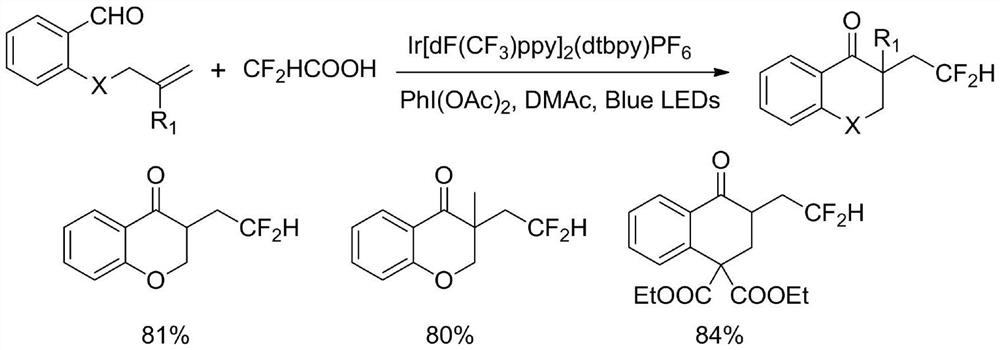
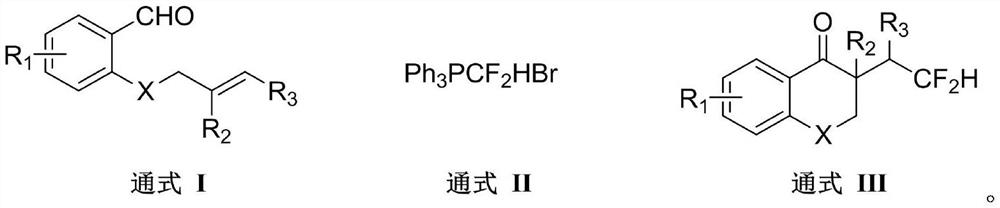
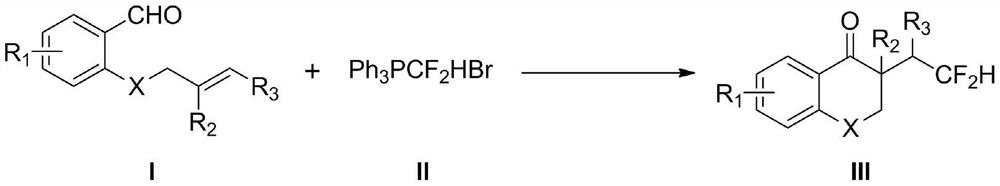
Method for synthesizing difluorohydromethylated 2, 3-dihydrobenzopyran-4-one derivative
A technology of dihydrobenzopyran and difluorohydromethyltriphenylphosphine bromide, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of high toxicity and environmental hazards of individual reagents Large, high reaction temperature and other issues, to achieve the effect of a wide range of substrates, safety and environmental friendliness, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] At room temperature, magneton, 2-allyloxybenzaldehyde (0.2mmol, 32.5mg), difluorohydromethyltriphenylphosphine bromide (0.3mmol, 117.6mg) and fac- Ir(ppy) 3 (0.004mmol, 2.62mg) after three vacuum changes of argon, add 2,6-Lutidine (0.3mmol, 32.2mg) and 2mL DMSO, react under 5W blue light irradiation for 24h, after the reaction, add 10mL water, ethyl acetate ( 15mL*3) extraction, the organic phases were combined, washed with water, washed with saturated brine, dried with anhydrous sodium sulfate, and separated and purified by column chromatography to obtain this example 3-(2,2-difluoroethyl) -2,3-Dihydrobenzopyran-4-one product 36.1 mg, yield 85%, colorless liquid. 1 H NMR (400MHz, CDCl 3 )δ7.89(dd,J=7.9,1.4Hz,1H),7.52–7.45(m,1H),7.03(t,J=7.5Hz,1H),6.98(d,J=8.4Hz,1H), 6.14(tdd, J=56.7,5.4,3.2Hz,1H),4.72-4.57(m,1H),4.23(t,J=11.7Hz,1H),3.08(td,J=11.8,5.9Hz,1H) ,2.67–2.39(m,1H),2.00–1.78(m,1H). 13 C NMR (101MHz, CDCl 3 )δ192.49,161.56, 136.17,127.38,121.60...
Embodiment 2
[0026]
[0027] At room temperature, in a 25ml reaction tube were sequentially added magneton, 2-allyloxy-3-methylbenzaldehyde (0.2mmol, 35.4mg), difluorohydromethyltriphenylphosphine bromide (0.3mmol, 117.6 mg) and fac-Ir (ppy) 3 (0.004mmol, 2.62mg) after three vacuum changes of argon, add 2,6-Lutidine (0.3mmol, 32.2mg) and 2mL DMSO, react under 5W blue light irradiation for 24h, after the reaction, add 10mL water, ethyl acetate ( 15mL*3) extraction, the organic phases were combined, washed with water, washed with saturated brine, dried with anhydrous sodium sulfate, and separated and purified by column chromatography to obtain the present example 8-methyl-3-(2,2- Difluoroethyl)-2,3-dihydrobenzopyran-4-one product 31.4 mg, yield 70%, colorless liquid. 1 H NMR (400MHz, CDCl 3 ) δ7.74(dd,J=7.9,1.2Hz,1H),7.42-7.30(m,1H),6.93(t,J=7.6Hz,1H),6.15(tdd,J=56.7,5.5,3.2Hz ,1H),4.67(dd,J=11.4,5.2Hz,1H),4.23(t,J=11.7Hz,1H),3.06(qd,J=6.7,1.0Hz,1H),2.52(ddddd,J= 23.8, 16.1, 15.0, 5.9...
Embodiment 3
[0029]
[0030] At room temperature, in a 25ml reaction tube were sequentially added magneton, 2-allyloxy-4-methylbenzaldehyde (0.2mmol, 35.4mg), difluorohydromethyltriphenylphosphine bromide (0.3mmol, 117.6 mg) and fac-Ir (ppy) 3 (0.004mmol, 2.62mg) after three vacuum changes of argon, add 2,6-Lutidine (0.3mmol, 32.2mg) and 2mL DMSO, react under 5W blue light irradiation for 24h, after the reaction, add 10mL water, ethyl acetate ( 15mL*3) extraction, the organic phases were combined, washed with water, washed with saturated brine, dried with anhydrous sodium sulfate, and separated and purified by column chromatography to obtain the present example 7-methyl-3-(2,2- Difluoroethyl)-2,3-dihydrobenzopyran-4-one product 29.0 mg, yield 64%, colorless liquid. 1 H NMR (400MHz, CDCl 3 ) δ7.77(d,J=8.0Hz,1H),6.85(d,J=8.1Hz,1H),6.78(s,1H),6.14(tdd,J=56.7,5.5,3.2Hz,1H), 4.59(dd,J=11.4,5.2Hz,1H),4.20(t,J=11.6Hz,1H),3.03(td,J=11.9,6.0Hz,1H),2.60 –2.42(m,1H),2.36 (s,3H),1.96–1.78(m,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com