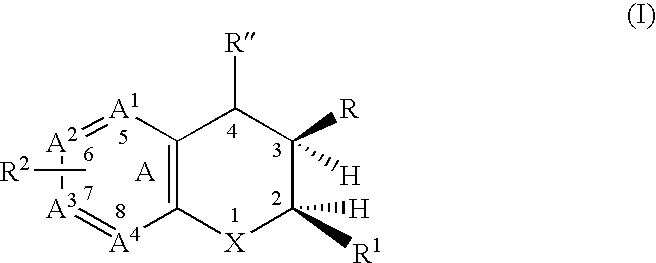
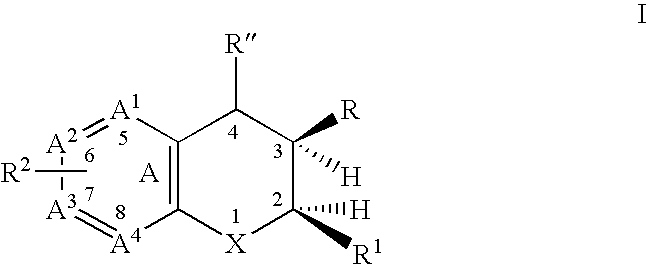
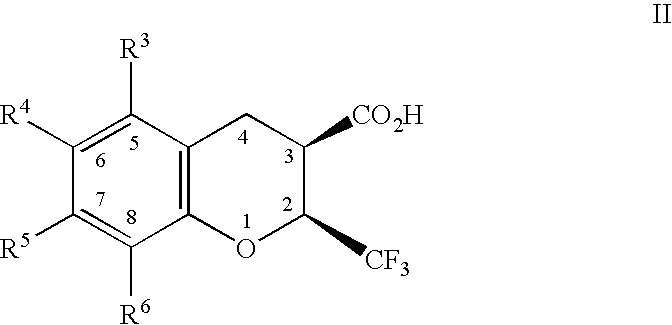
Dihydrobenzopyrans, dihydrobenzothiopyrans, and tetrahydroquinolines for the treatment of COX-2-mediated disorders
a technology of dihydrobenzopyrans and dihydrobenzothiopyrans, which is applied in the field of compounds, can solve the problems of limiting the therapeutic potential of compounds and more drastic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
rel-(2R,3S)-6,8-dichloro-3,4-dihydro-2-(trifluoromethyl)-2H-1-benzothiopyran-3-carboxylic acid
6,8-Dichloro-2-trifluoromethyl-2H-1-benzothiopyran-3-carboxylic acid, prepared as described in WO98 / 47890, (0.32 g, 0.97 mmol) was placed in a Fisher & Porter™ tube with THF (30 mL), and platinium(IV) oxide (0.47 g). The tube was pressurized to 34 psi with hydrogen gas and the reaction mixture stirred at room temperature for about 23 hours. The reaction mixture was filtered through diatomaceous earth, concentrated in vacuo and passed through a column of silica gel with ethyl acetate-hexane-acetic acid (20:8.0:2) as the eluent, to give a white solid which was recrystallized from isooctane-hexane yielding a white crystalline solid (0.10 g, 31%): mp 165.0-170.2° C. 1H NMR (acetone-d6 / 300 MHz) 7.34 (s, 1H), 7.28 (s, 1H), 4.63-4.69 (m, 1H), 3.40 (d, 1H, J=13.5 Hz), 3.29 (d, 1H, J=18.1 Hz), 3.04-3.16 (m, 1H). 19F NMR (acetone-d6 / 282 MHz) −67.3 (d, J=8.7 Hz). FABLRMS m / z 329 (M−H). ESHRMS m / z 34...
example 2
(2S,3R)-6,8-dichloro-3,4-dihydro-2-(trifluoromethyl)-2H-1-benzopyran-3-carboxylic acid
A 500 mL Parr™ shaker bottle was charged with platinum (IV) oxide (2.99 g) and acetic acid (120 mL) pressurized with hydrogen gas (50 psi) and shaken for 0.75 hours. The hydrogen was vented and the reactor was charged with (2S)-6,8-dichloro-2-(trifluoromethyl)-2H-1-benzopyran-3-carboxylic acid (10.21 g, 32.62 mmol) and pressurized with hydrogen gas (25 psi). After 3 h, the reactor was vented, additional platinum (IV) oxide (1.59 g) was added, the reactor was pressurized with hydrogen gas (25 psi) and shaken for 2 h longer. The crude product mixture was filtered through diatomaceous earth and the filtrate concentrated in vacuo.
The resulting crude product was purified by flash silica chromatography (hexanes-ethyl acetate, 3:1) followed by reverse phase chromatography [C18 stationary phase, CH3CN—H2O with 0.1% TFA (gradient 15:85 to 50:50)] providing 5.02 g of a white, oily foam. This foam was fur...
example 3
rel-(2R,3S)-5,6-dichloro-3,4-dihydro-2-(trifluoromethyl)-2H-1-benzopyran-3-carboxylic acid
Prepared by a procedure similar to that described in EXAMPLE 1. mp 173.0-174.4° C. 1H NMR (acetone-d6 / 300 MHz) 7.43 (d, 1H, J=8.9 Hz), 7.03 (d, 1H, J=8.9 Hz), 5.19-5.22 (m, 1H), 3.59-3.69 (m, 1H), 3.23 (dd, 1H, J=17.9 Hz, 6.2 Hz), 3.13 (dd, 1H, J=17.9 Hz, 7.5 Hz). 19F NMR (acetone-d6 / 282 MHz)-74.5 (d, J=7.2 Hz). FABLRMS m / z 313 (M−H). ESHRMS m / z 312.9635 (M−H, Calc'd 312.9646). Anal. Calc'd for C11H7Cl2F3O3: C, 41.93; H, 2.24. Found: C, 41.52; H, 2.53.
PUM
| Property | Measurement | Unit |
|---|---|---|
| swelling | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com