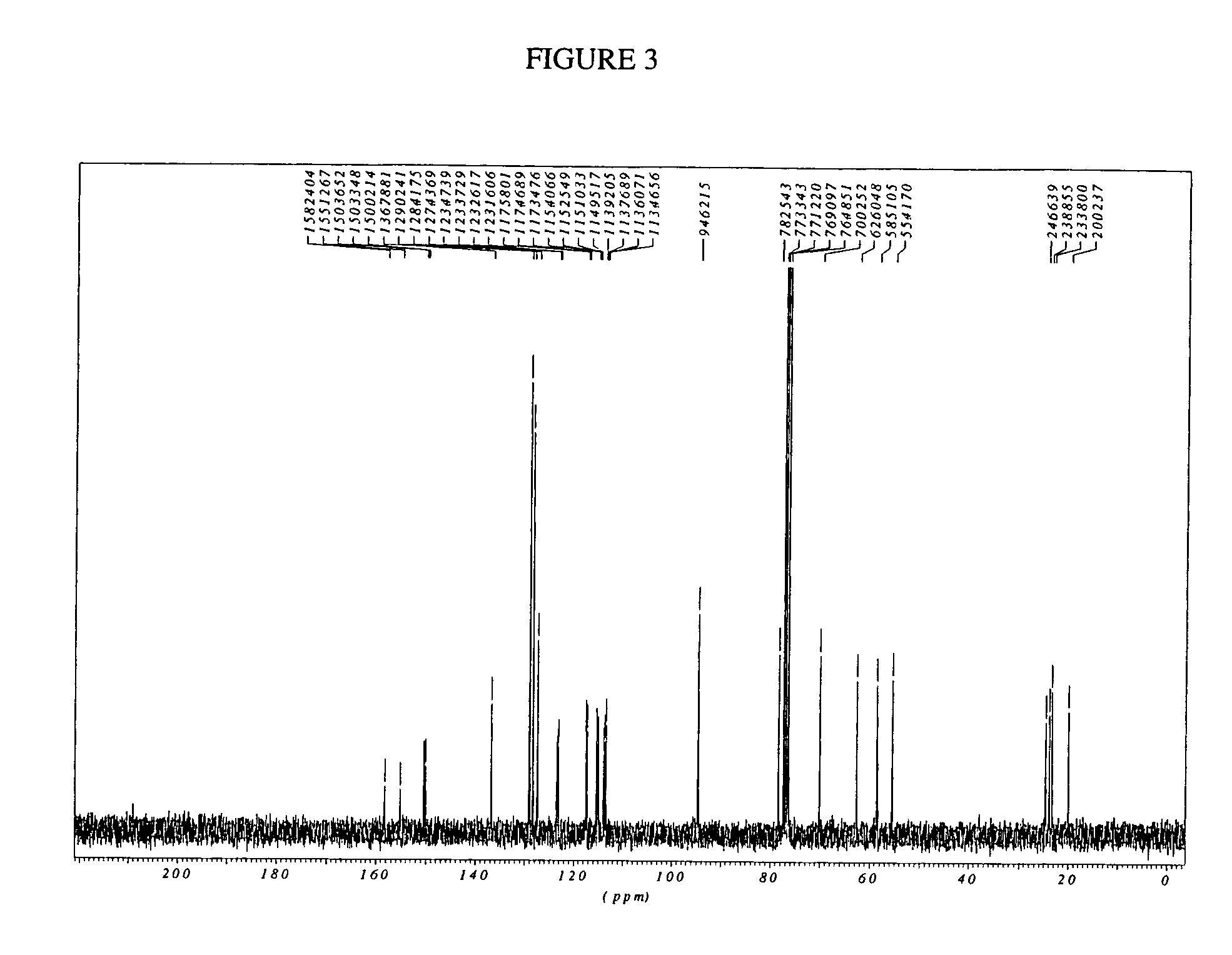
Dongil Choi, Naoki Shiga, Robert Franzén and Tetsuhiro Nemoto, Acid‐Promoted Cascade Cyclization to Produce 2‐(4′‐Alkoxyaryl)‐3,4‐Fused Tricyclic Dihydrobenzopyrans via a Vinylidene para‐Quinone Methide Intermediate, European Journal of Organic Chemistry, 2018, 15, (1785-1788), (2018).
Anticachectic composition
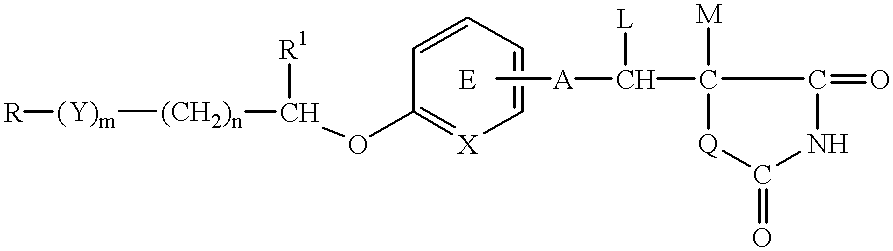
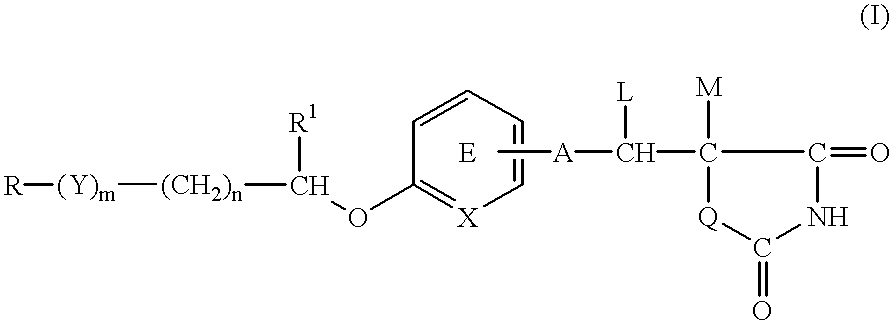
InactiveUS6110948AReduce fasting insulin levelIncrease insulin sensitivityBiocideOrganic chemistrySulfurAliphatic hydrocarbon
PCT No. PCT / JP97 / 01148 Sec. 371 Date Sep. 30, 1998 Sec. 102(e) Date Sep. 30, 1998 PCT Filed Apr. 3, 1997 PCT Pub. No. WO97 / 37656 PCT Pub. Date Oct. 16, 1997A medicinal composition for the prophylaxis and treatment of cachexia which comprises a compound of the formula: wherein R represents a hydrocarbon group that may be substituted or a heterocyclic group that may be substituted; Y represents a group of the formula -CO-, -CH(OH)-, or -NR3- (R3 represents an alkyl group that may be substituted); m is 0 or 1; n is 0, 1 or 2; X represents CH or N; A represents a bond or a bivalent aliphatic hydrocarbon group having 1 to 7 carbon atoms; Q represents oxygen or sulfur; R1 represents hydrogen or an alkyl group; ring E may have further 1 to 4 substituents, which may form a ring in combination with R1; L and M respectively represent hydrogen or may be combined with each other to form a bond, provided that when m and n are 0, X represents CH, A represents a bond, Q represents sulfur, R1, L and M respectively represent hydrogen, and ring E does not have further substituents, R does not represent dihydrobenzopyranyl; or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
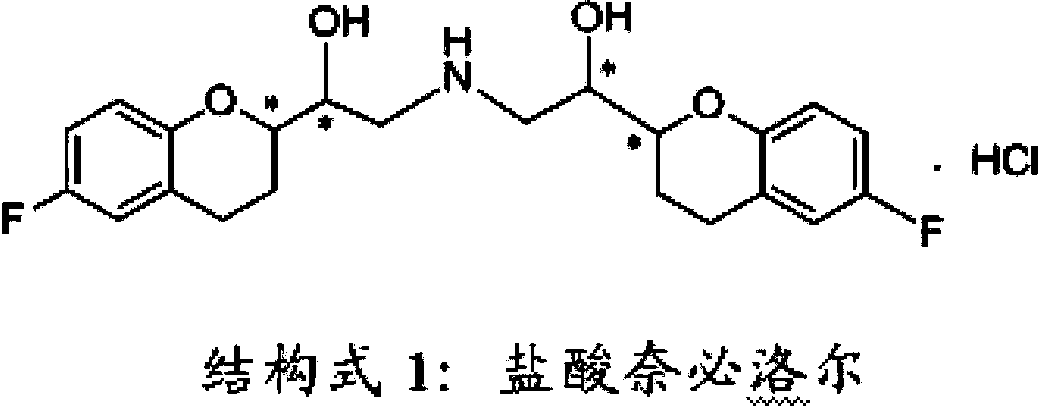
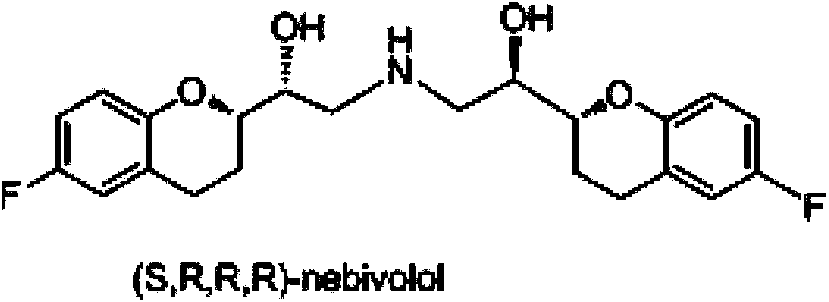
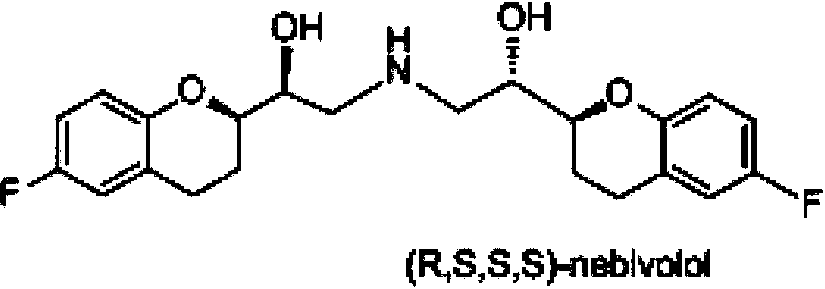
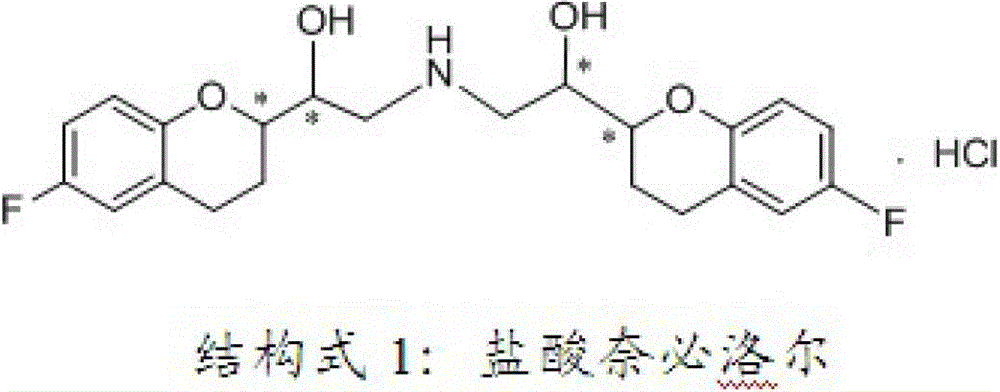
Process for preparation of racemic Nebivolol
Owner:UNIV ZURICH +1
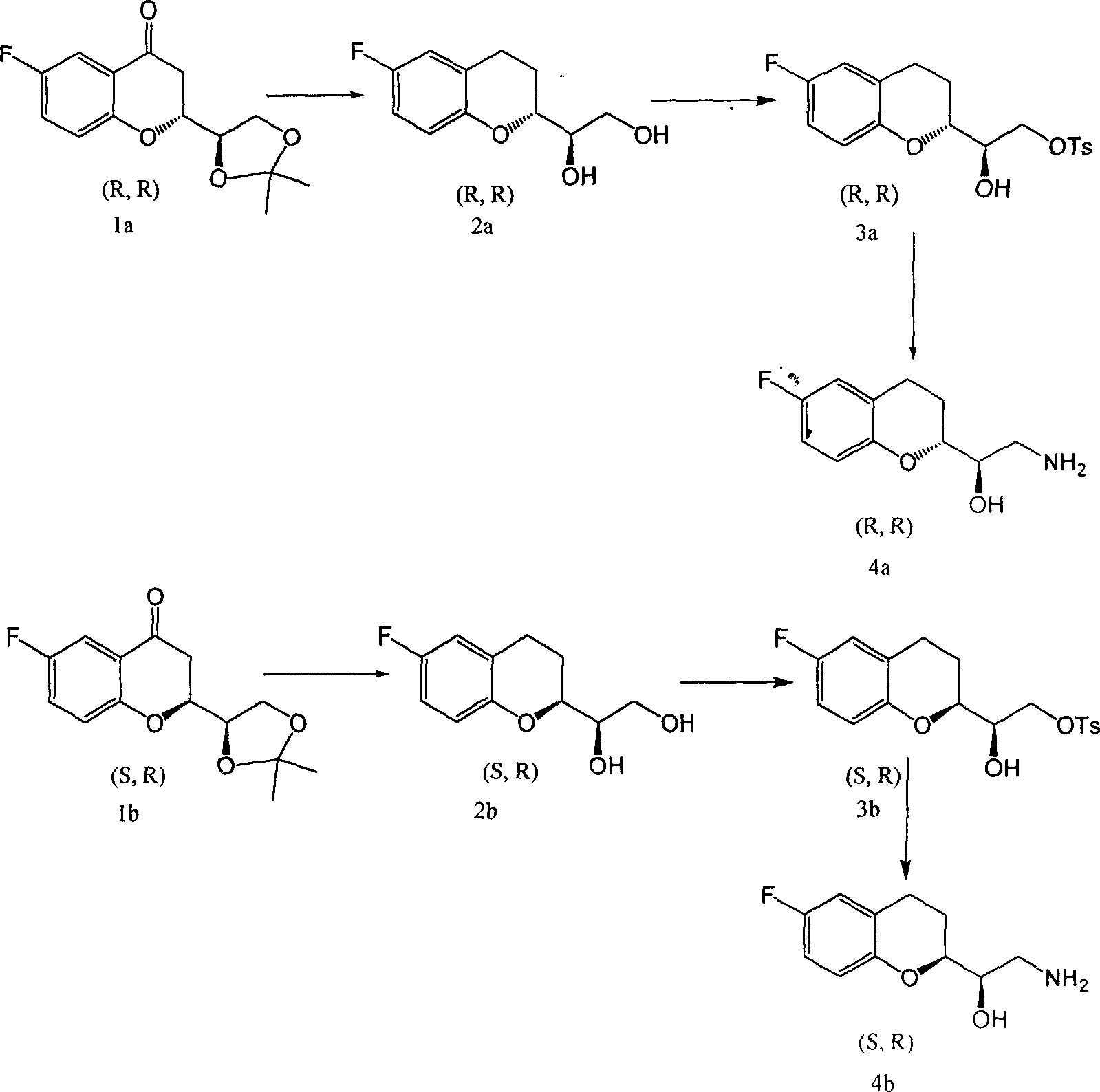
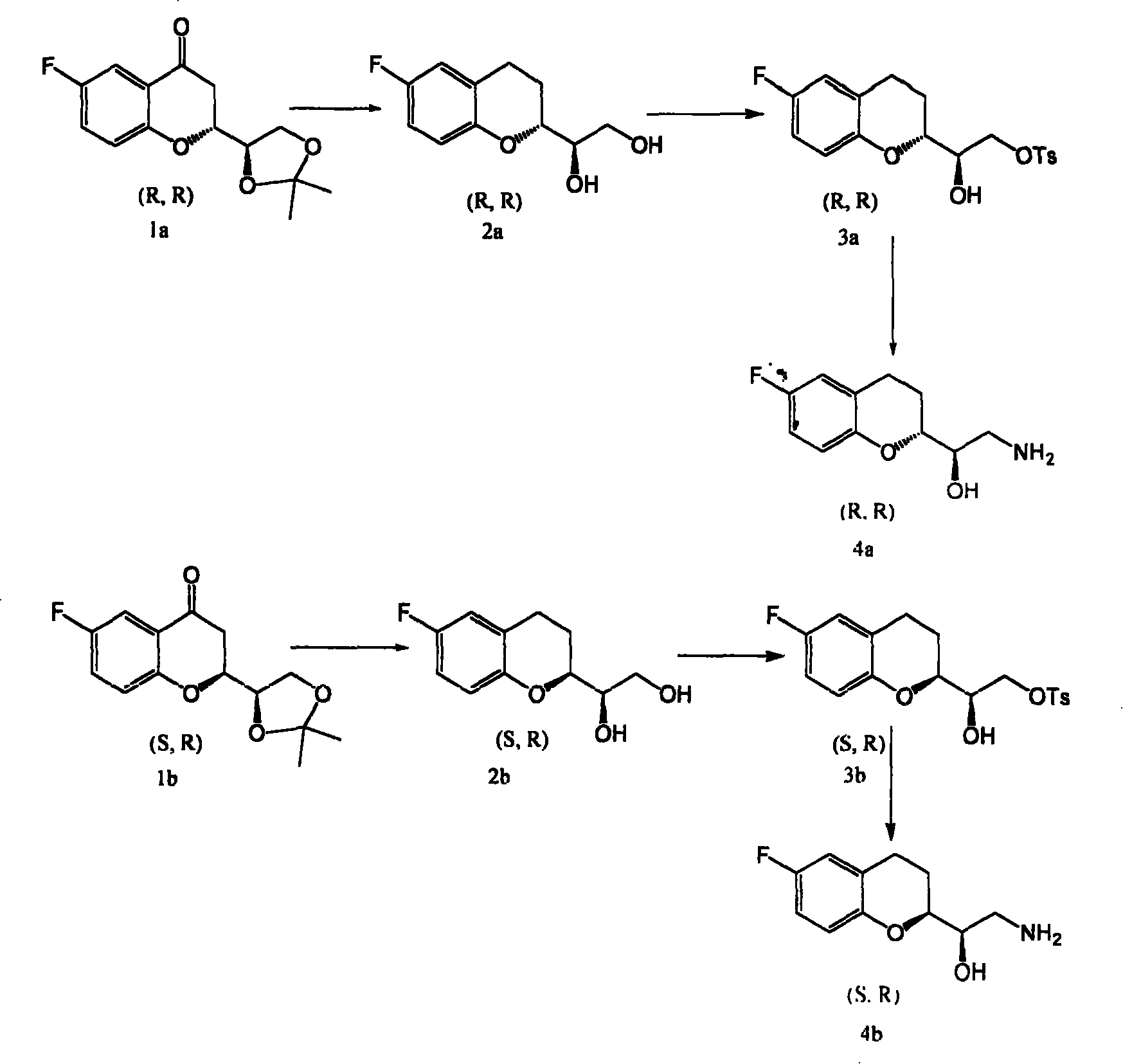
Synthetic process of chiral 2-amido-1-(6-fluorine-3,4-dihydrobenzopyranyl) alCohol
This invention is attributed to the field of organic chemistry and specifically relates to the method to synthesize drug intermediates (R)-2-amino-1-((R)-6-fluoro-3, 4-dihydrochromeno) ethanol and (R)-2-amino-1-((S)-6-fluoro-3, 4-dihydrochromeno) ethanol. The method is that, drug intermediates (2R)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone and (2S)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone are adopted as raw materials and reduced by Clemmensens method with the products reacting with p-toluenesulfonyl chloride in pyridine. The consequent products are dissolved in solvent and dry ammonia is introduced for heating reflux. The total yield can be as much as 32%.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Insecticidal (dihalopropenyl) phenylalkyl substituted dihydrobenzofuran and dihydrobenzopyran derivatives
ActiveUS6987194B2Easy to controlReduce application rateBiocideOrganic chemistryDihydrobenzopyransInsect
Owner:FMC CORP
Novel process for preparation of nebivolol intermediates
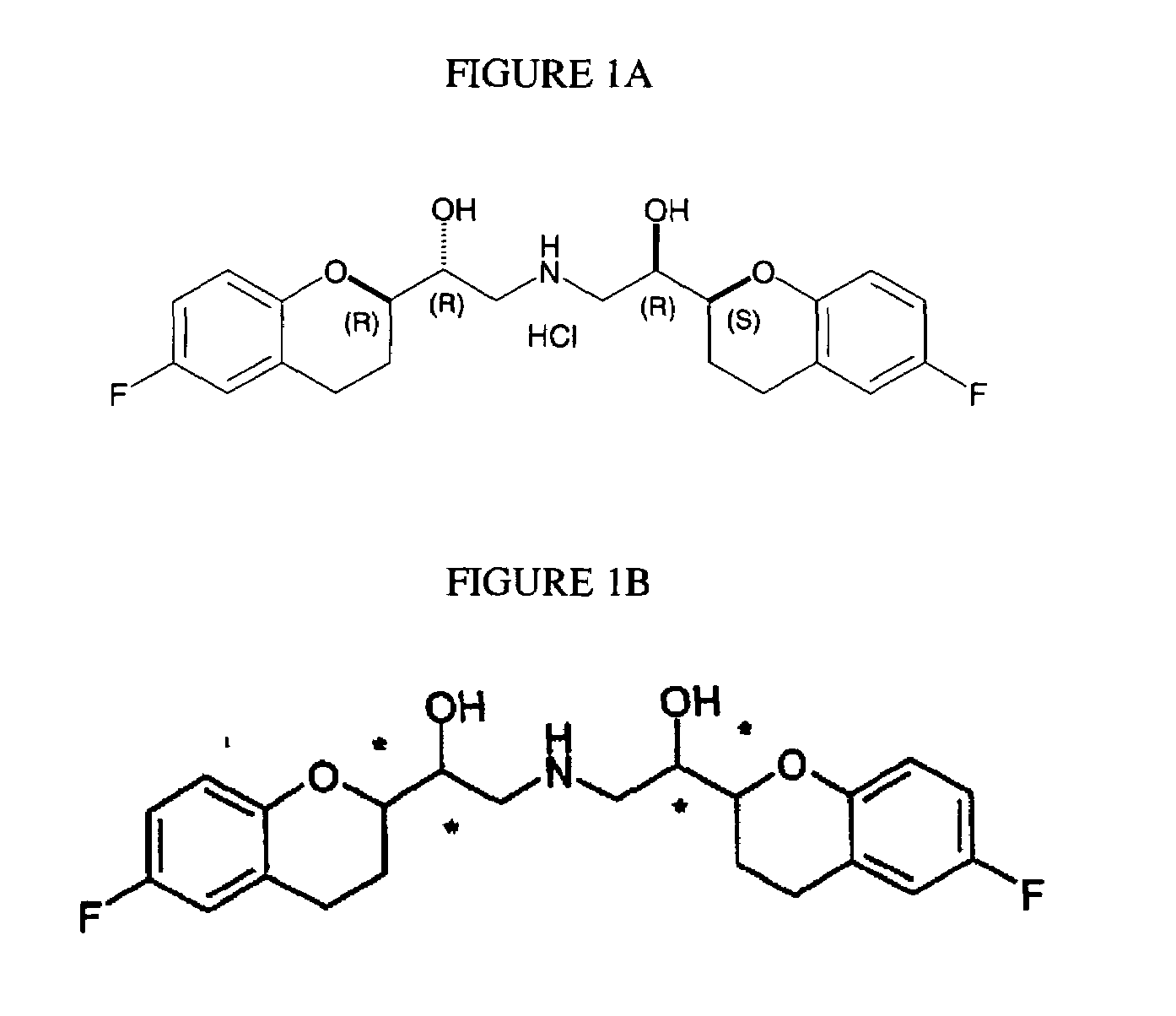
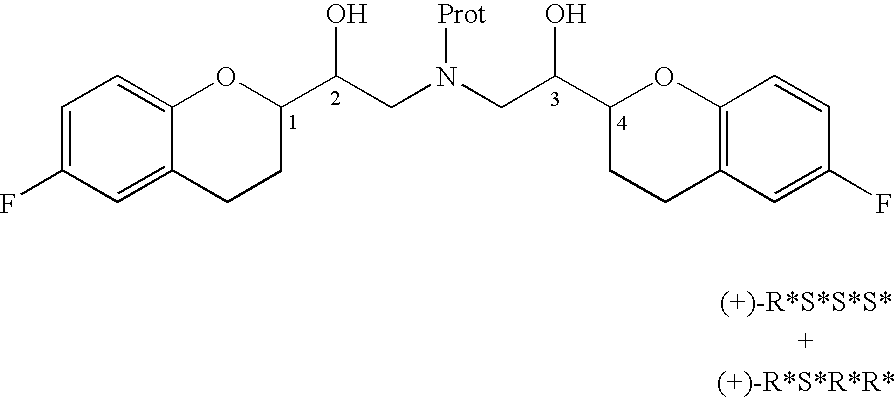
