Phosphatidylinositol-3-kinase inhibitor and application thereof
A technology of kinase inhibitor and phosphatidylinositol, which is applied in the field of tumor treatment drugs, can solve the problems of unseen function and application reports, and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
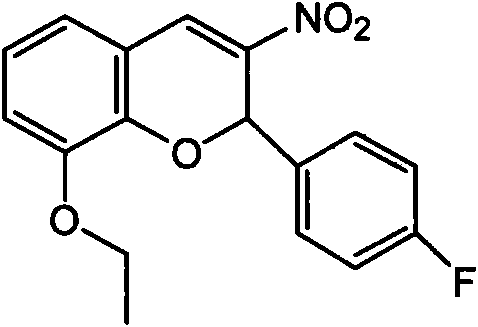
[0032] Example 1: Synthesis of PI3K inhibitors
[0033] First synthesize p-fluoro-β-nitrostyrene with fluorobenzaldehyde and nitromethane, then synthesize 2-p-fluorophenyl- 3-nitro-8-ethoxy-2H-benzopyran. The specific method is as follows:
[0034] Preparation of p-fluoro-β-nitrostyrene:
[0035] Dissolve p-fluorobenzaldehyde (50.0 g, 0.4 mol) in 3 liters of nitromethane, add ammonium acetate (34.0 g, 0.44 mol), and react under reflux for 24 h. After concentration under reduced pressure, a 1:1 mixture of dichloromethane and water was added, and the aqueous layer was extracted three times with dichloromethane. The organic layers were combined, washed with saturated brine, and dried over anhydrous magnesium sulfate. Filter and concentrate. Recrystallized from ethyl acetate-petroleum ether to obtain 37.3 g (92%) of p-fluoro-β-nitrostyrene as a yellow solid, melting point: 98-100°C;
