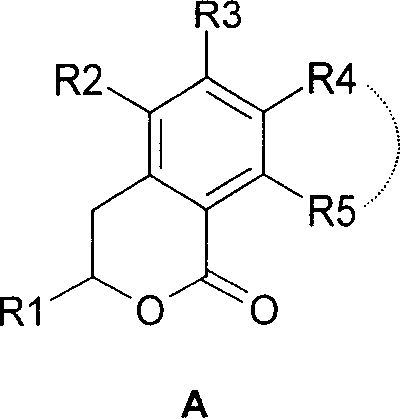
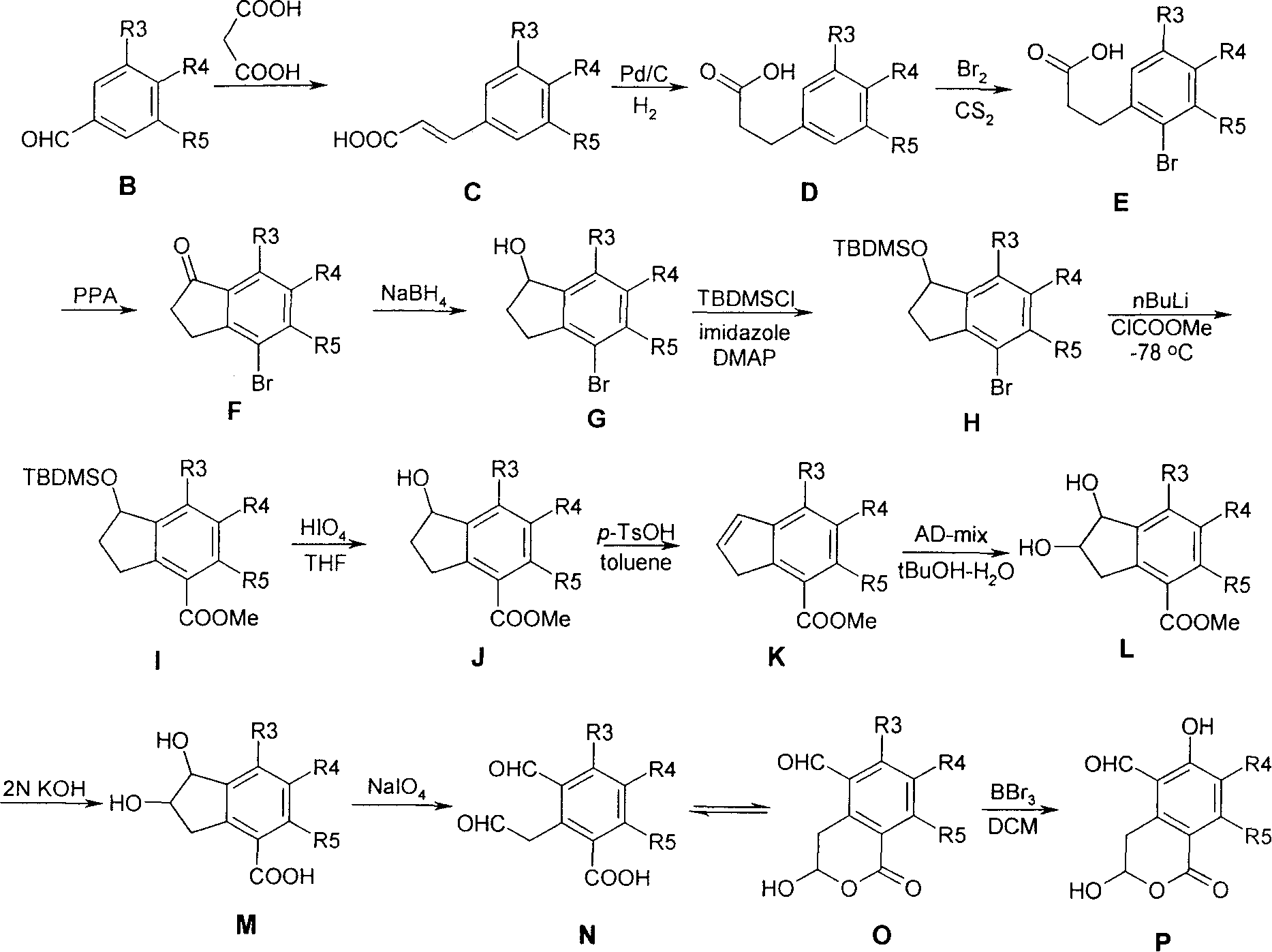
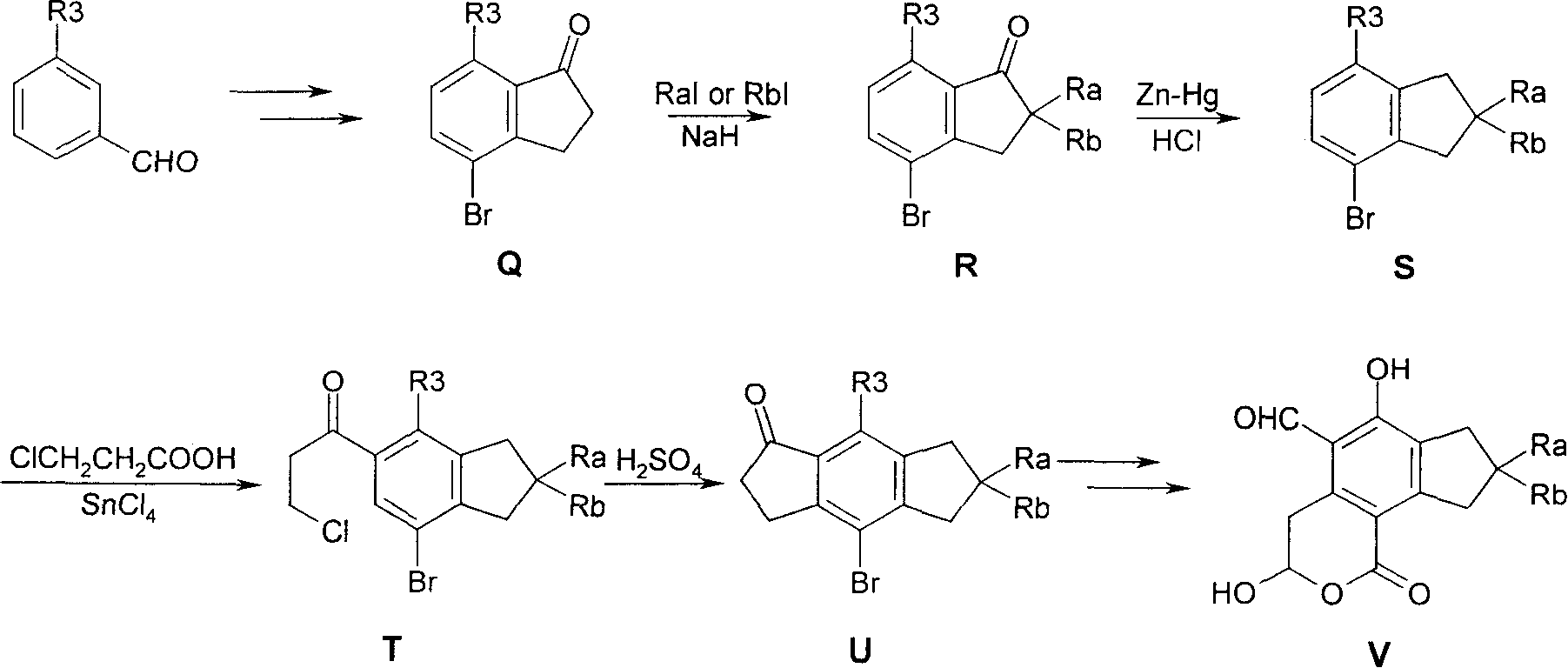
3,4-dihydro-iii 2 benzopyran-1 ketone kind compound, its preparation method and use
A technology of benzopyran, -1H-2-, applied in 3 fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0045] In all embodiments, the melting point is measured with the SWG X-4 micro melting point instrument of Shanghai Precision Scientific Instrument Co., Ltd., and the thermometer is not corrected; 1H-NMR is recorded with a Varian Mercury 300 nuclear magnetic resonance instrument, and the chemical shift is expressed in δ (ppm). The silica gel not specified is 200-300 mesh.
[0046] I. Below we prepare compound 3-hydroxyl-5 formyl-6-methoxy-1H-2-benzopyran-1-ketone (structural formula 14) and 3-hydroxyl-5 formyl-6-hydroxyl- 1H-2-benzopyran-1-one (structural formula 15) is a specific example to further illustrate the first preparation method above, but does not limit this patent in any way.
[0047]
[0048] 1. Synthesis of Compound 2
[0049] Weigh 33g of malonic acid, dissolve it with 75ml of pyridine, add 19ml of m-methoxybenzaldehyde, and 1.5ml of piperidine. The resulting mixture was reacted with stirring at 100°C for 3h, then at 130°C for 10min. Cool to room temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com