The present invention relates to a process for separation of desired diastereomeric pair from a mixture of diastereomeric pairs thereby obtaining nebivolol intermediates. Thus, the mixture of (+)-[1S*(R*)]-6-fluoro-3,4-di-hydro-α-[[(phenylmethyl)amino]methyl]-2H-1-benzopyran-2-methanol, (+)-[1S*(S*)]-6-fluoro-3,4-dihydro-2-oxi -ranyl-2H-1-benzopyran and ethanol is heated to reflux temperature and stirred for 8 hours at the same temperature to obtain (±)-[2R*[1S*,5S*(S*)]]+[2R*[1S*,5R*(R*)]]-α,α′-[phenylmethyliminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran ran-2-methanol]. Then the reaction mass is cooled to 10° C., the pH is adjusted to 2 with HCl gas and stirred for 45 minutes at 25° C. to 30° C. Then the separated solid is filtered and dried to give (+)-[2R*[1S*,5S*(S*)]]-α,α′-[phenylmethyliminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol] hydrochloride salt, which can be converted into nebivolol.
Owner:HETERO DRUG
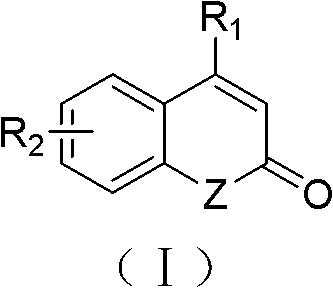
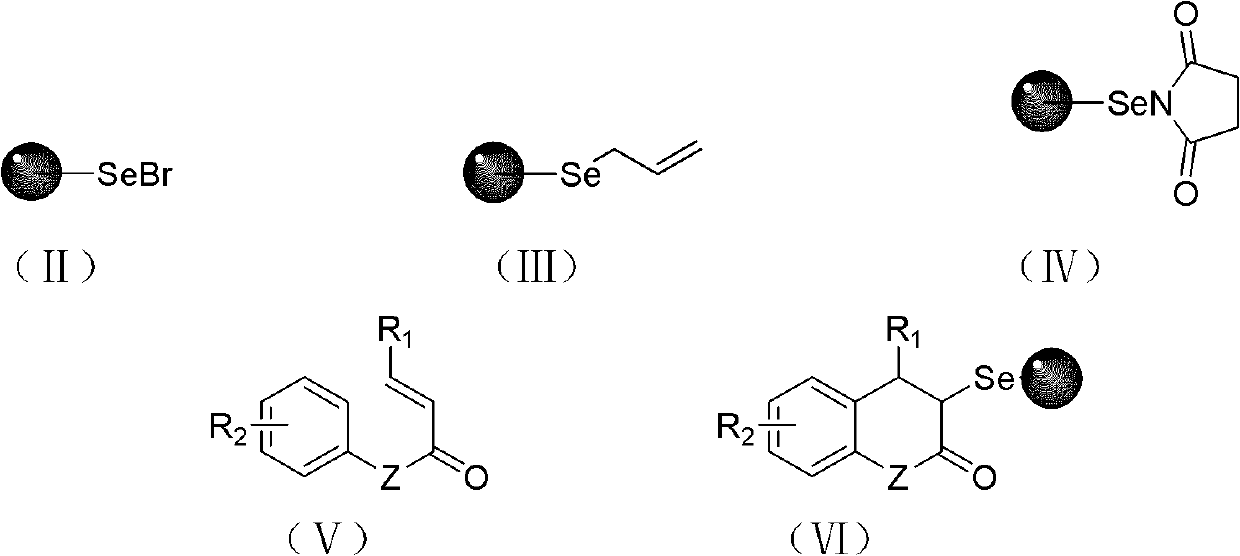
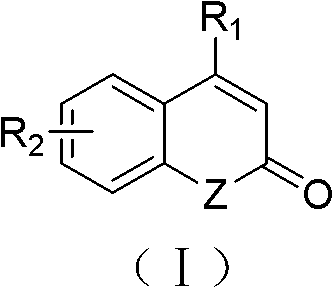
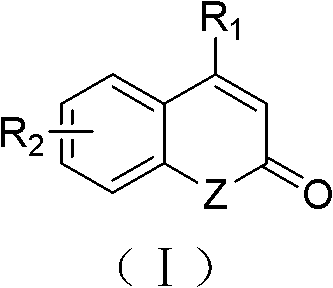
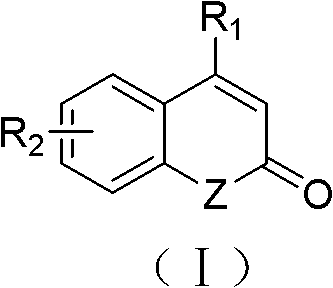
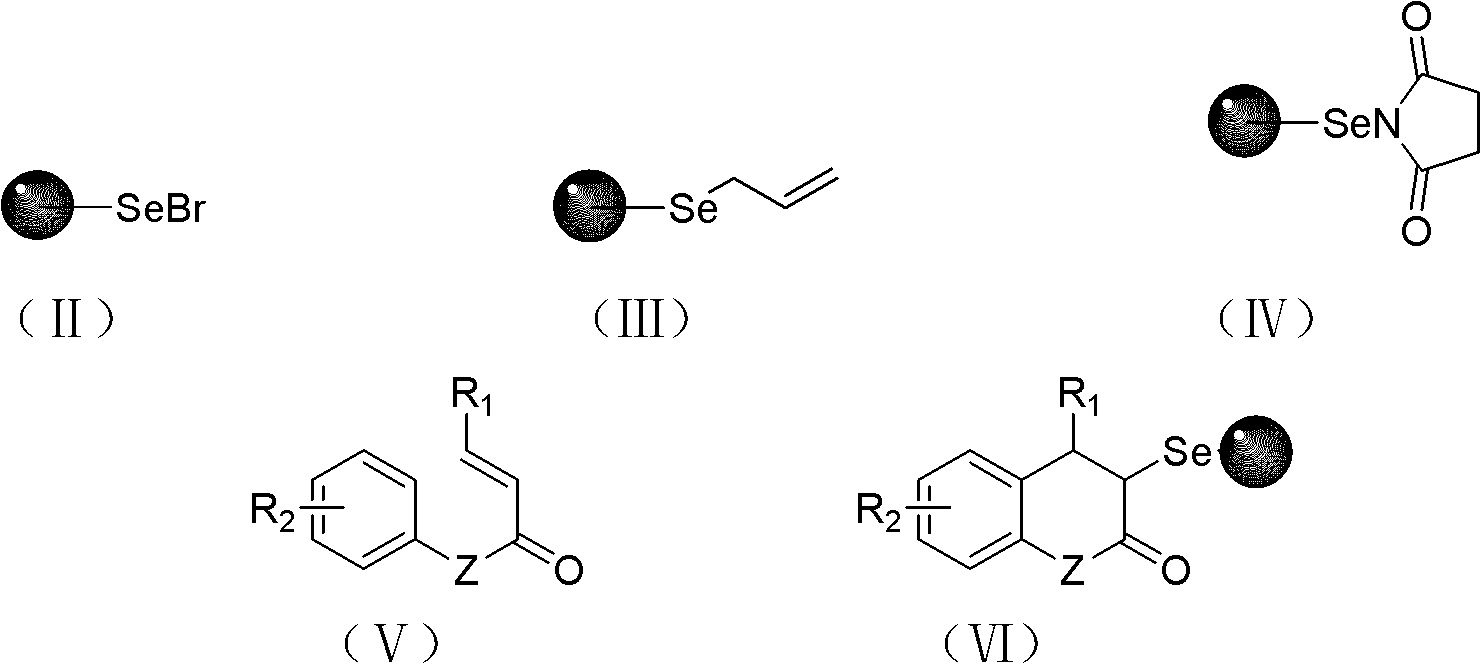
Solid-phase synthesis method of coumarin and analogue thereof
InactiveCN102532015AHigh yieldOvercome the disadvantage of low yield of cyclization reactionOrganic chemistryPolystyreneKetone
The invention relates to a solid-phase synthesis method of coumarin and an analogue (I) thereof and belongs to the field of organic chemistry. The method comprises the following steps: 1) taking 1% of cross-linked polystyrene resin as a carrier to prepare a polystyrene-supported seleno-succimide reagent (III); 2) using the III to induce phenyl acrylate (V) to perform intramolecular cyclization under the catalysis of trimethylsilyl trifluoromethanesulfonate so as to form 3-polystyrene-supported seleno-3,4-dihydro-benzopyran-2-ketone (VI); and 3) performing oxidation elimination on the VI via an oxidant so as to directly get the coumarin (I) without further separation. When the phenyl acrylate (V) is replaced by N-phenyl acrylamide, the analogue of the coumarin, namely a 2-quinolone compound, can be prepared through the same steps. The solid-phase synthesis method disclosed by the invention has the advantages of easily available raw materials, good product yield, high purity, simplicityand convenience in operation, simple post-treatment and great industrial application prospects.
Owner:YUNNAN UNIV
3,4-dihydro-iii 2 benzopyran-1 ketone kind compound, its preparation method and use
The present invention relates to one kind of 3, 4-dihydro-1H-2-benzopyran-1-ketone compounds, and provides the preparation process of these compounds. These compounds have LAR and PTPsigma inhibiting effect, and may be used in treating various types of diabetes, obesity and their complication as well as other diseases caused by excessive activity or over expression of PTP-LAR and / or PTPsigma, such as tumor and nervous degenerative disease.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Process for preparation of racemic Nebivolol
InactiveUS20070149612A1Increase productionLow costBiocideOrganic chemistryMethanolMedicinal chemistry
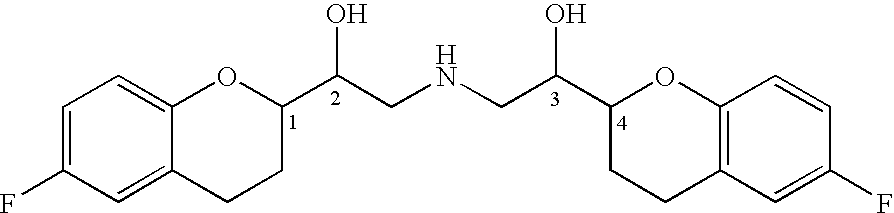
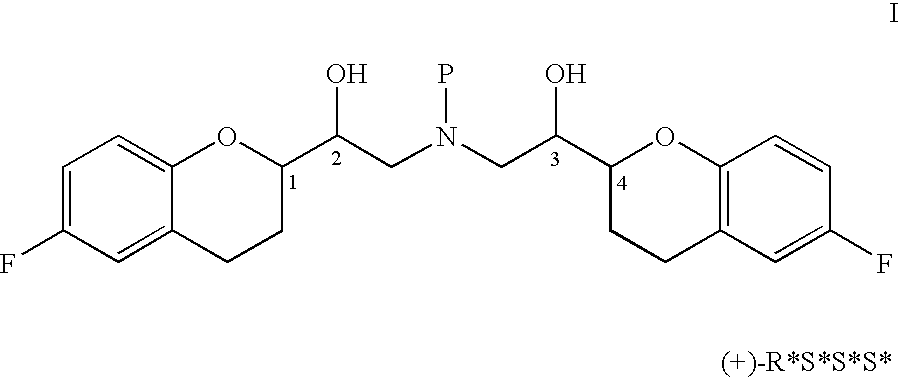
A process of making racemic [2S*[R*[R*[R*]]]] and [2R*[S*[S*[S*]]]]-(±)α,α′-[iminobis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol] of the compound of the formula (I) and its pure [2S*[R*[R*[R*]]]]- and [2R*[S*[S*[S*]]]]-enantiomer compounds and pharmaceutically acceptable salts thereof.
