Dihydrobenzopyrans ketone compound and uses thereof
A technology of benzopyrans and compounds, which is applied in the field of new dihydrobenzopyrones, can solve the problems of unclear function of non-classical estrogen membrane signaling pathway and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: (S)-2,3-dihydro-7-hydroxyl-2-(4-hydroxyphenyl)-6-[2-(1-pyrrolidinyl)ethyl]-4H-1-benzene Preparation of pyran-4-one (compound 1)
[0045]
[0046] Preparation of Compound B
[0047] Compound A (320 mg, 1.0 mmol) was dissolved in 20 ml of methanol at -78°C, and then ozone was passed through for 20 minutes. Remove the cooling bath, then add 0.5 mL dimethyl sulfide to the above mixture, and stir for 10 minutes. After removing the solvent, the product was separated by column chromatography to obtain product B (260 mg, 88%).
[0048] Preparation of compound 1
[0049] A mixture of compound B (250mg, 0.71mmol), pyrrolidine hydrochloride (252mg, 2.34mmol), sodium acetate (107mg, 1.30mmol), sodium cyanoborohydride (80mg, 1.28mmol) and methanol (4mL) at room temperature Stirring was continued for 5 hours. Then 5 ml of water was added to the above mixture and stirred for 30 minutes. Then the above mixture was extracted with ethyl acetate (3X10mL). The extracts...
Embodiment 2
[0050] Example 2: (S)-2,3-dihydro-7-hydroxyl-2-(4-hydroxyphenyl)-6-[2-(dimethylamino)ethyl]-4H-1-benzo Preparation of pyran-4-one (compound 2)
[0051]
[0052] A mixture of compound B (120 mg, 0.34 mmol), dimethylamine hydrochloride (65 mg, 0.80 mmol), sodium acetate (43 mg, 0.52 mmol), sodium cyanoborohydride (32 mg, 0.51 mmol) and methanol (2 mL) Stir at room temperature for 8 hours. Then 5 ml of water was added to the above mixture and stirred for 30 minutes. Then the above mixture was extracted with ethyl acetate (3X10mL). The extracts were combined and dried over anhydrous sodium sulfate. After removing the solvent, the crude product was purified by column chromatography to obtain the product compound 1 (45 mg, 41%). 1 H NMR (acetone-d6, 300MHz): δ7.48(s, 1H), 7.37((d, J=8.1Hz, 1H), 6.89(d, J=8.7Hz, 1H), 6.23(s, 1H) , 5.37 (dd, J 1 =2.7Hz,J 2 =12.6Hz, 1H), 2.96(dd, J 1 =12.8Hz,J 2 =16.7Hz, 1H), 2.84-2.80(m, 1H), 2.74-2.72(m, 1H), 2.62(dd, J 1 =2.9Hz,J 2 =16....
Embodiment 3
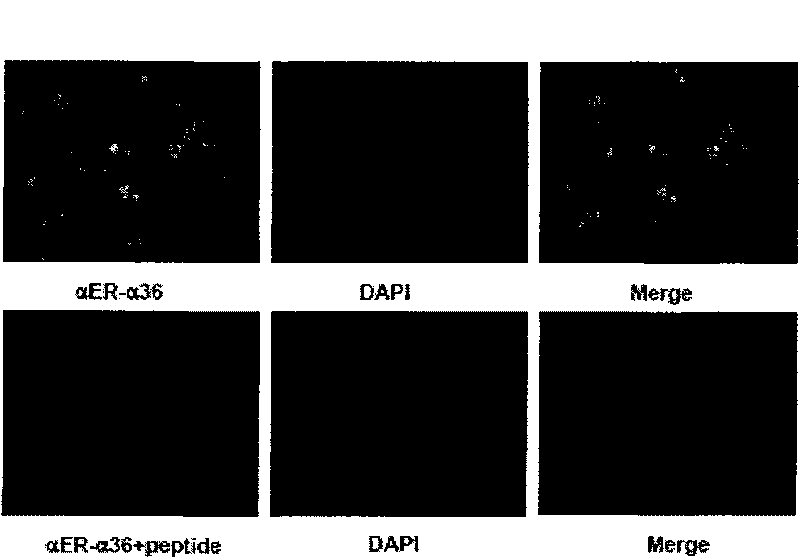
[0053] The in vitro biological test of embodiment 3 formula (I) compound
[0054] Test 3-1:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com