[0036] Synthesis of 2-p-fluorophenyl-3-nitro-8-ethoxy-2H-benzopyran
[0037] Add 3-ethoxy ...
Embodiment 2
[0038] Example 2: Inhibiting the proliferation of malignant hematological tumor cells
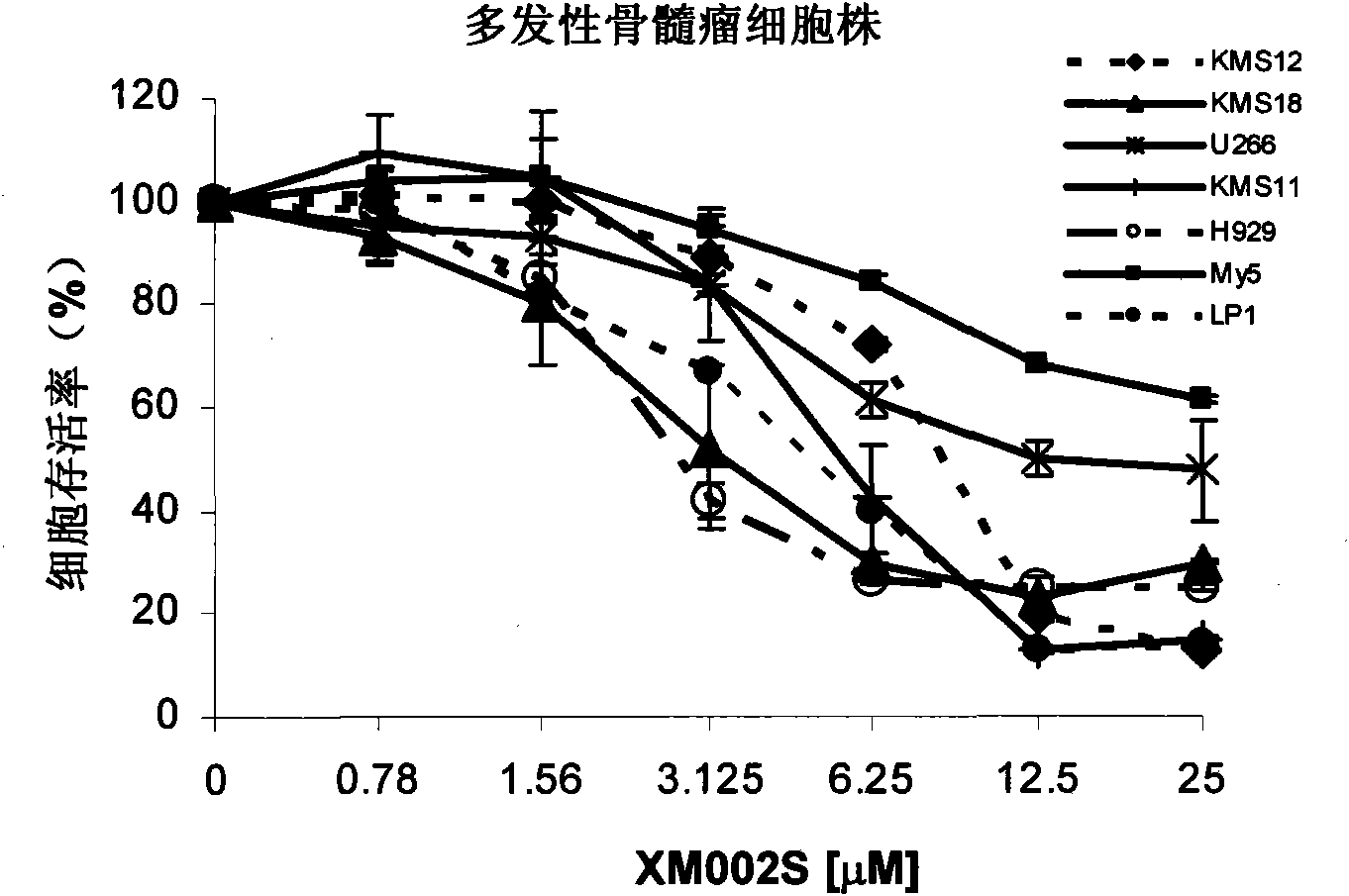
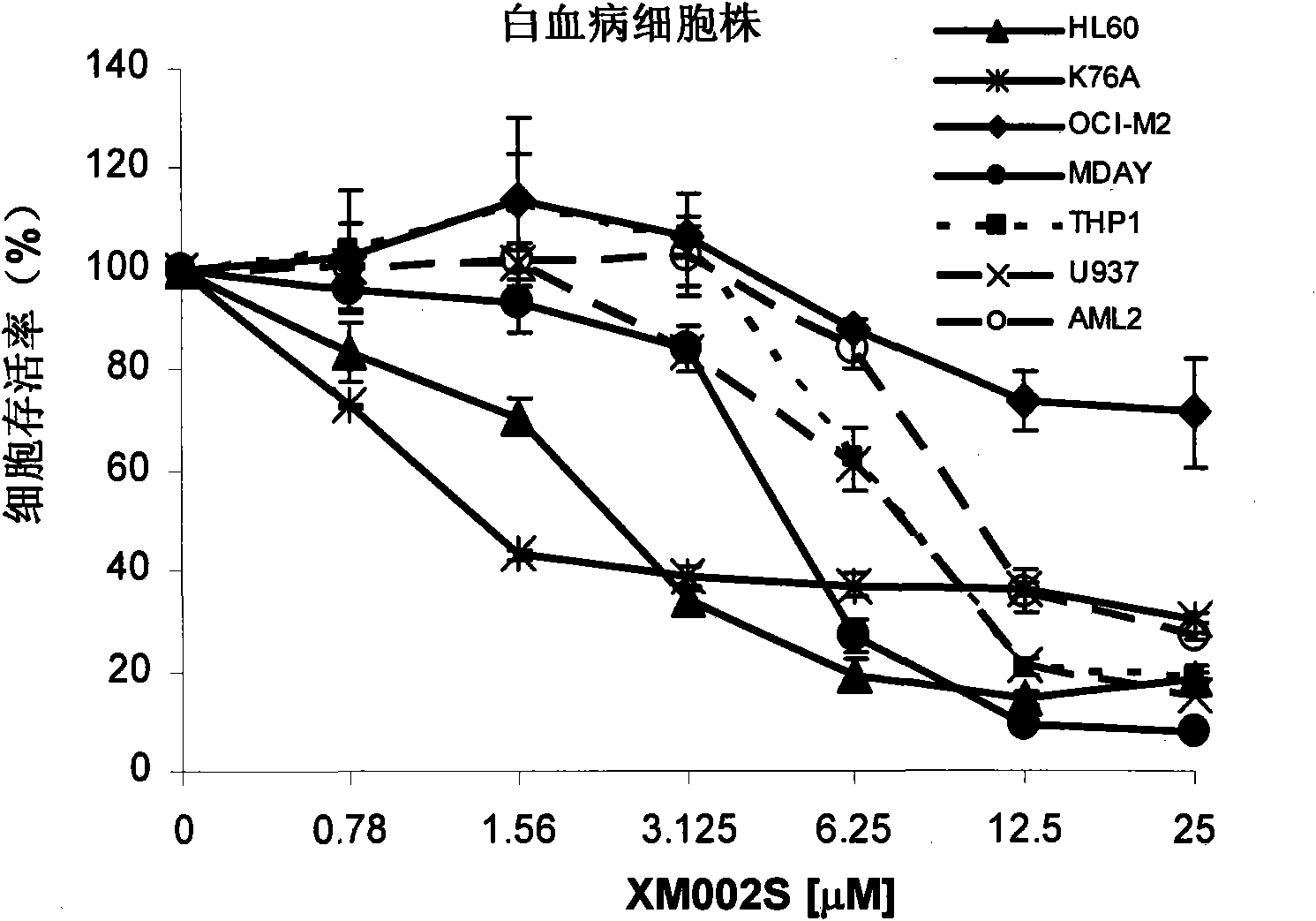
[0039] Culture myeloma cell lines, leukemia cell lines, primary acute myeloid leukemia cells, and peripheral blood stem cells in RPMI-1640 or IMDM cell culture medium, add 10% fetal bovine serum, 100 μg / ml ampicillin, 100 units / ml streptomycin. The cell culture environment was 37 degrees Celsius, 5% carbon dioxide; different concentrations of XM002S were given to incubate for 72 hours, and the cell viability was analyzed by MTS / PMS staining method according to conventional techniques (the wavelength for reading optical density was 490nm), and the attached Figure 2a (myeloma cell line), with Figure 2b (leukemia cell line), with Figure 2c (acute myeloid leukemia primary cells), with Figure 2d (peripheral blood stem cells).
[0040] from Figure 2a-2d It can be seen that the toxicity of XM002S to malignant hematological tumor cells is dose-dependent, but it has no obvious effect on t...
Embodiment 3
[0041] Example 3: Inhibition of tumor growth in tumor-bearing mice
[0042] 5-6 weeks old female NOD / SCID mice were subcutaneously injected with K562 cells (5×10 5 ) or U937 cells (1×10 6 ), when the tumor grew to be palpable, XM002S (100mg / kg / d) was injected intraperitoneally for intervention, and the control group was injected with the same amount of XM002S solvent (10% DMSO) for 10 consecutive days. The change in tumor volume was measured every other day, and the body weight of the mice was monitored at the same time to obtain image 3 a (cell tumor-bearing mice, cell tumor-bearing mice), image 3 b (U937 cell tumor-bearing mice), where the left graph is the curve of tumor size change, and the right graph is the curve of body weight change.
[0043] according to image 3 a, 3b: XM002S significantly inhibited the growth of tumors in the two tumor-bearing mice. From the 6th day to the end of the experiment, the tumor volume of the XM002S intervention group was significantly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com