Owner:UNIV ZURICH +1
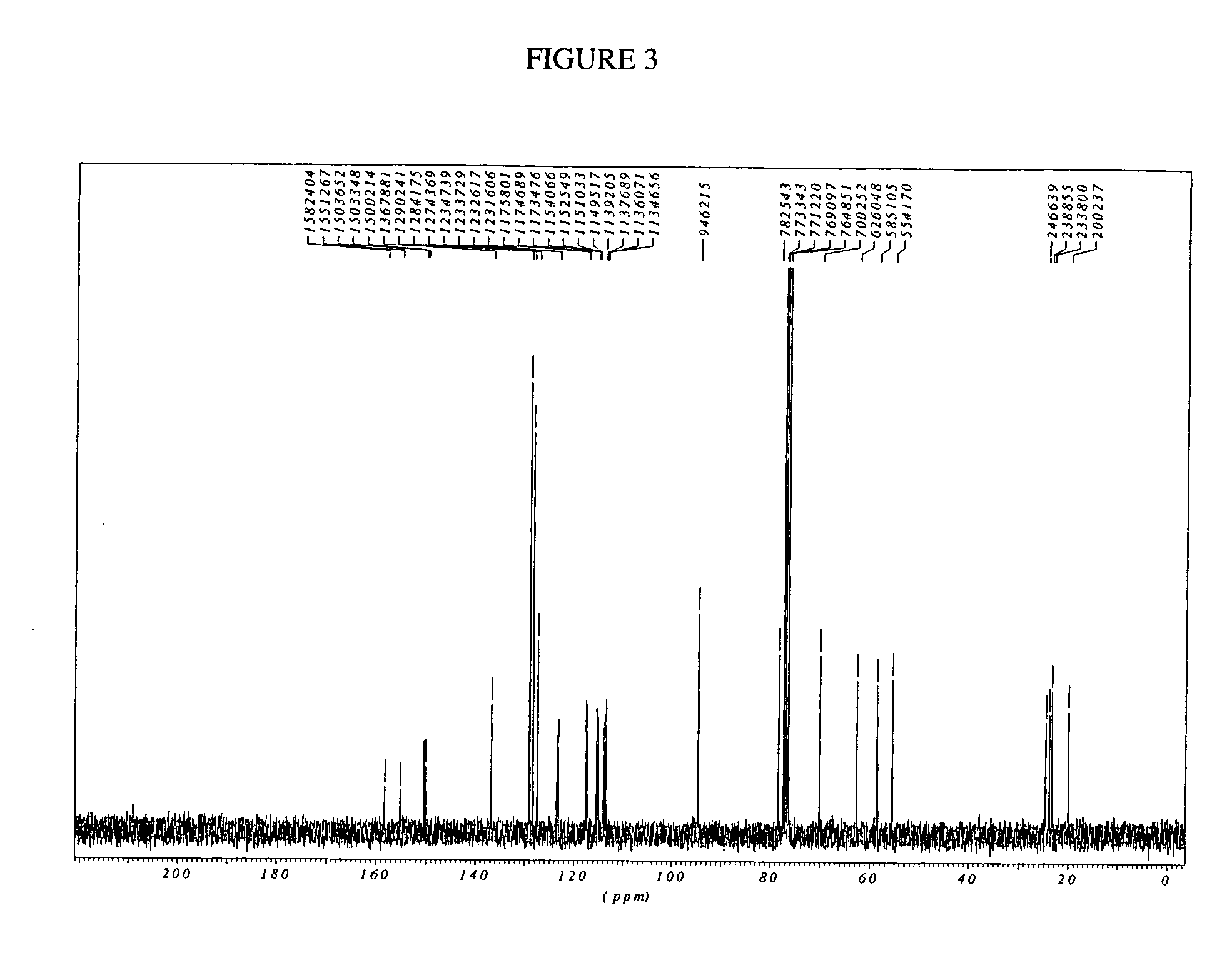
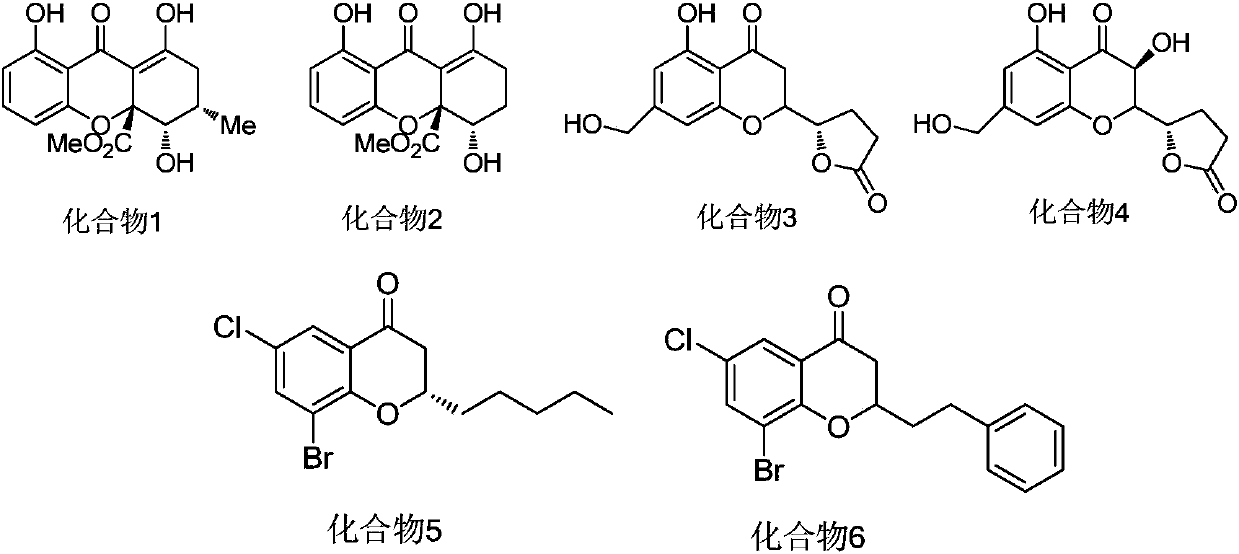
Dihydrobenzopyrans ketone compound and uses thereof
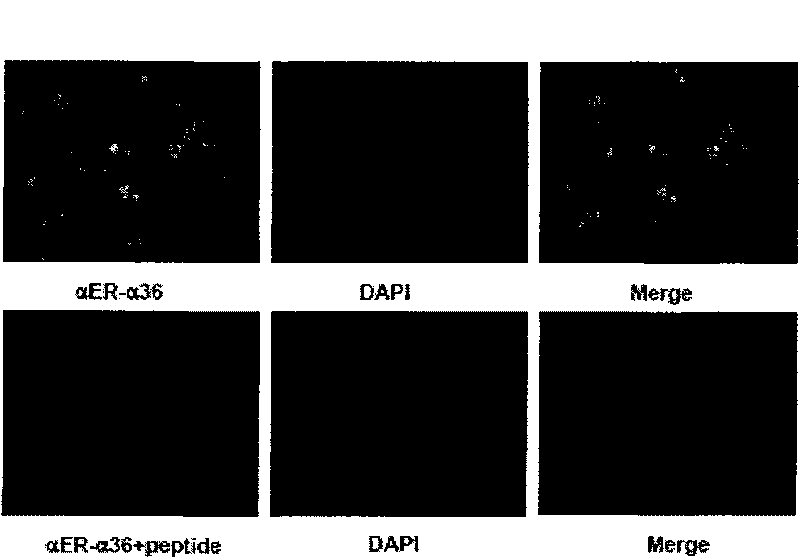
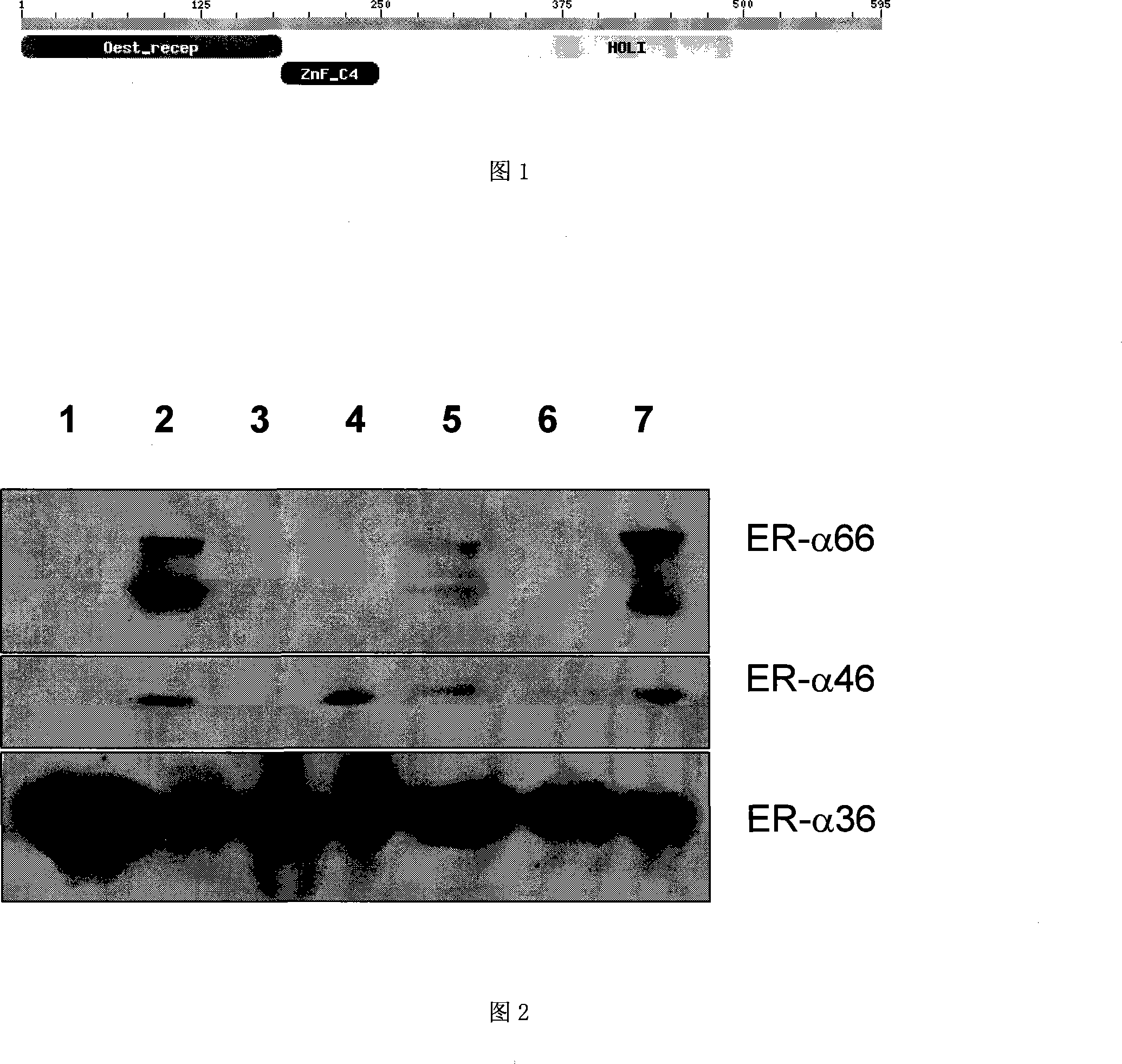
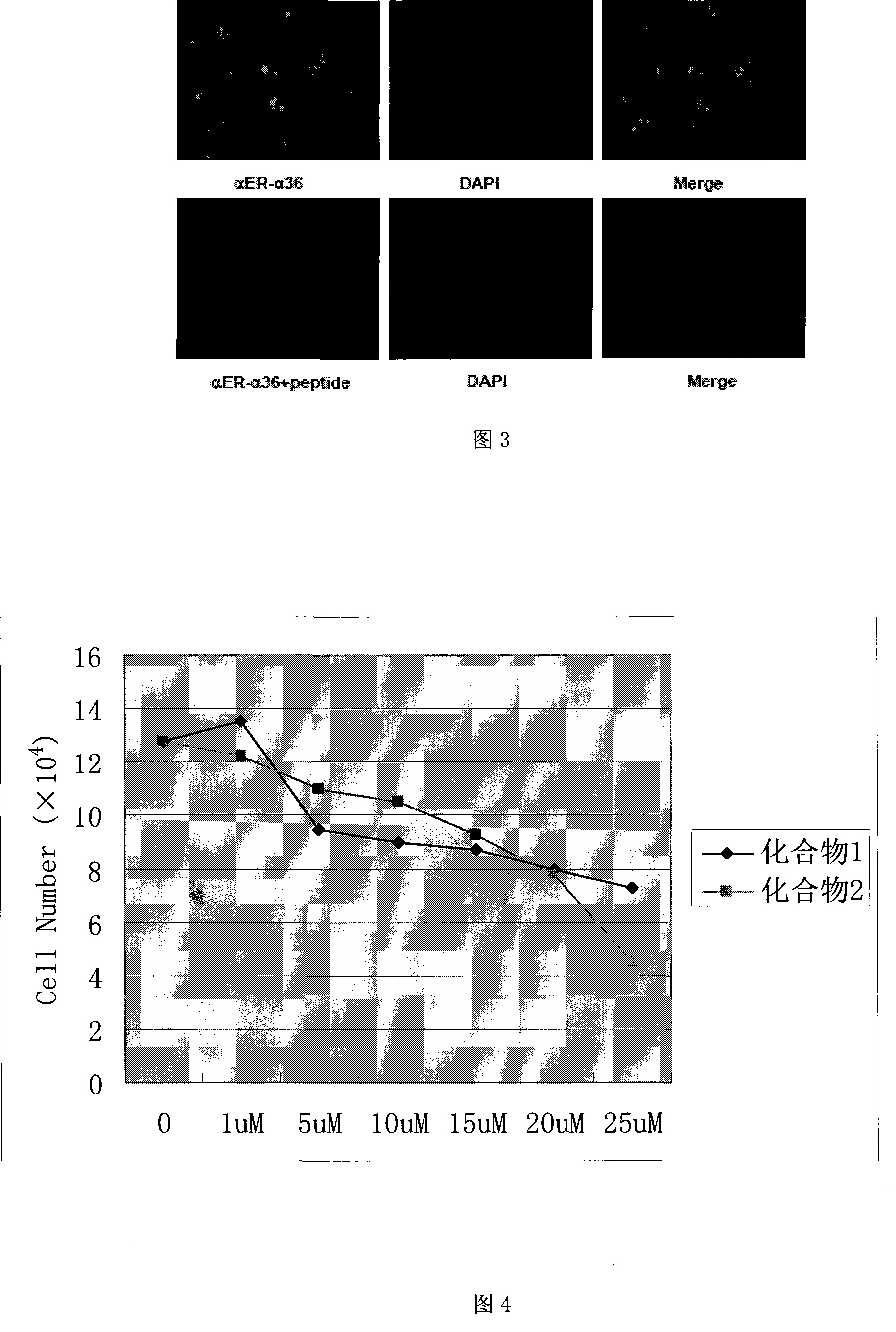
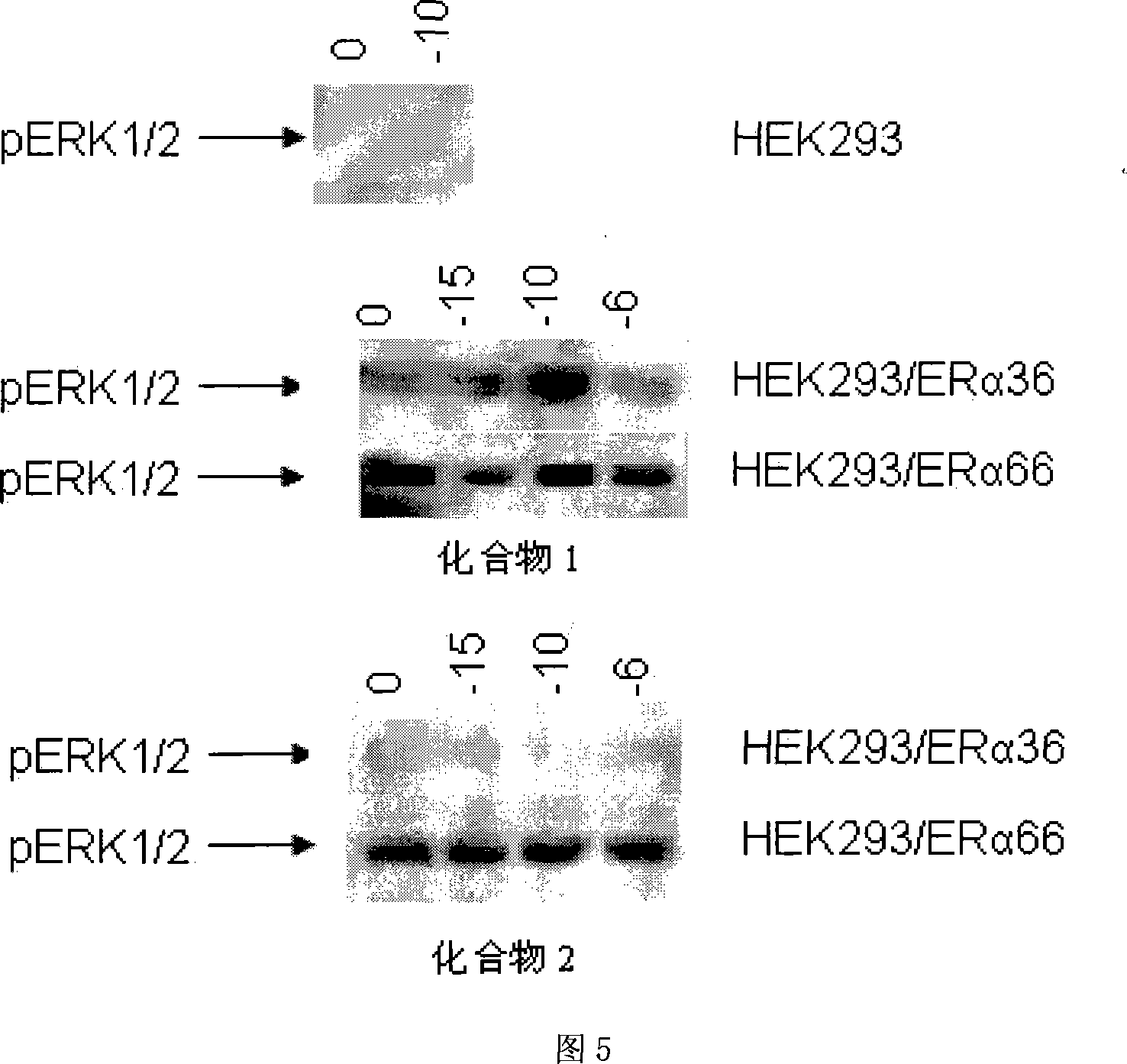
The invention relates to the new synthesized compound i.e. dihydrobenzo pyrone type and the application thereof, and belongs to the field of the chemical drug. The experiment results shows that the compound (I) can kill the breast cancer cell MCF7 expressing ER Alpha 66, FR Alpha 46 and ER Alpha 36. Moreover, the compound (I) can be served as the regulator of the estrogen receptor ER Alpha 36 for treatment of the illness caused by the unusual expression of the estrogen receptor ER Alpha 36, such as the tumor, the osteoporosis, the asthma, the heart disease and the senile dementia, etc.
Owner:BEIJING SHENOGEN BIOMEDICAL
Method for preparing 2,3-dihydrobenzopyran-4-one derivative
ActiveCN107586285ASolve the cumbersome synthesis stepsSolve yieldOrganic chemistryEnvironmental resistanceSynthesis methods
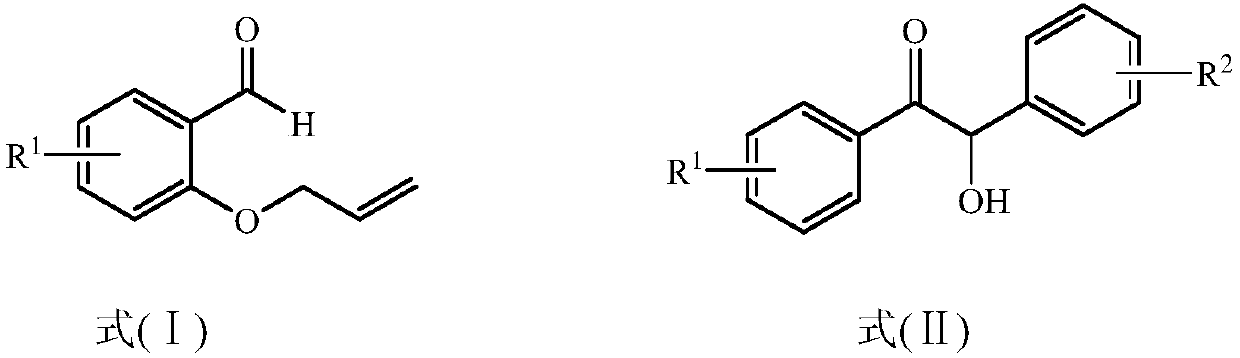
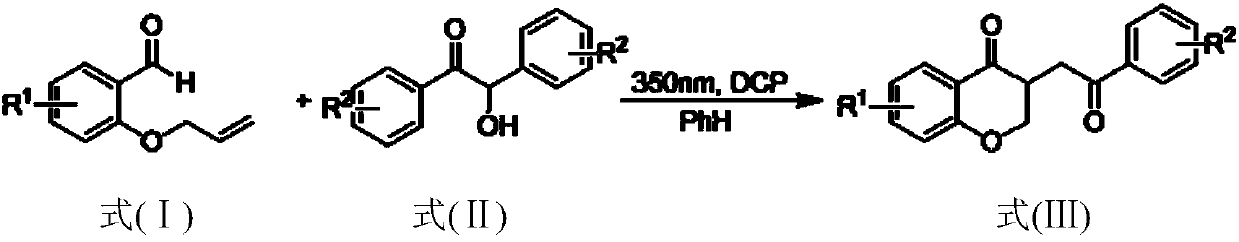
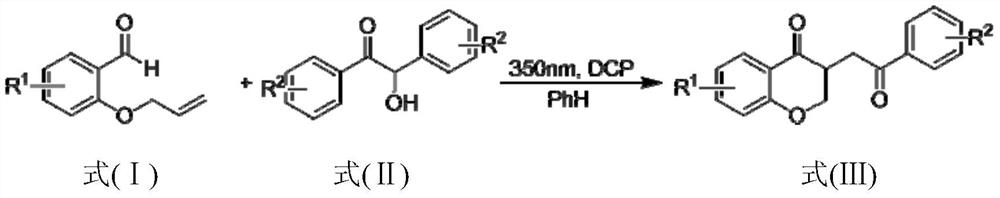
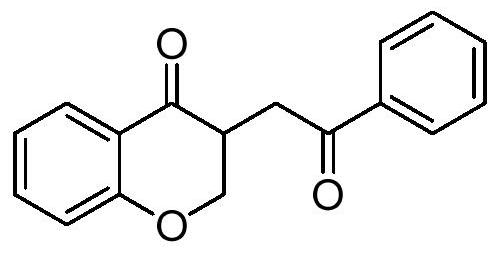
The present invention provides a method for preparing a 2,3-dihydrobenzopyran-4-one derivative, and the method comprises the following steps: under nitrogen atmosphere at room temperature, dissolvinga 2-allyloxybenzaldehyde derivative represented by formula (I) and a benzoin derivative represented by formula (II) in an organic solvent, adding a photosensitizer, evenly mixing, placing under an ultraviolet lamp for light irradiation reaction, removing the solvent by rotary evaporation, and separating and purifying by silica gel column chromatography to obtain the resulting product 2,3-dihydrobenzopyran-4-one derivative. The preparation method solves the problems of cumbersome steps, low yield and poor environmental protection property of the existing synthesis method, can react under normaltemperature and normal pressure, and has the advantages of mild reaction conditions, no need of transition metal catalysis, simple operation, no pollution, safety, environmental protection, low costand the like. R1 and R2 are hydrogen, halogen or alkyl.
Owner:ZHEJIANG SHENGXIAO CHEM
Compound, preparation method thereof and application thereof as feed additive
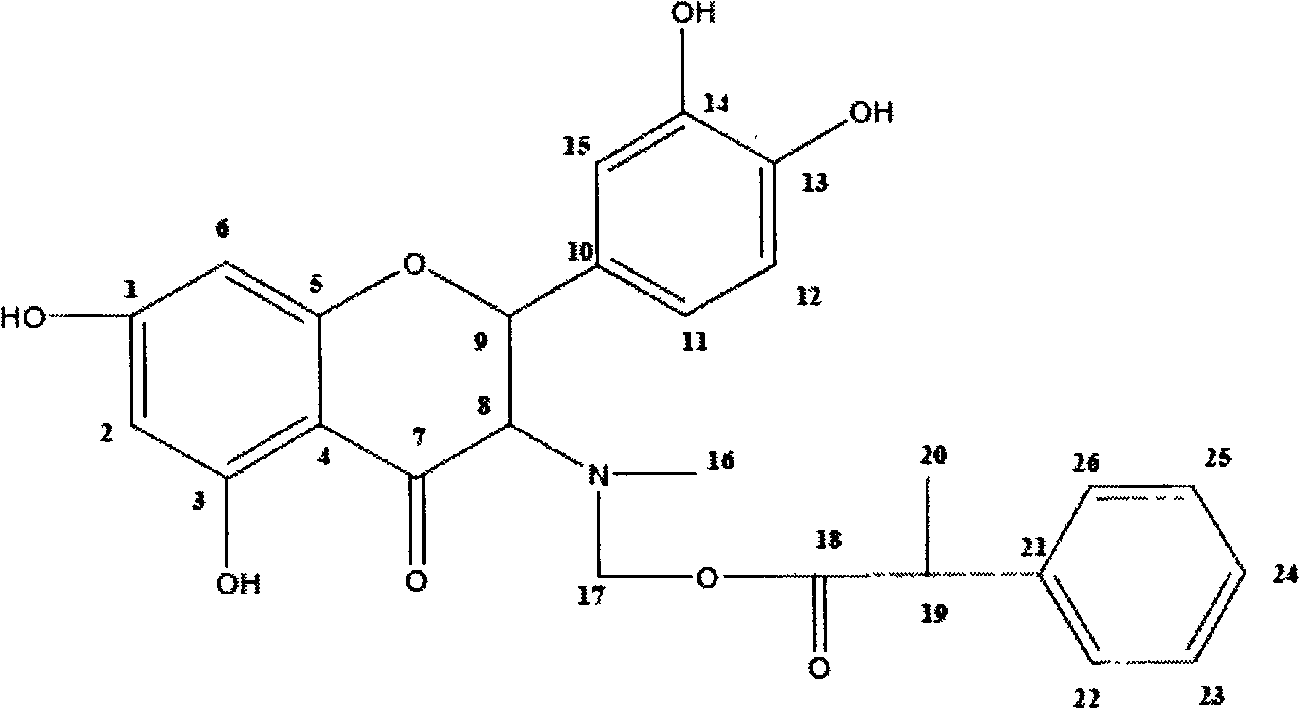
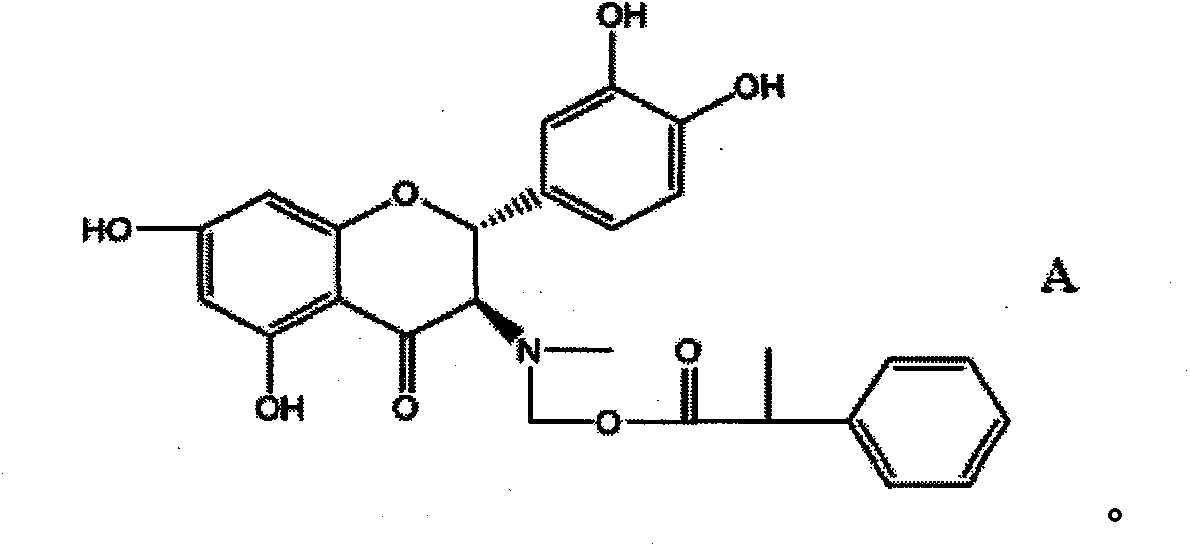
ActiveCN102464642AImprove developmentIncrease daily weight gainOrganic chemistryDigestive systemDiseaseFood additive
The invention discloses a compound, namely (2R, 3R)-((2-(3,4-dihydroxyphenyl)-5,7-dihydroxyl-4-oxo-2,3-dihydrobenzopyran-3-yl) methylamino) methyl-2-phenylpropionate. The invention also discloses a preparation method for the compound and the application of the compound as a feed additive. When serving as the feed additive, the compound can specifically promote the growth of intestinal mucosa villus of livestocks, promote the growth of intestinal linings, reduce the intestinal tension and inhibit abnormal peristalsis, can reduce the feed discharging speed of intestinal tracts of the livestocks so as to enhance the digestive absorption of the intestinal tracts to nutritional components, and can obviously increase the daily weight increasing of the livestocks. The reduction of the intestinal tension contributes to water absorption in the intestinal tracts, and plays an obvious role of convergence and reducing functional diarrhea. The compound provided by the invention also has an effect of repairing intestinal mucosa, and can also obviously improve the immunity function of intestinal mucosa and disease resistance of the livestocks.
Owner:谢联金
Anticachectic composition
InactiveUS6365607B1Toxic potential of the compound of the present invention is lowPrevent and delay onsetBiocideOrganic chemistryHydrogenSulfur
A medicinal composition for the prophylaxis and treatment of cachexia which comprises a compound of the formula:wherein R represents a hydrocarbon group that may be substituted or a heterocyclic group that may be substituted; Y represents a group of the formula -CO-, -CH(OH)-, or -NR3- (R3 represents an alkyl group that may be substituted); m is 0 or 1; n is 0, 1 or 2; X represents CH or N; A represents a bond or a bivalent aliphatic hydrocarbon group having 1 to 7 carbon atoms; Q represents oxygen or sulfur; R1 represents hydrogen or an alkyl group; ring E may have further 1 to 4 substituents, which may form a ring in combination with R1; L and M respectively represent hydrogen or may be combined with each other to form a bond, provided that when m and n are 0, X represents CH, A represents a bond, Q represents sulfur, R1, L and M respectively represent hydrogen, and ring E does not have further substituents, R does not represent dihydrobenzopyranyl; or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
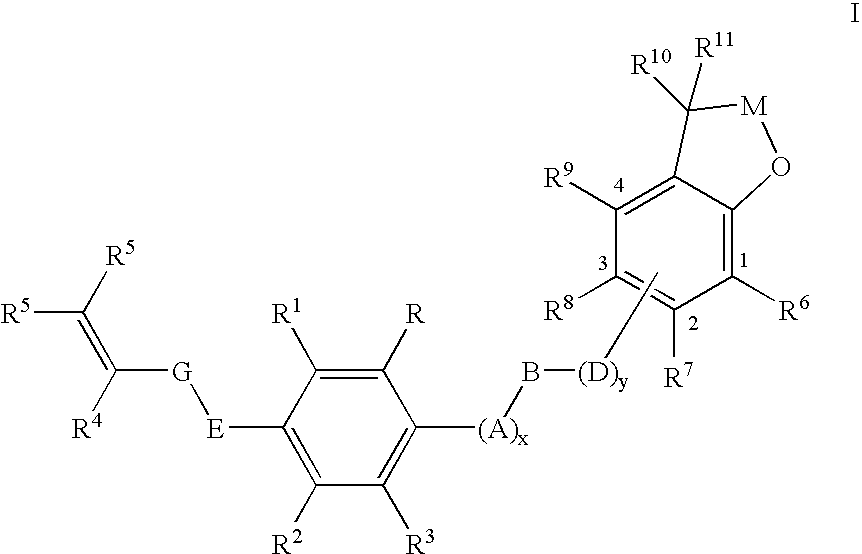
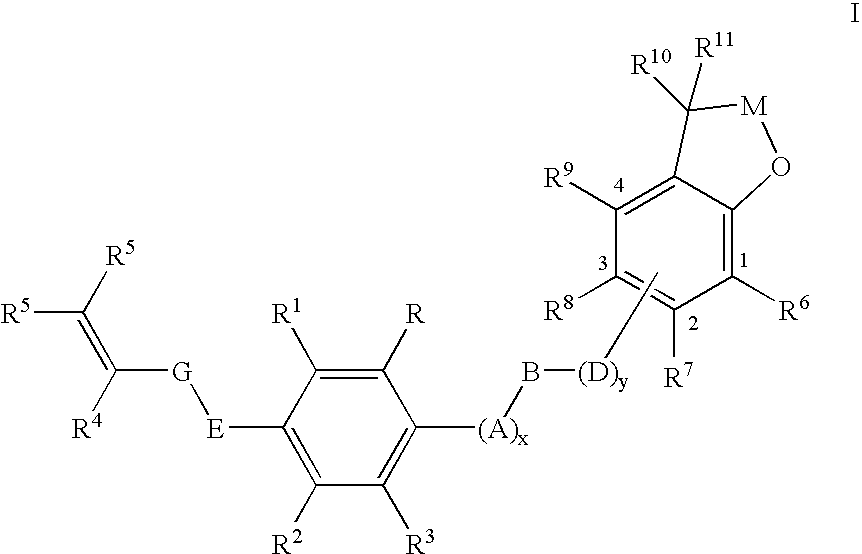
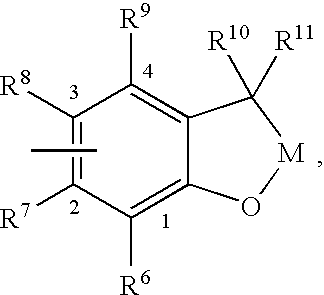
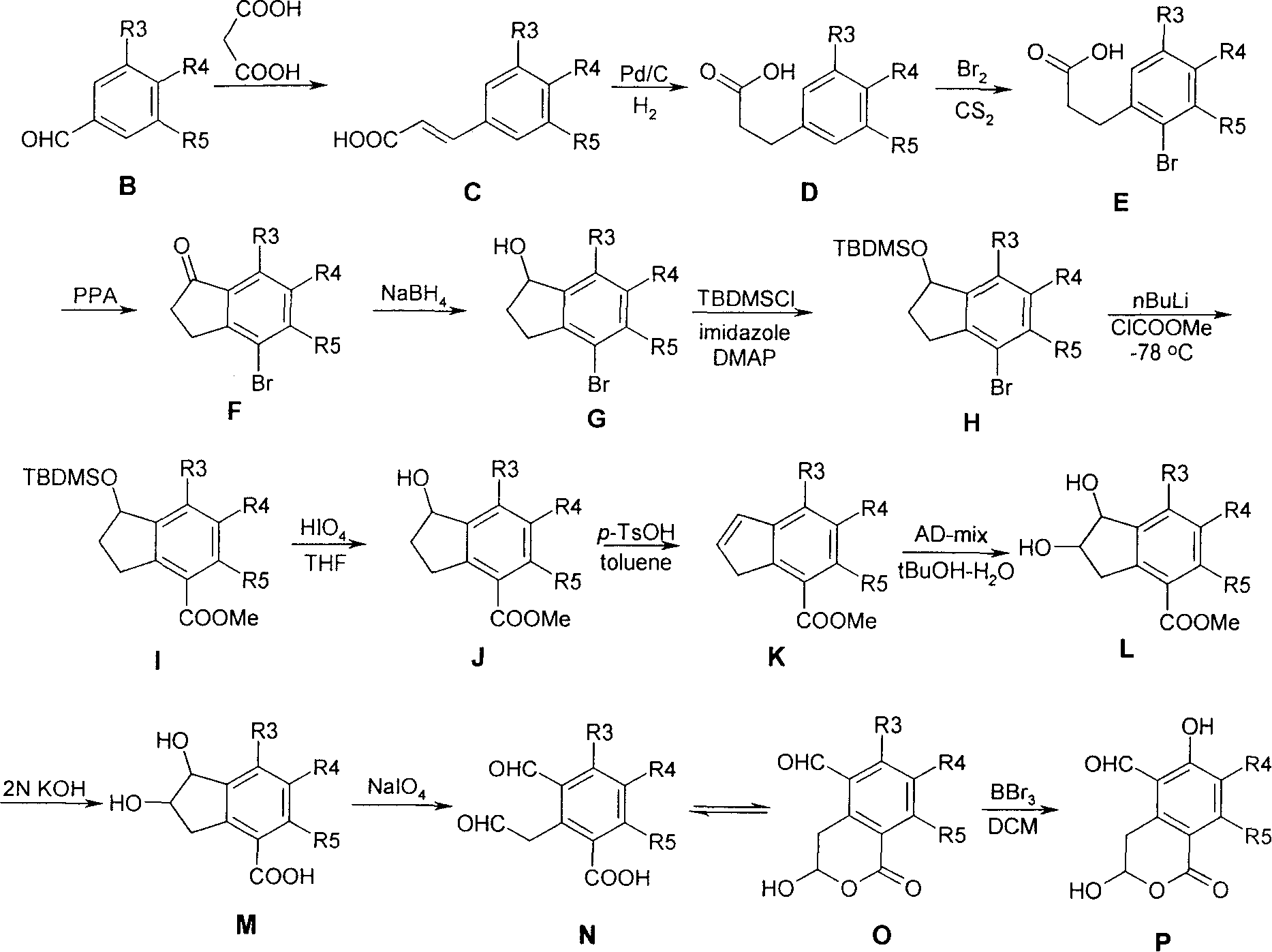
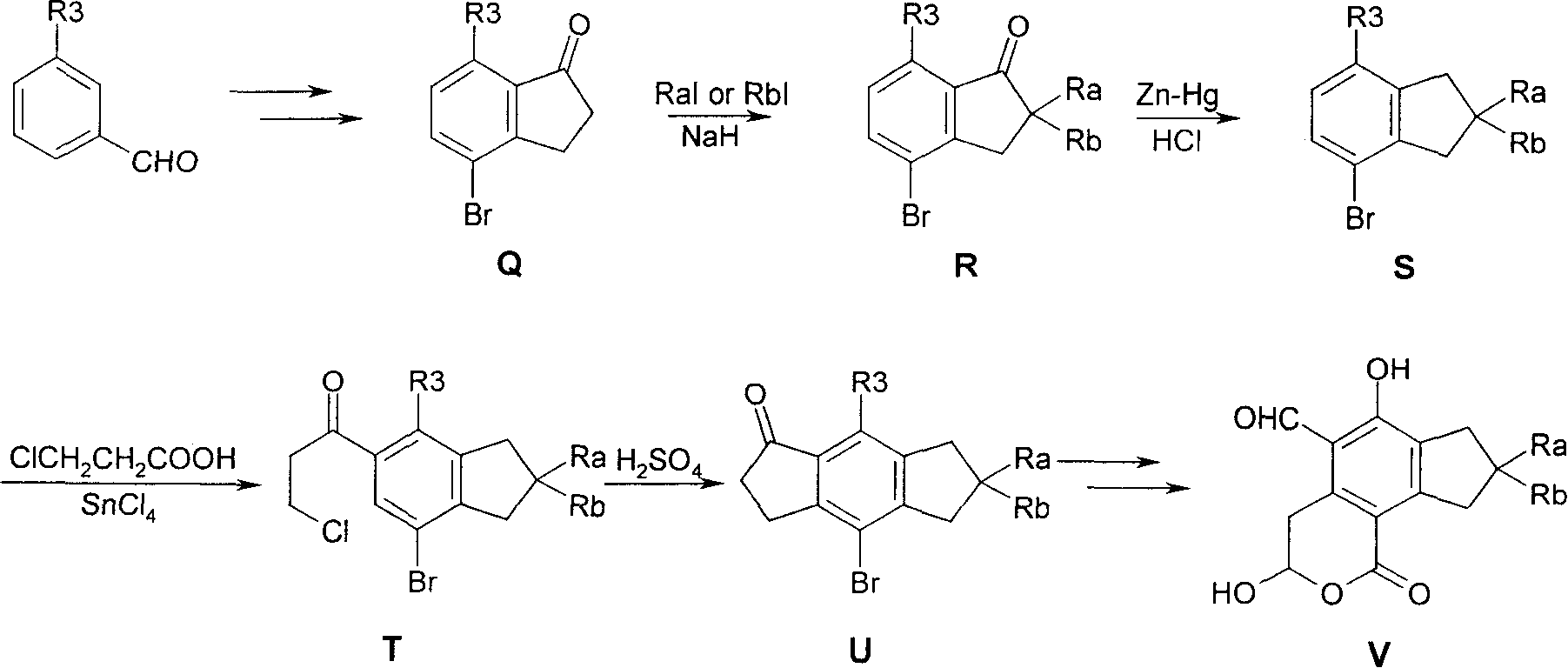
Substituted 2,3-dihydro-1H-benzo[a]pyrano[2,3-c]phenazines as anti-angiogenic and anti-cancer agents
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN +1
Solid-phase synthesis method of coumarin and analogue thereof
InactiveCN102532015BHigh yieldOvercome the disadvantage of low yield of cyclization reactionOrganic chemistryMeth-Polystyrene
The invention relates to a solid-phase synthesis method of coumarin and an analogue (I) thereof and belongs to the field of organic chemistry. The method comprises the following steps: 1) taking 1% of cross-linked polystyrene resin as a carrier to prepare a polystyrene-supported seleno-succimide reagent (III); 2) using the III to induce phenyl acrylate (V) to perform intramolecular cyclization under the catalysis of trimethylsilyl trifluoromethanesulfonate so as to form 3-polystyrene-supported seleno-3,4-dihydro-benzopyran-2-ketone (VI); and 3) performing oxidation elimination on the VI via an oxidant so as to directly get the coumarin (I) without further separation. When the phenyl acrylate (V) is replaced by N-phenyl acrylamide, the analogue of the coumarin, namely a 2-quinolone compound, can be prepared through the same steps. The solid-phase synthesis method disclosed by the invention has the advantages of easily available raw materials, good product yield, high purity, simplicity and convenience in operation, simple post-treatment and great industrial application prospects.
Owner:YUNNAN UNIV
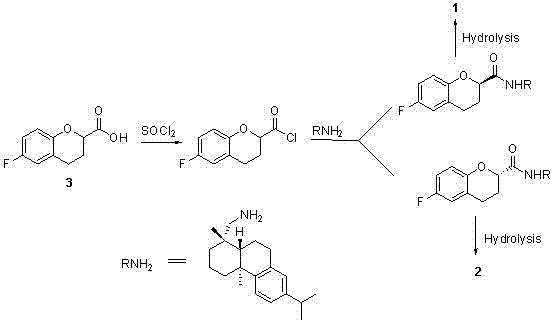
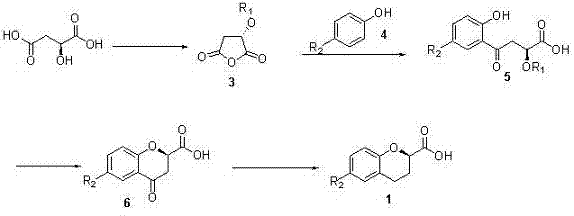
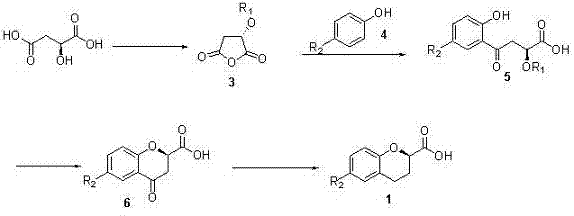
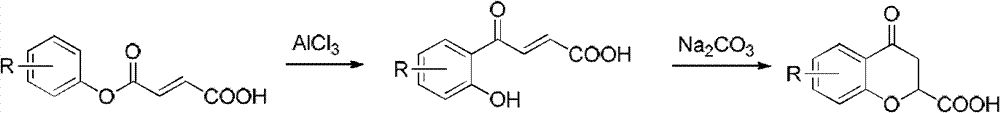
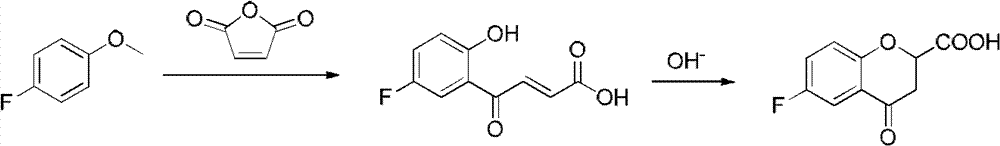
Synthesis method of (R) or (S)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid
The invention relates to a synthesis method of (R) or (S)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid and in particular relates to a synthesis method of an intermediate of 2-hydroxyl-2-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)acetonitrile (nebivolol hydrochloride). The method comprises the following steps: using optically pure (R) or (S)-malic acid and the corresponding acyl chloride or anhydride containing R1 groups to react to obtain a compound 3; using the compound 3 and a p-substituted phenol compound to perform a Friedel-Crafts reaction in the presence of an lewis acid to obtain a compound 5; using the compound 5 to perform an intramolecular SN2 substitution reaction under the action of a base and obtain a cyclized product 6; and hydrogenizing the carbonyl groups of the compound 6 in the presence of palladium-charcoal and performing benzyl reduction to obtain the target product 1. The method has the advantages of cheap and available starting material, fewer reaction steps, high synthesis efficiency and the like; and the defects of the prior art can be better overcome.
Owner:SHANGHAI RECORD PHARM CO LTD +1
Crystalline Forms and Processes for Their Preparation
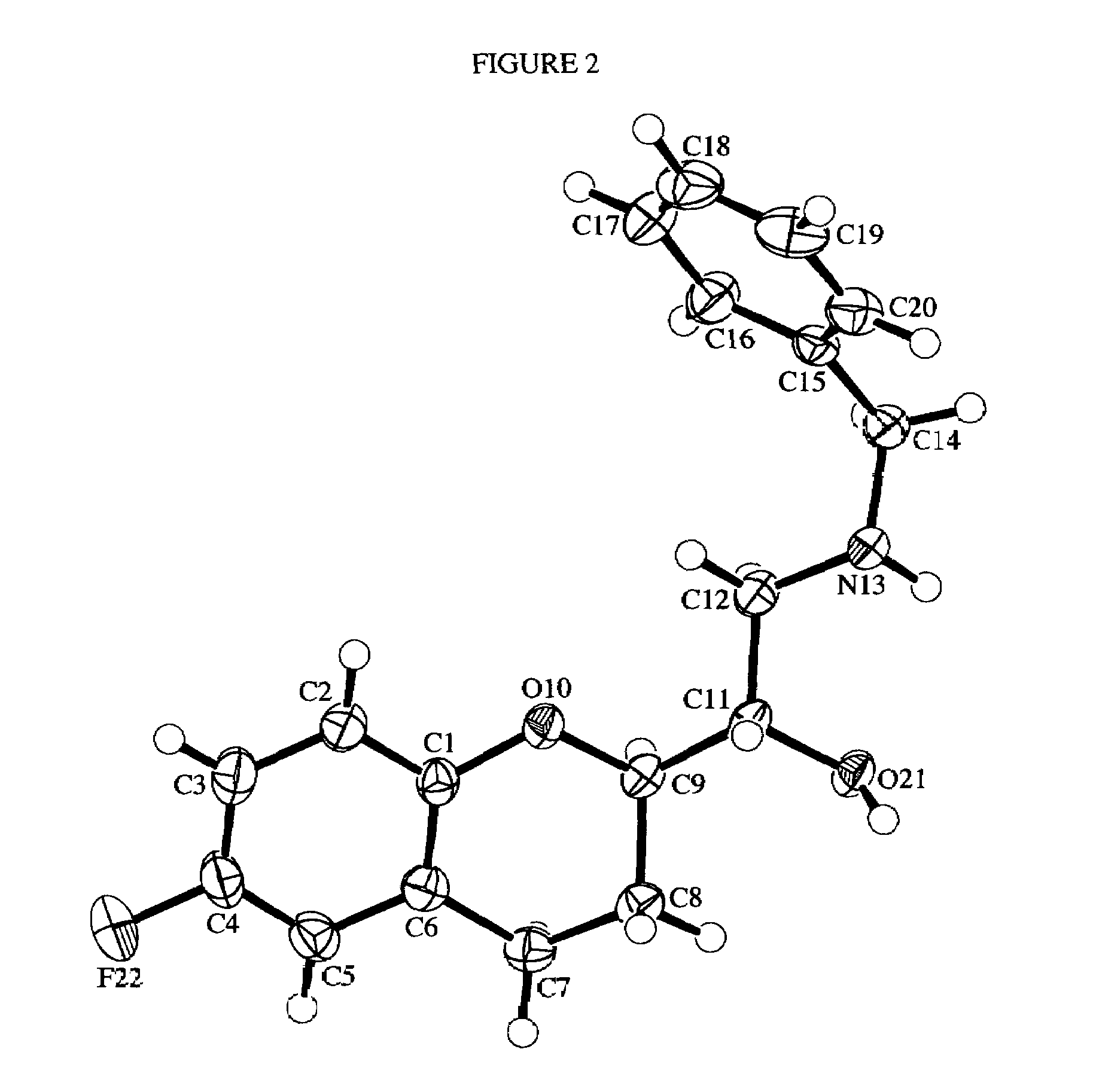
InactiveUS20140031561A1Improve solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistryBenzopyranMedicine
The present invention relates to crystalline Form A of 1-[(3R)-6,8-difluoro-3,4-dihydro-2H-1-benzopyran-3-yl]-1,3-dihydro-5-[2-[(phenylmethyl)amino]ethyl]-2H-imidazole-2-thione and crystalline Form B of 1-[(3R)-6,8-difluoro-3,4-dihydro-2H-1-benzopyran-3-yl]-1,3-dihydro-5-[2-[(phenylmethyl)amino]ethyl]-2H-imidazole-2-thione, processes for preparing the forms and their uses in medicine. The present invention also relates to the amorphous form of 1-[(3R)-6,8-difluoro-3,4-dihydro-2H-1-benzopyran-3-yl]-1,3-dihydro-5-[2-[(phenylmethyl)amino]ethyl]-2H-imidazole-2-thione processes for preparing it and its uses in medicine.
Owner:BIAL PORTELA & CA SA
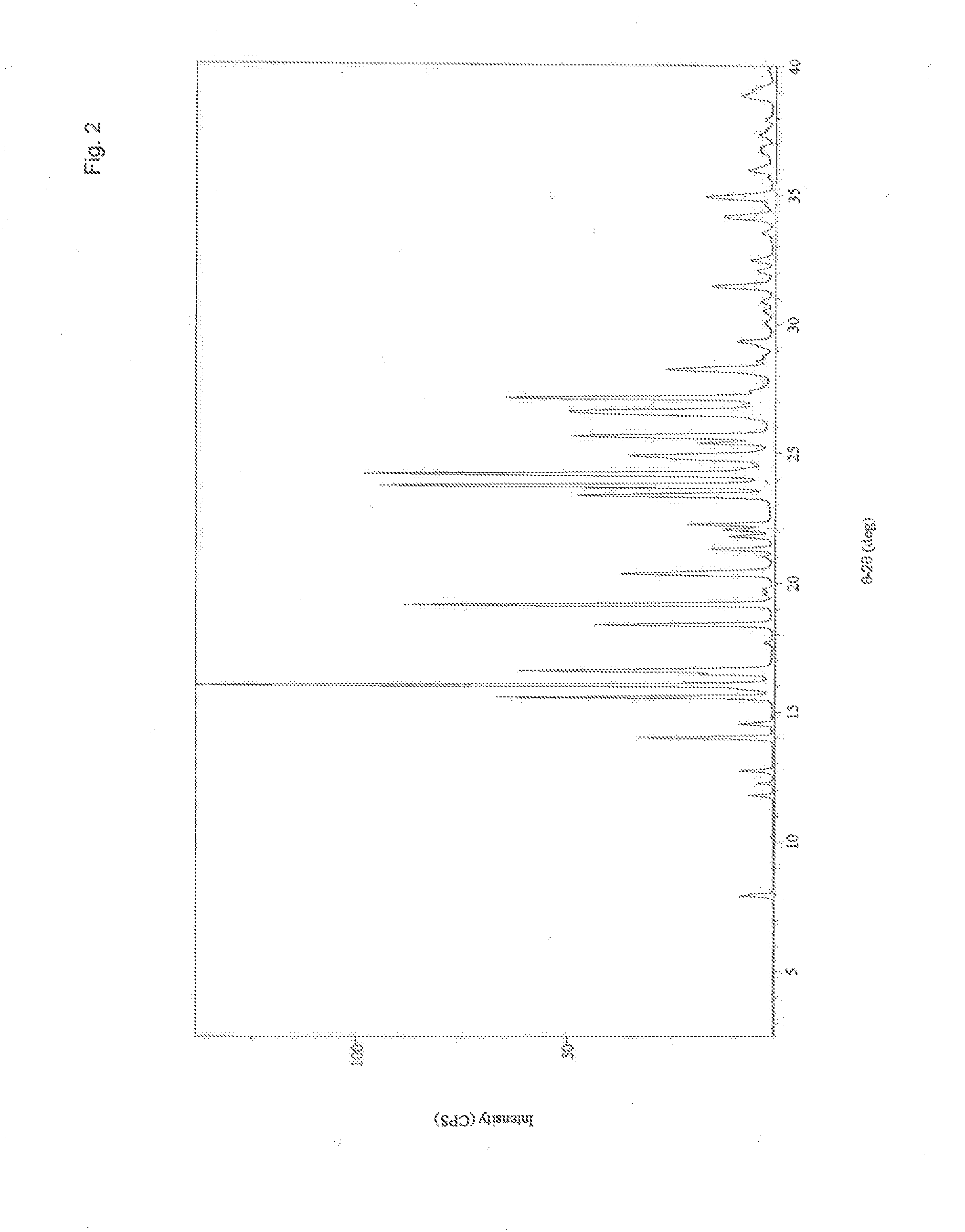
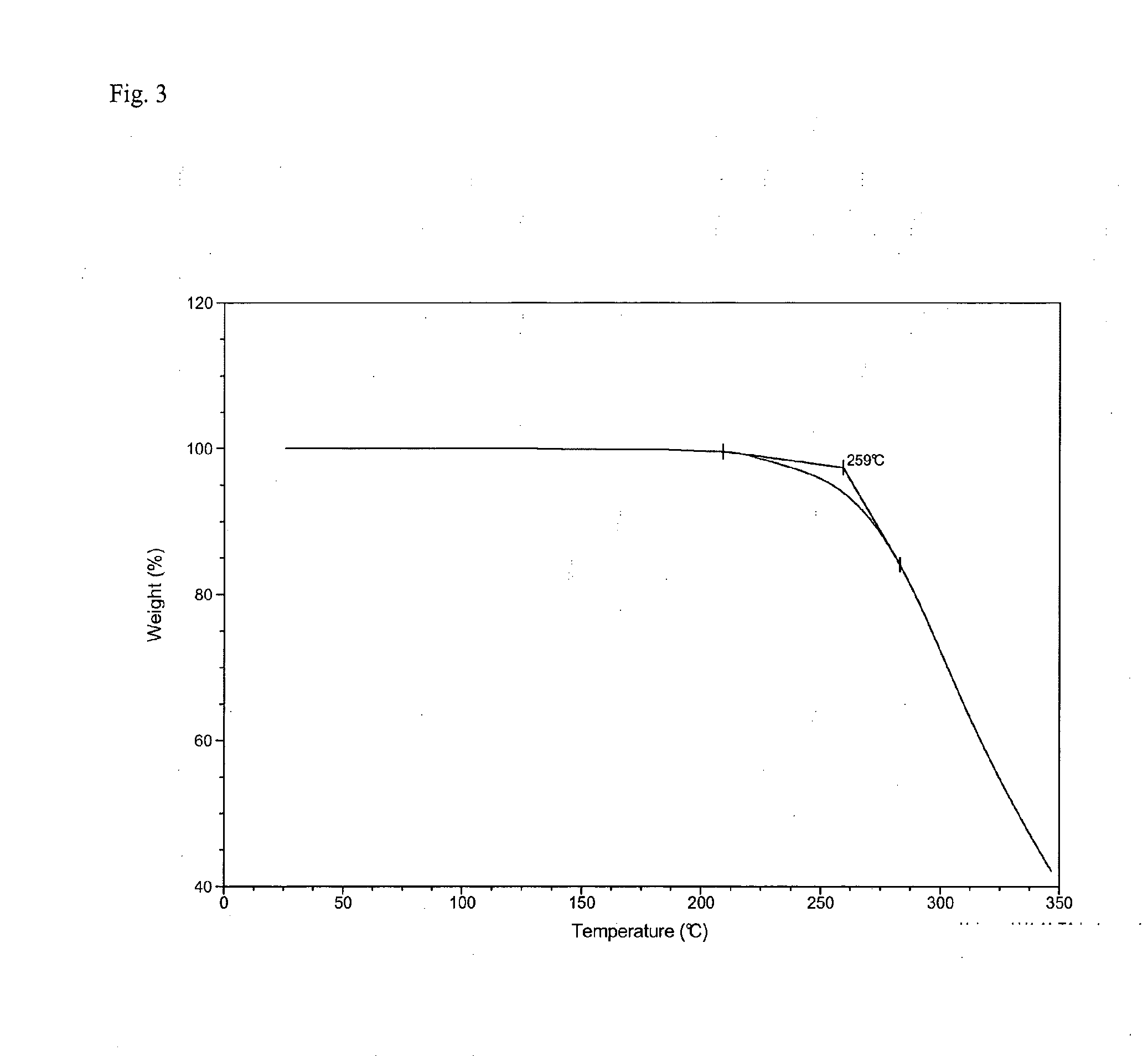
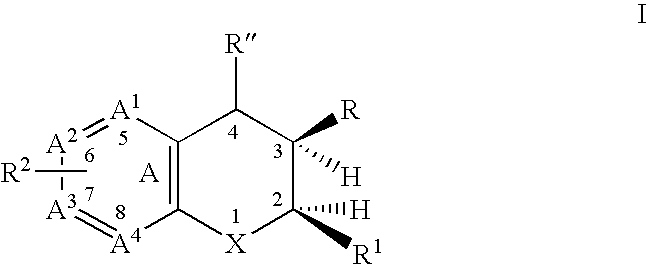
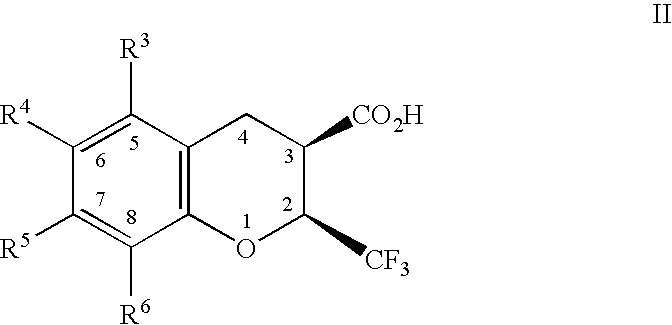
Dihydrobenzopyrans, dihydrobenzothiopyrans, and tetrahydroquinolines for the treatment of COX-2-mediated disorders
A class of dihydrobenzopyrans, dihydrobenzothiopyrans, tetrahydroquinolines, tetrahydronaphthalenes, and analogs thereof, is described for use in treating cyclooxygenase-2 mediated disorders. Compounds of particular interest are defined by Formula (I) wherein X, A1, A2, A3, A4, R, R″, R1 and R2 are as described in the specification.
Owner:GD SEARLE & CO
Medicine for reducing blood pressure
InactiveCN107868094AInhibition releaseOrganic active ingredientsOrganic chemistryExternal calciumKetone
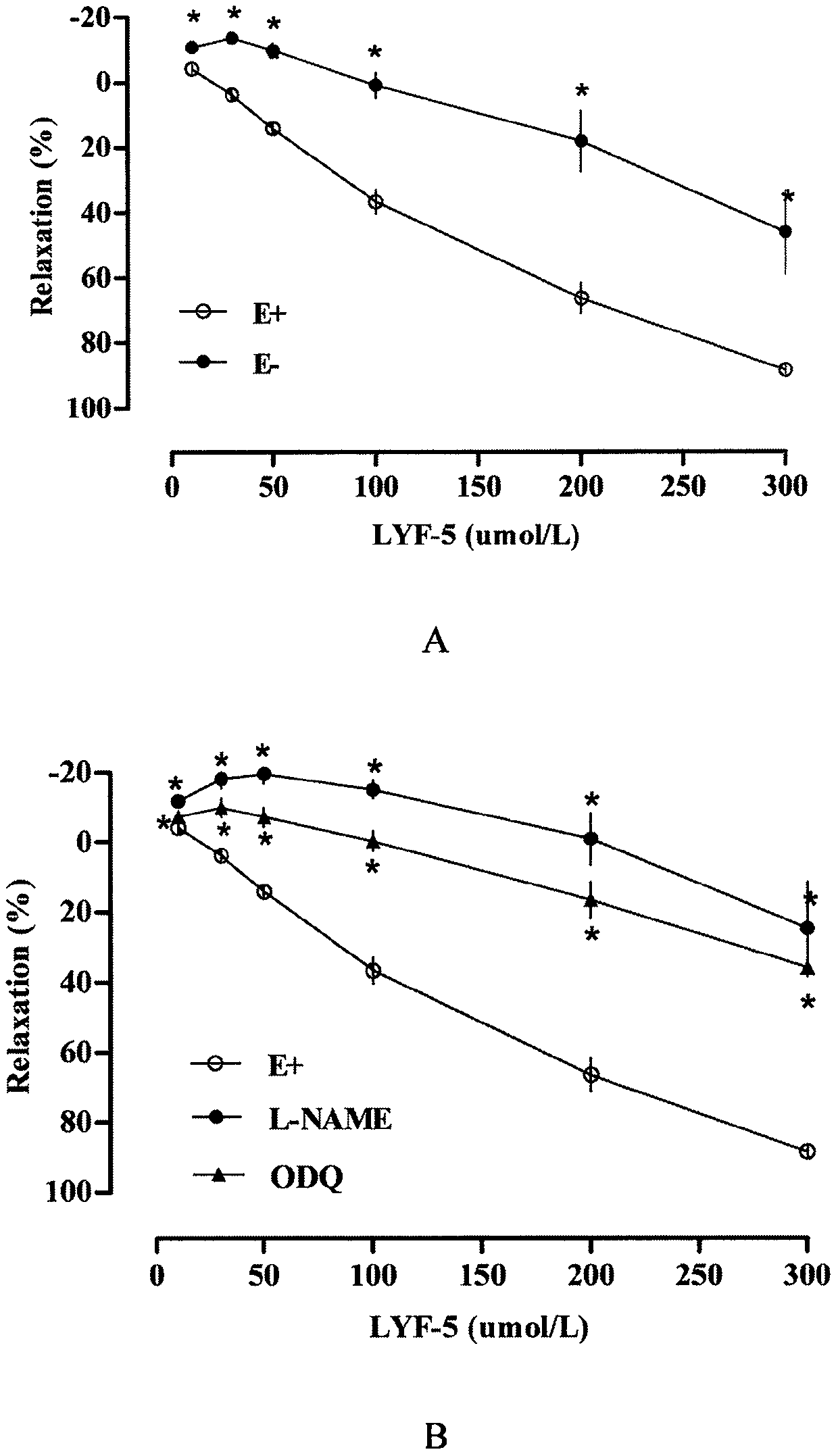
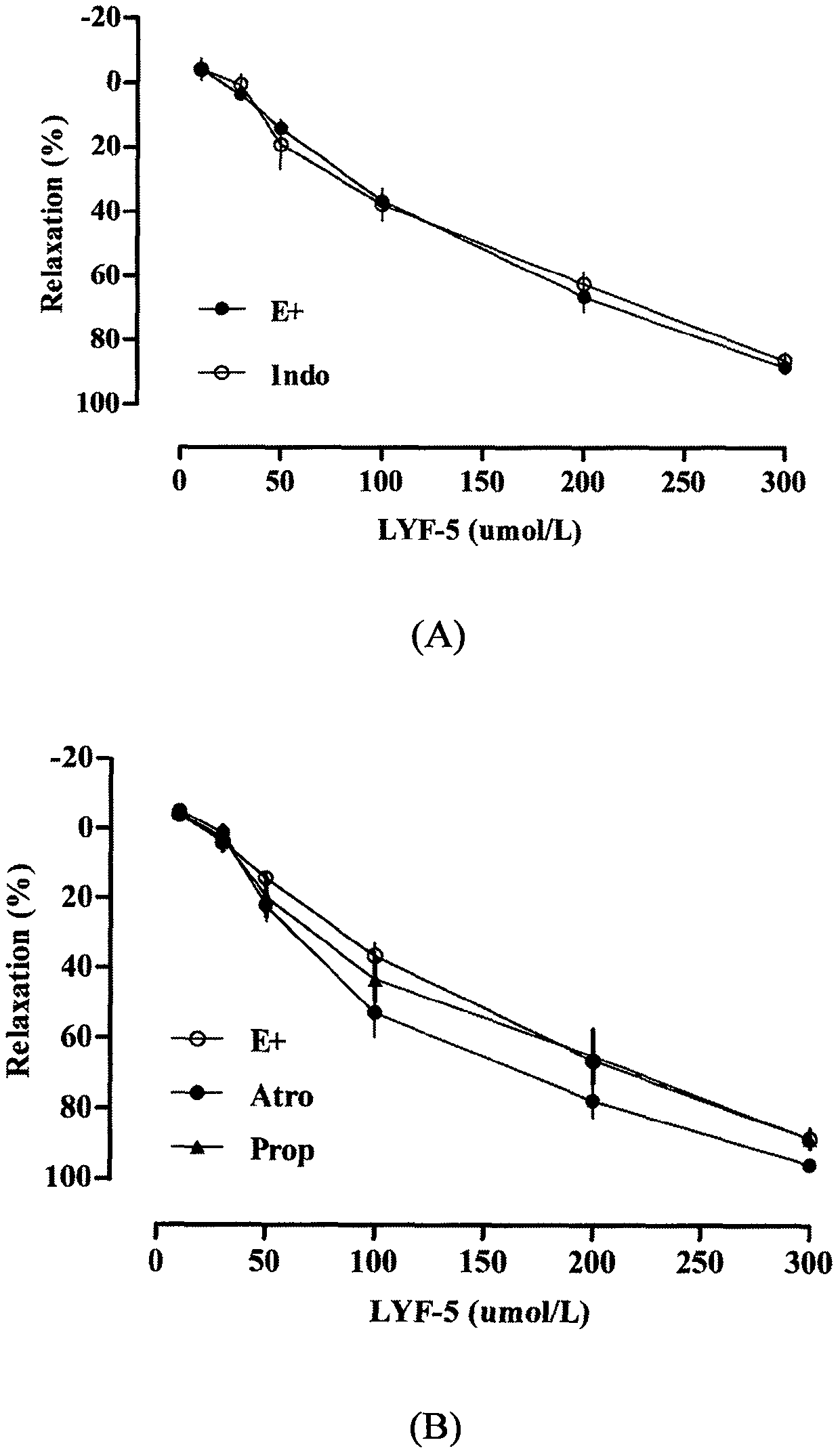
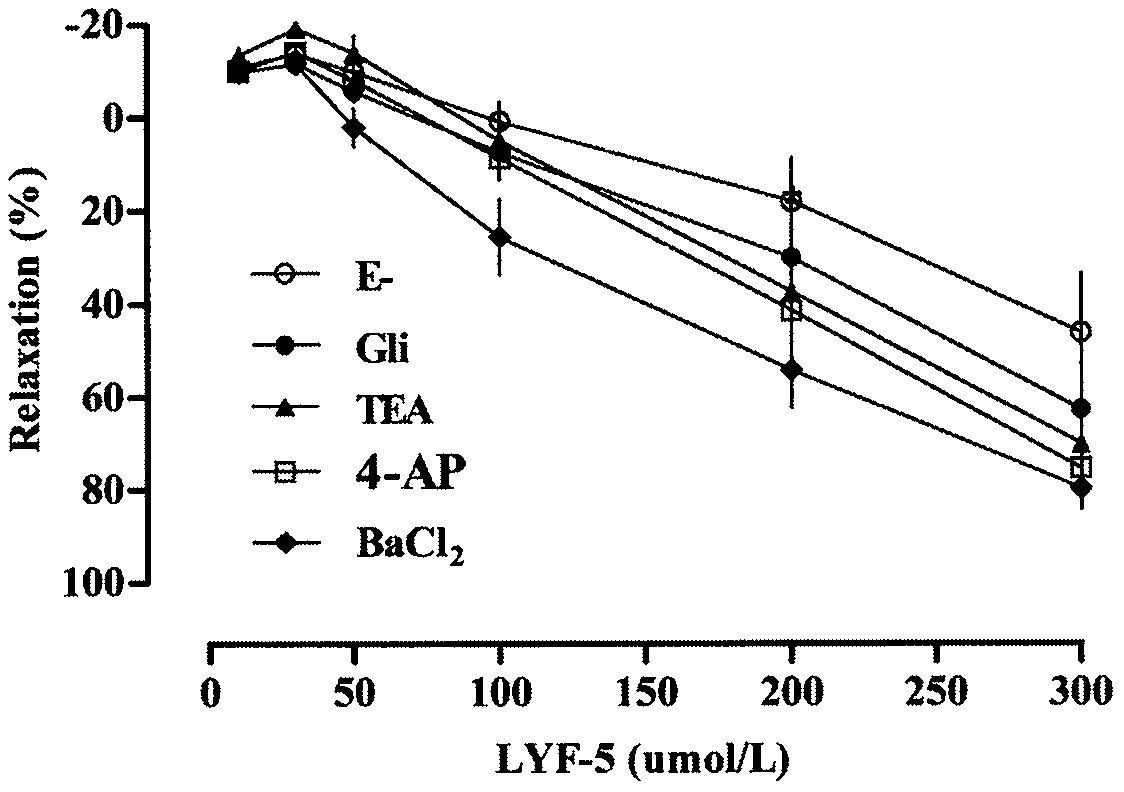
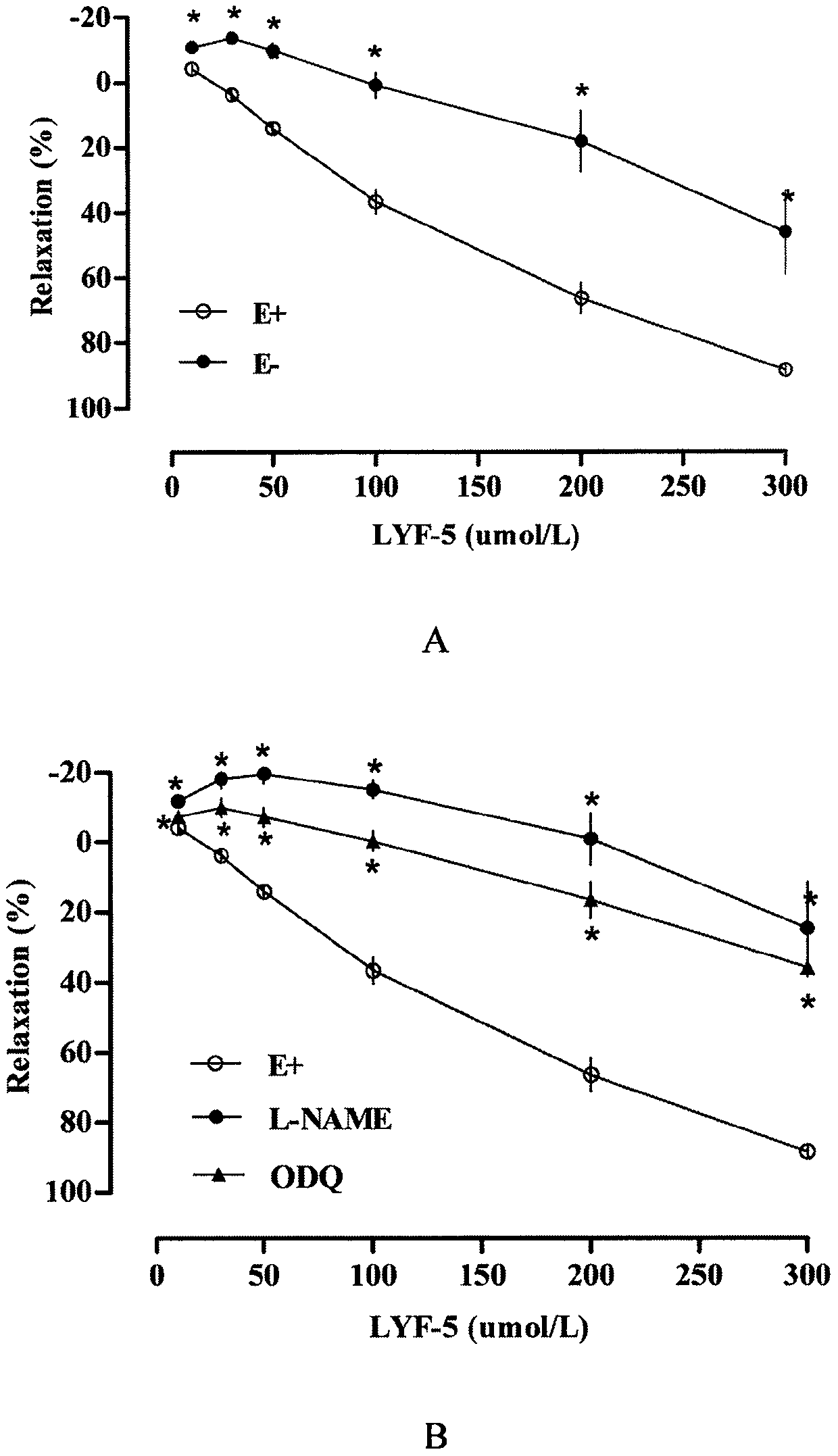
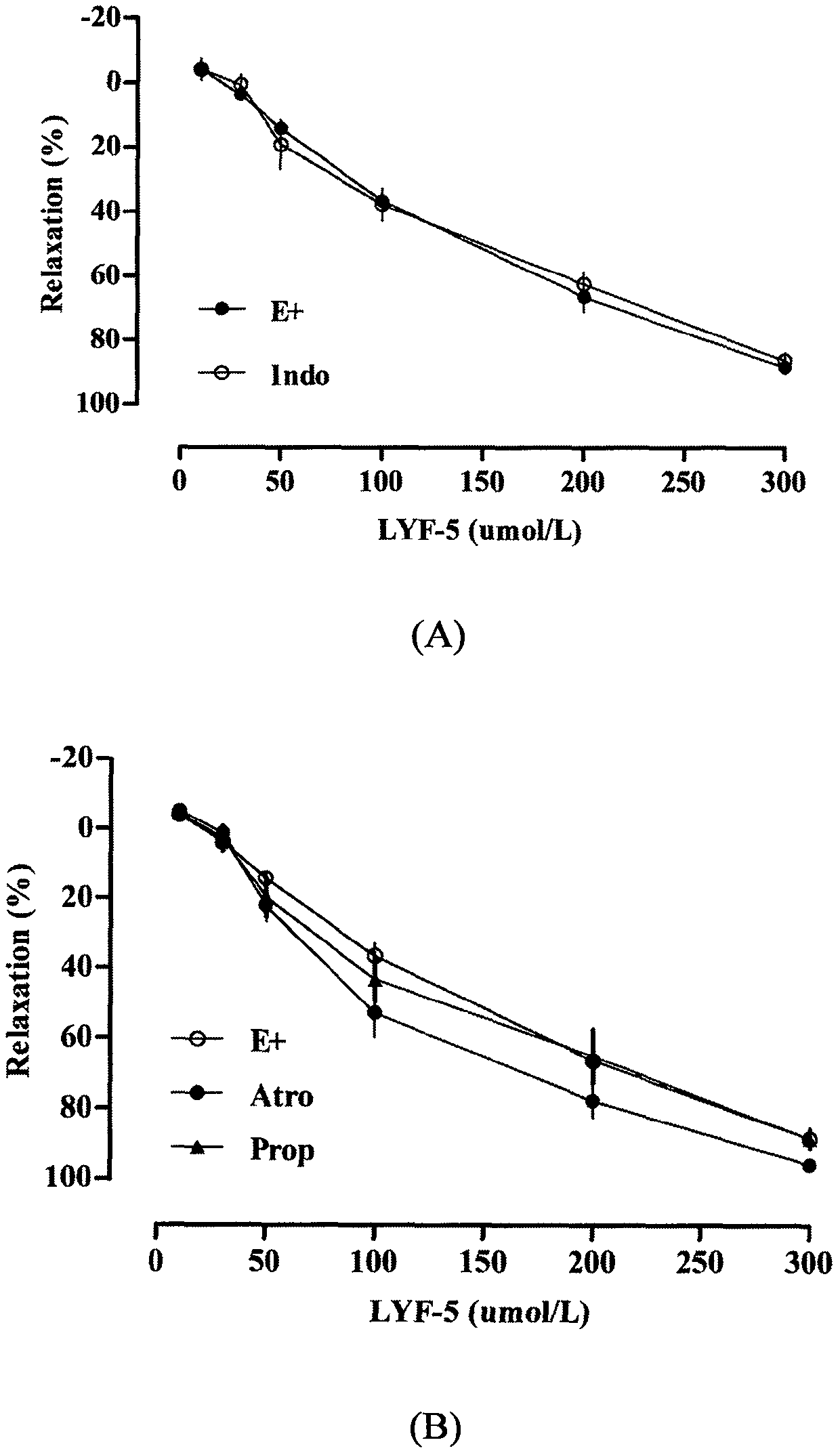
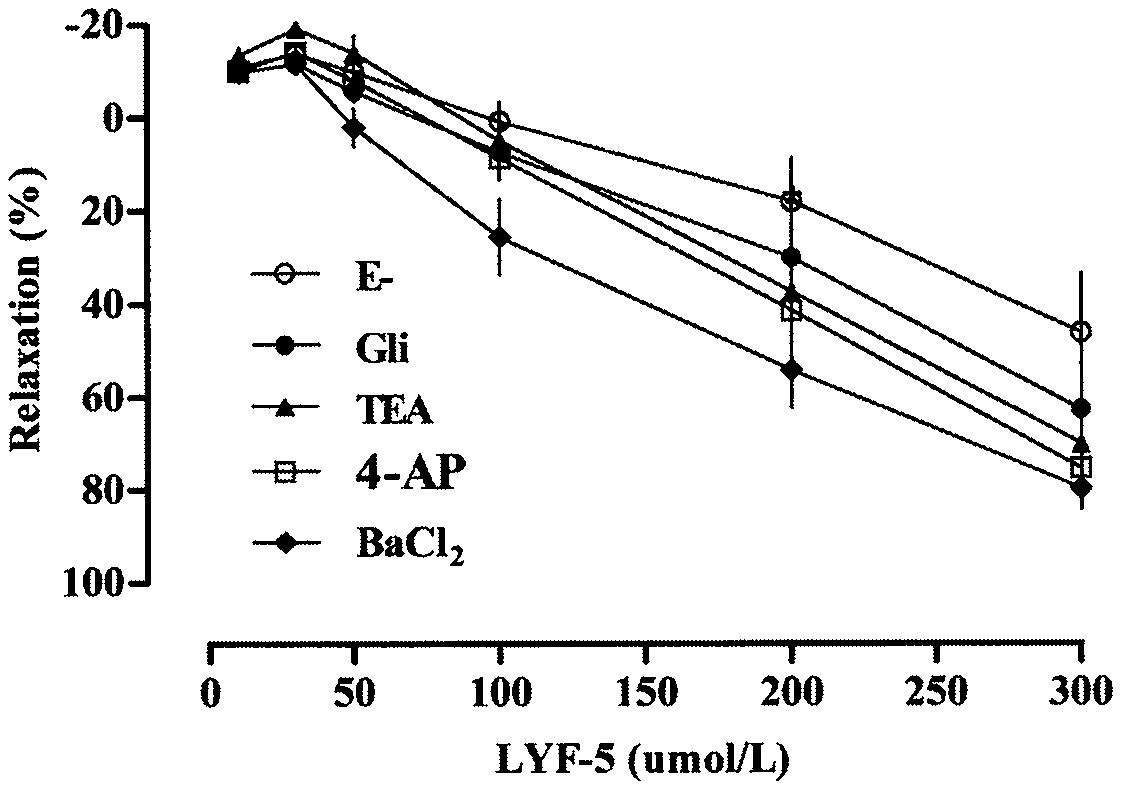
The invention belongs to the technical field of medicine and discloses a medicine for reducing blood pressure. The molecular formula of the medicine for reducing blood pressure is 9-octyl-9,10-dihydrobenzopyran [8,7-e][1,3]oxazine-2(8H)-ketone(LYF-5). The LYF-5 has endothelium and non-endothelium dependent vasodilatation effect; the mechanism and the endothelium dependent NO-cGMP signal pathway and the non-endothelium dependent signal pathway mainly inhibit external calcium from flowing inside and inhibit calcium on a sarcoplasmic reticulum from releasing through a VDCC; and the result of theinvention provides a clue for developing the LYF-5 into a new medicine for reducing blood pressure.
Owner:YANBIAN UNIV
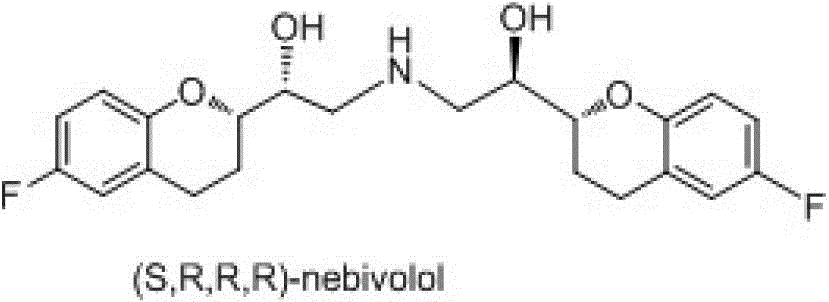
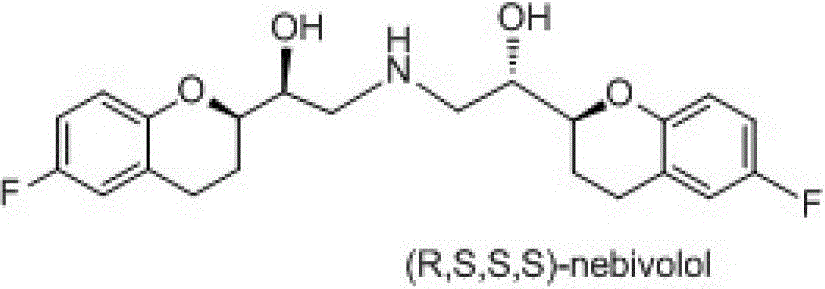
Preparation method of nebivolol and its intermediate compound
The invention discloses a preparation method of nebivolol used for preparing medicines for treating hypertension of slight or medium degrees, and an intermediate compound. The preparation method comprises the following steps: taking 6-fluoro-2-(1-hydroxy-2-paratoluensulfonyl oxygroup-ethyl)-3,4-dihydrobenzopyrans as an initial raw material, introducing amino, then coupling with 6- fluoro-3,4-dihydro-2-epoxy ethyl-2H-1-benzopyran, and preparing (S,R,R,R) and (R,S,S,S)-nebivolol. Compared with a prior art, the preparation method has the advantages of novel design, simple operation and high yield, the usage of hazardous reagent such as ssodium azide and sodium hydride can be avoided, a column chromatography purifying method is avoided, so that the preparation method conforms to industrial production.
Owner:福安药业集团重庆博圣制药有限公司 +2
Synthetic process of chiral 2-amido-1-(6-fluorine-3,4-dihydrobenzopyranyl) alCohol
This invention is attributed to the field of organic chemistry and specifically relates to the method to synthesize drug intermediates (R)-2-amino-1-((R)-6-fluoro-3, 4-dihydrochromeno) ethanol and (R)-2-amino-1-((S)-6-fluoro-3, 4-dihydrochromeno) ethanol. The method is that, drug intermediates (2R)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone and (2S)-2-[(1R)-4, 4-dimethyl-3, 5-dioxocyclopentyl]-6-fluoro-4-chromanone are adopted as raw materials and reduced by Clemmensens method with the products reacting with p-toluenesulfonyl chloride in pyridine. The consequent products are dissolved in solvent and dry ammonia is introduced for heating reflux. The total yield can be as much as 32%.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
A kind of preparation method of nebivolol and intermediate compound thereof
The invention discloses a preparation method of nebivolol used for preparing medicines for treating hypertension of slight or medium degrees, and an intermediate compound. The preparation method comprises the following steps: taking 6-fluoro-2-(1-hydroxy-2-paratoluensulfonyl oxygroup-ethyl)-3,4-dihydrobenzopyrans as an initial raw material, introducing amino, then coupling with 6- fluoro-3,4-dihydro-2-epoxy ethyl-2H-1-benzopyran, and preparing (S,R,R,R) and (R,S,S,S)-nebivolol. Compared with a prior art, the preparation method has the advantages of novel design, simple operation and high yield, the usage of hazardous reagent such as ssodium azide and sodium hydride can be avoided, a column chromatography purifying method is avoided, so that the preparation method conforms to industrial production.
Owner:福安药业集团重庆博圣制药有限公司 +2
Compound, preparation method thereof and application thereof as feed additive
ActiveCN102464642BIncrease daily weight gainPromote digestion and absorptionOrganic chemistryDigestive systemDiseaseFunctional diarrhea
The invention discloses a compound, namely (2R, 3R)-((2-(3,4-dihydroxyphenyl)-5,7-dihydroxyl-4-oxo-2,3-dihydrobenzopyran-3-yl) methylamino) methyl-2-phenylpropionate. The invention also discloses a preparation method for the compound and the application of the compound as a feed additive. When serving as the feed additive, the compound can specifically promote the growth of intestinal mucosa villus of livestocks, promote the growth of intestinal linings, reduce the intestinal tension and inhibit abnormal peristalsis, can reduce the feed discharging speed of intestinal tracts of the livestocks so as to enhance the digestive absorption of the intestinal tracts to nutritional components, and can obviously increase the daily weight increasing of the livestocks. The reduction of the intestinal tension contributes to water absorption in the intestinal tracts, and plays an obvious role of convergence and reducing functional diarrhea. The compound provided by the invention also has an effect of repairing intestinal mucosa, and can also obviously improve the immunity function of intestinal mucosa and disease resistance of the livestocks.
Owner:谢联金
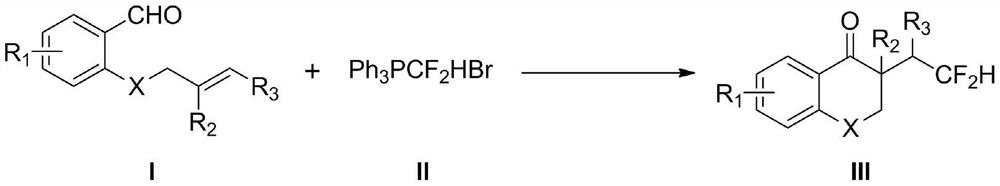
Method for synthesizing difluorohydromethylated 2, 3-dihydrobenzopyran-4-one derivative
PendingCN114853707AAvoid strong corrosiveAvoid cumbersome synthesisOrganic compound preparationCarboxylic acid esters preparationFluoroacetic acidEngineering
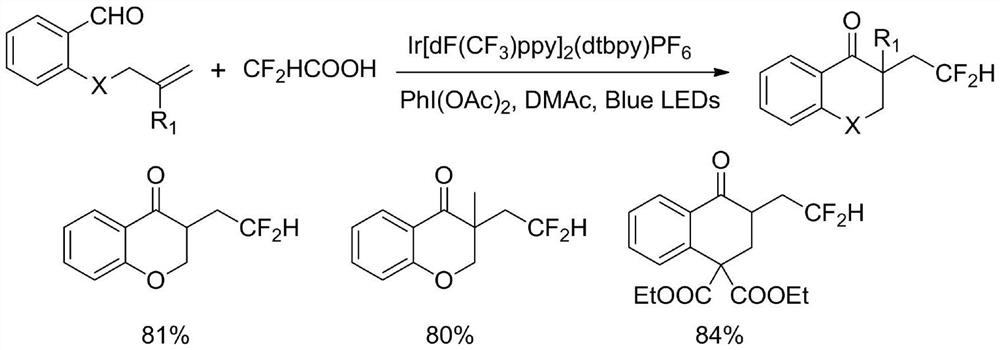
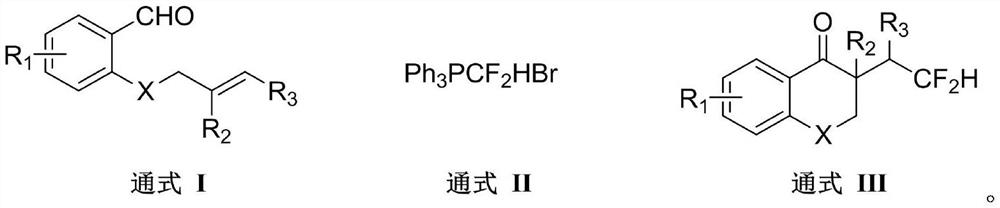
According to the method for synthesizing the difluorohydromethylated 2, 3-dihydrobenzopyran-4-one derivative, difluorohydromethyl triphenylphosphonium bromide is adopted as a difluorohydromethylation reagent, tris (2-phenylpyridine) iridium is adopted as a photocatalyst, and the difluorohydromethylated 2, 3-dihydrobenzopyran-4-one derivative is synthesized under the conditions of alkali and illumination. According to the invention, difluoromethyl triphenylphosphonium bromide which is stable and easy to obtain is used as a difluoromethylation reagent, so that the defects of strong corrosivity, irritation and toxicity of difluoroacetic acid are overcome. The stable, easily available and commercialized tris (2-phenylpyridine) iridium is used for replacing (Ir [dF (CF3) ppy] 2 (dtbpy) PF6) as a photocatalyst, so that the tedious synthesis of a complex photocatalyst is avoided. The method has the advantages of simple process flow, easily available raw materials, mild reaction conditions, simple and efficient operation, wide substrate application range, stable and easily controllable process, safety and environmental friendliness, and is suitable for industrial production.
Owner:SHANGRAO NORMAL UNIV
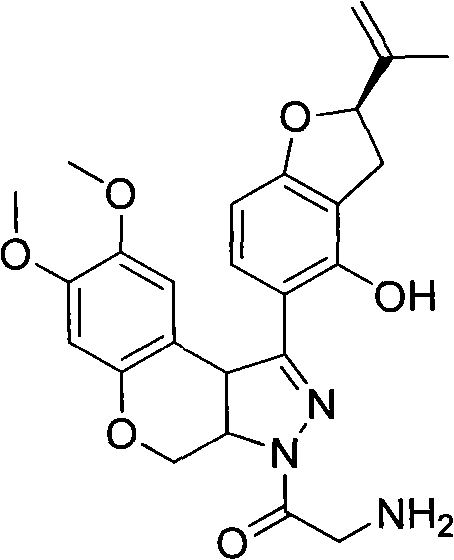
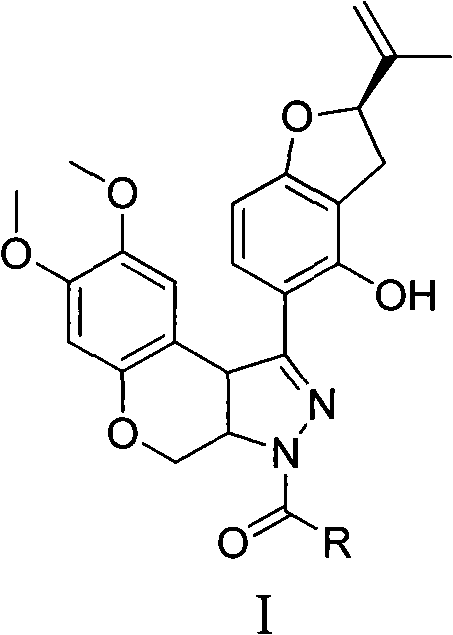
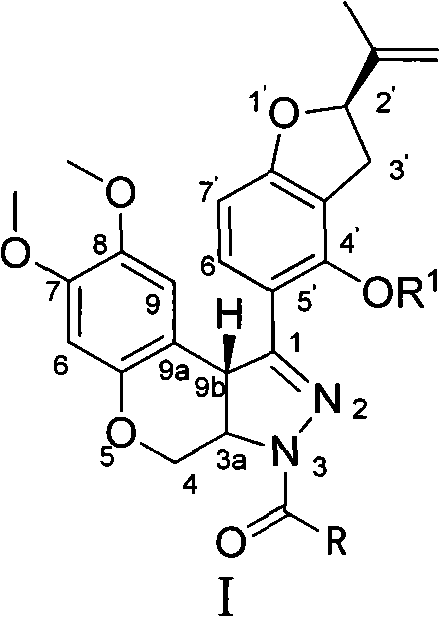
N-acylpyrazole derritol, preparation method thereof, and application thereof
InactiveCN102399229BHigh anti-neuraminidase activityGood anti-neuraminidase activityOrganic active ingredientsOrganic chemistry2-groupPhenol
The invention discloses N-acylpyrazole derritol with a chemical formula represented by the formula I. In the formula, R is selected from C1-C2 alkyl, C3-C4 straight-chain or branched-chain alkyl, and C6H5; X(CH2)n, X=OH, NH2, NHAc, Cl, Br, or C6H5; and n=1, 2, 3 or 4. The preparation method of N-acylpyrazole derritol comprises steps that: (2R)-5-[7,8-dimethoxy-3,3a,4,9b-tetrahydrobenzopyrano[3,4-c]pyrazole-1-group]-2-(propylene-2-group)-2,3-dihydrobenzopyran-4-phenol and acyl chloride or anhydride are subject to a reaction according to a molar ratio of 1:1, such that N-acylpyrazole derritol is obtained. The invention also provides an application of N-acylpyrazole derritol in the preparations of neuraminidase inhibitors.
Owner:HUNAN UNIV
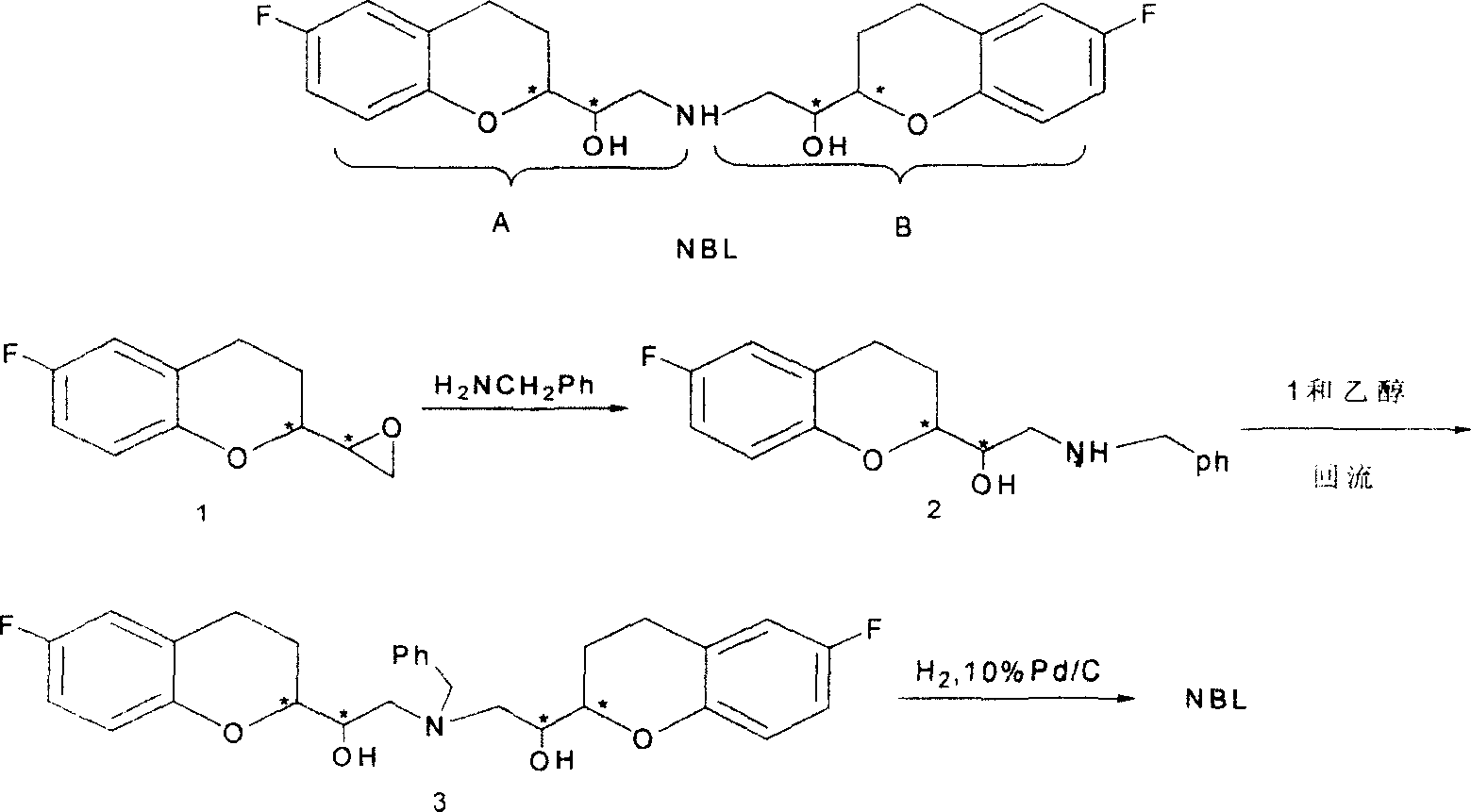
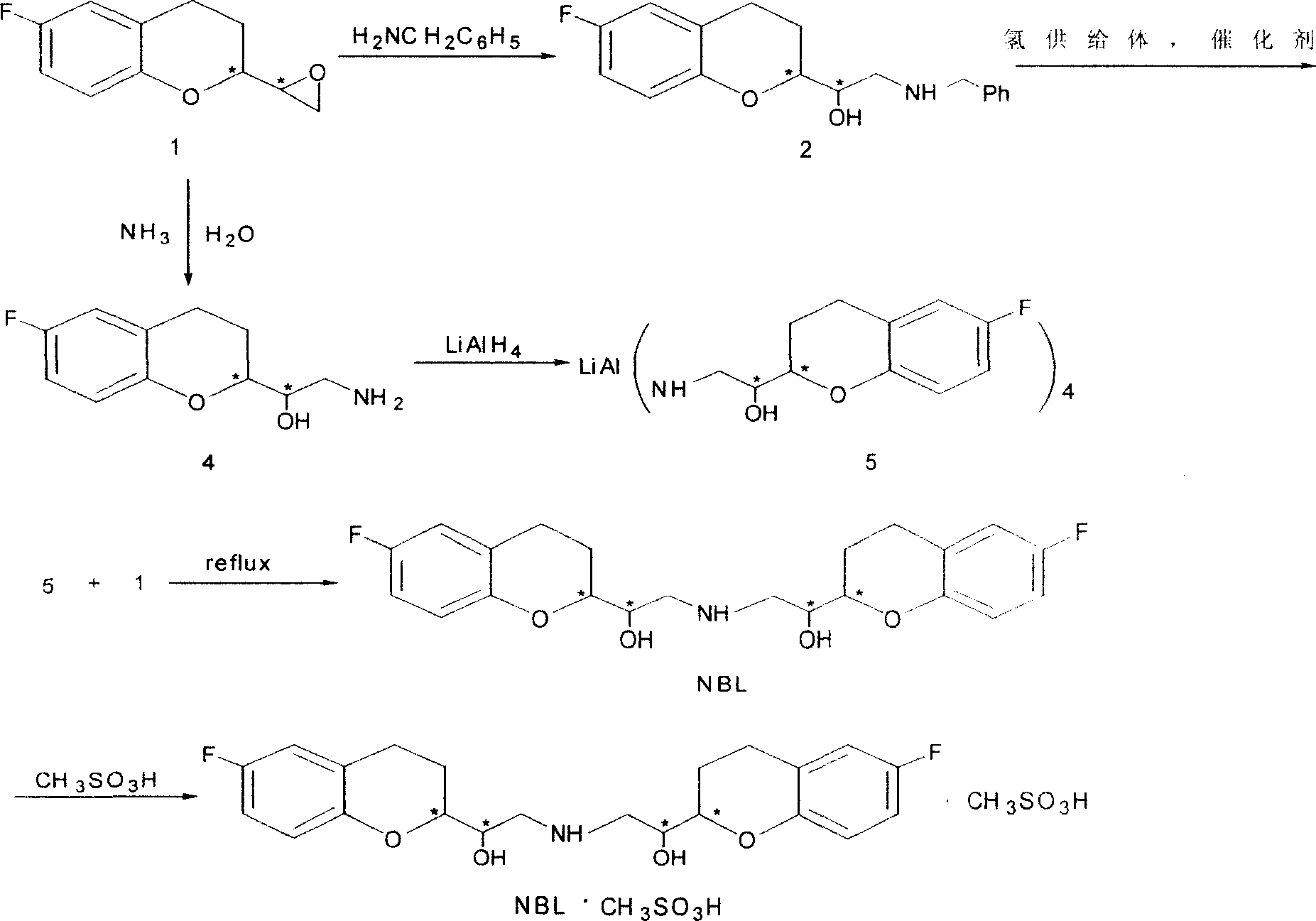
2,2'-[iminodi(methylene)di-(6-fluoro-3,4-dihydro-2H-1-benzenepyran-2- methanol) methane sulfosalt
InactiveCN100341866CSynthetic high purityHigh purityOrganic chemistryMethyl palmoxirateReceptor blockade
This invention relates to a synthetic method for beta1-receptor blocking agent and 2, 2'-[imino di(methylene)]-di-(6-fluorine-3, 4-dihydro-2H-1-benzopyrane-2-methanol) methanesulfonate. First, prepare 6-fluorine-3, 4-dihydro-1-[amino (methylene)]-2H-1-benzopyrane-2-methanol using 6-fluorine-3, 4-dihydro-2- oxirane group -2H-1- benzopyrane as raw material; then, use LiAlH4 to dispose and get the compound Li-Al amidate; finally, enable Li-Al amide to react with 6-fluorine-3, 4-dihydro-2-oxirane group-2H-1- benzopyrane to get NBL. Advantages:low cost, simple operation and high purity of NBL methanesulfonate.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Method for synthesizing 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid and alkyl ester thereof
The invention relates to a method for synthesizing 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid and alkyl ester thereof. 2,4-dibromoalkylbutyrate is used as a raw material, and the products are obtained by three-step reaction. According to the synthesizing method, the 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid and the alkyl ester thereof serving as target products are obtained without hydrogenation reduction of expensive Pd / C catalyst; and the reaction process is simple, the conditions are mild, the raw materials are easily obtained, and the yield is high.
Owner:上海立科化学科技有限公司
A kind of preparation method of 2,3-dihydrochromen-4-one derivative
The present invention provides a method for preparing a 2,3-dihydrobenzopyran-4-one derivative, and the method comprises the following steps: under nitrogen atmosphere at room temperature, dissolvinga 2-allyloxybenzaldehyde derivative represented by formula (I) and a benzoin derivative represented by formula (II) in an organic solvent, adding a photosensitizer, evenly mixing, placing under an ultraviolet lamp for light irradiation reaction, removing the solvent by rotary evaporation, and separating and purifying by silica gel column chromatography to obtain the resulting product 2,3-dihydrobenzopyran-4-one derivative. The preparation method solves the problems of cumbersome steps, low yield and poor environmental protection property of the existing synthesis method, can react under normaltemperature and normal pressure, and has the advantages of mild reaction conditions, no need of transition metal catalysis, simple operation, no pollution, safety, environmental protection, low costand the like. R1 and R2 are hydrogen, halogen or alkyl.
Owner:ZHEJIANG SHENGXIAO CHEM
a drug used to lower blood pressure
InactiveCN107868094BInhibition releaseOrganic active ingredientsOrganic chemistryPharmacologyLower blood pressure
Owner:YANBIAN UNIV
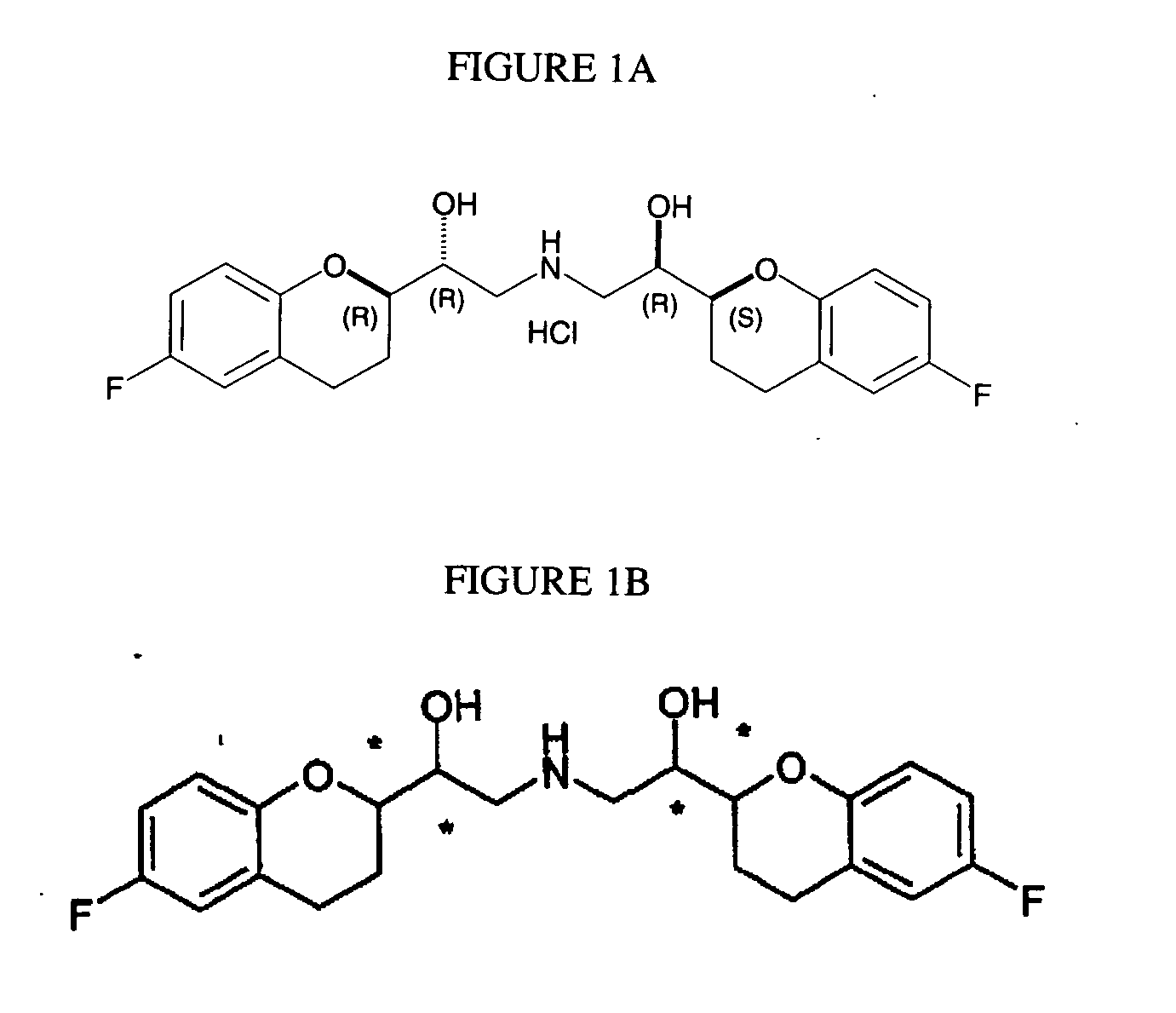
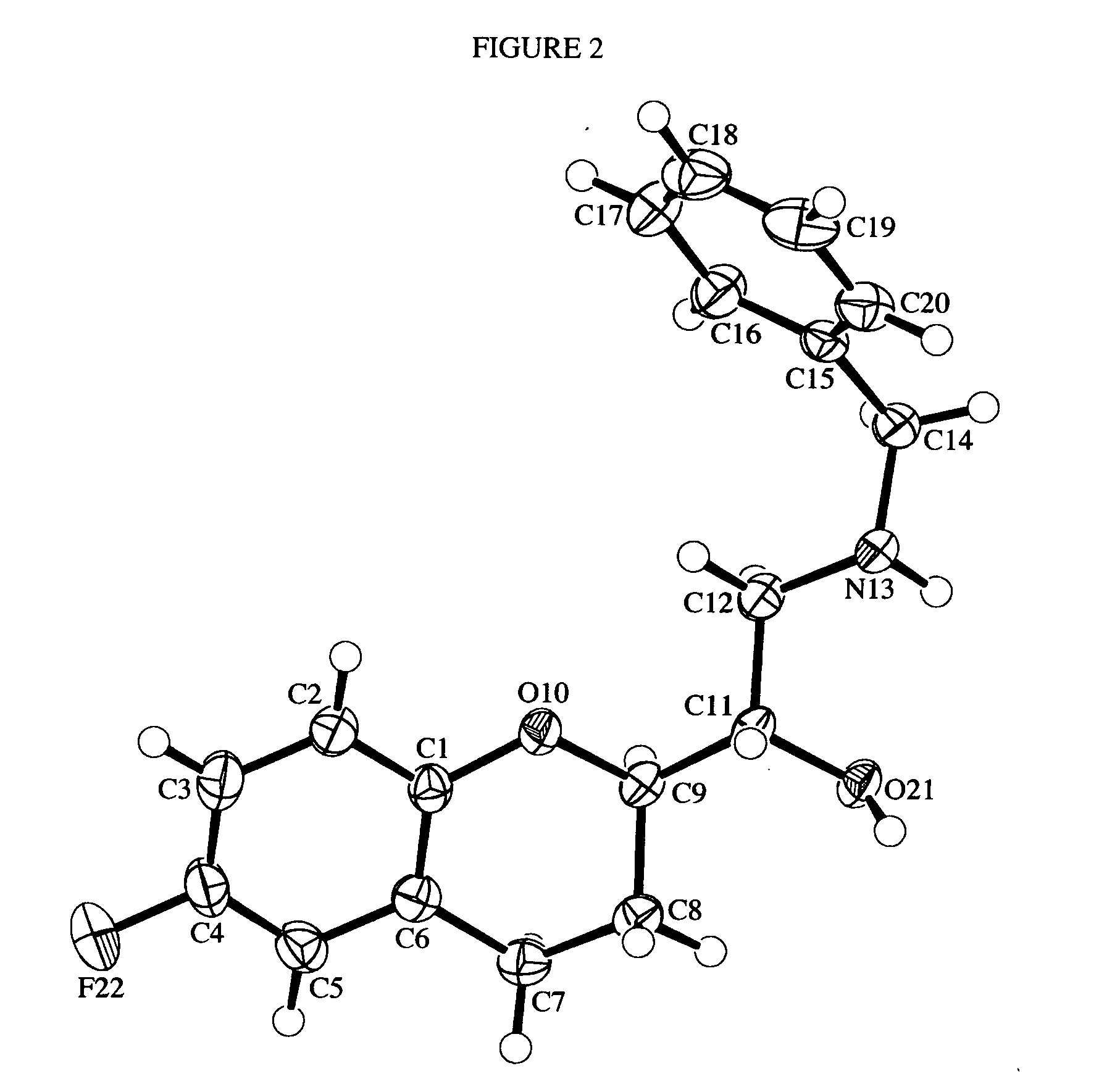
Substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound and preparation method thereof
InactiveCN105111220ALow priceReduce pollutionOrganic chemistryAntineoplastic agentsAcetic acidSilica column
The invention discloses a substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound. The structural formula is as shown in specifications. The invention further discloses a preparation method of the substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound. The preparation method includes the steps that olefine azido compounds and 4-hydroxycoumarin react for 8-12 hours at 80-100 DEG C under the existence of solvent and a metal catalyst; obtained reaction liquid is cooled to indoor temperature, then water and ethyl acetate are used for extraction, and an obtained organic layer is concentrated after being rinsed; obtained concentrate is subjected to silica column chromatography to obtain the substituted 1, 4-dihydro-chromene [2, 3-b] pyrrole compound. The compound is an anti-tumor lead compound.
Owner:ZHEJIANG UNIV
Dihydrobenzopyrans ketone compound and uses thereof
The invention relates to the new synthesized compound i.e. dihydrobenzo pyrone type and the application thereof, and belongs to the field of the chemical drug. The experiment results shows that the compound (I) can kill the breast cancer cell MCF7 expressing ER Alpha 66, FR Alpha 46 and ER Alpha 36. Moreover, the compound (I) can be served as the regulator of the estrogen receptor ER Alpha 36 fortreatment of the illness caused by the unusual expression of the estrogen receptor ER Alpha 36, such as the tumor, the osteoporosis, the asthma, the heart disease and the senile dementia, etc.
Owner:BEIJING SHENOGEN BIOMEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
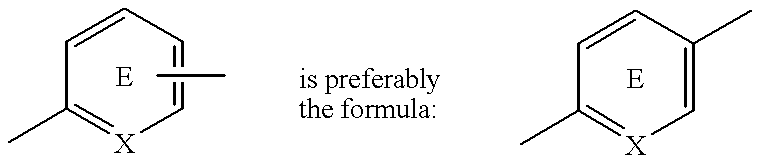
![Substituted 2,3-dihydro-1H-benzo[a]pyrano[2,3-c]phenazines as anti-angiogenic and anti-cancer agents Substituted 2,3-dihydro-1H-benzo[a]pyrano[2,3-c]phenazines as anti-angiogenic and anti-cancer agents](https://images-eureka.patsnap.com/patent_img/5ac36d10-ade6-40c6-8b41-c87dab499ab6/US09181265-20151110-D00001.PNG)
![Substituted 2,3-dihydro-1H-benzo[a]pyrano[2,3-c]phenazines as anti-angiogenic and anti-cancer agents Substituted 2,3-dihydro-1H-benzo[a]pyrano[2,3-c]phenazines as anti-angiogenic and anti-cancer agents](https://images-eureka.patsnap.com/patent_img/5ac36d10-ade6-40c6-8b41-c87dab499ab6/US09181265-20151110-C00001.PNG)
![Substituted 2,3-dihydro-1H-benzo[a]pyrano[2,3-c]phenazines as anti-angiogenic and anti-cancer agents Substituted 2,3-dihydro-1H-benzo[a]pyrano[2,3-c]phenazines as anti-angiogenic and anti-cancer agents](https://images-eureka.patsnap.com/patent_img/5ac36d10-ade6-40c6-8b41-c87dab499ab6/US09181265-20151110-C00002.PNG)
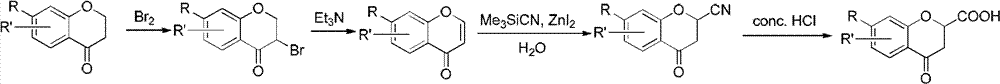
![Substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound and preparation method thereof Substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/891f5ab0-6cdf-4a87-8230-94ee6ba1687e/BDA0000801341030000011.PNG)
![Substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound and preparation method thereof Substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/891f5ab0-6cdf-4a87-8230-94ee6ba1687e/BDA0000801341030000021.PNG)
![Substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound and preparation method thereof Substituted 1,4-dihydro-chromene [2, 3-b] pyrrole compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/891f5ab0-6cdf-4a87-8230-94ee6ba1687e/BDA0000801341030000022.PNG)