Synthetic process of chiral 2-amido-1-(6-fluorine-3,4-dihydrobenzopyranyl) alCohol
A technology of dihydrobenzopyranyl and synthesis method, applied in directions such as organic chemistry, can solve problems such as low product purity, high production cost, harsh conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
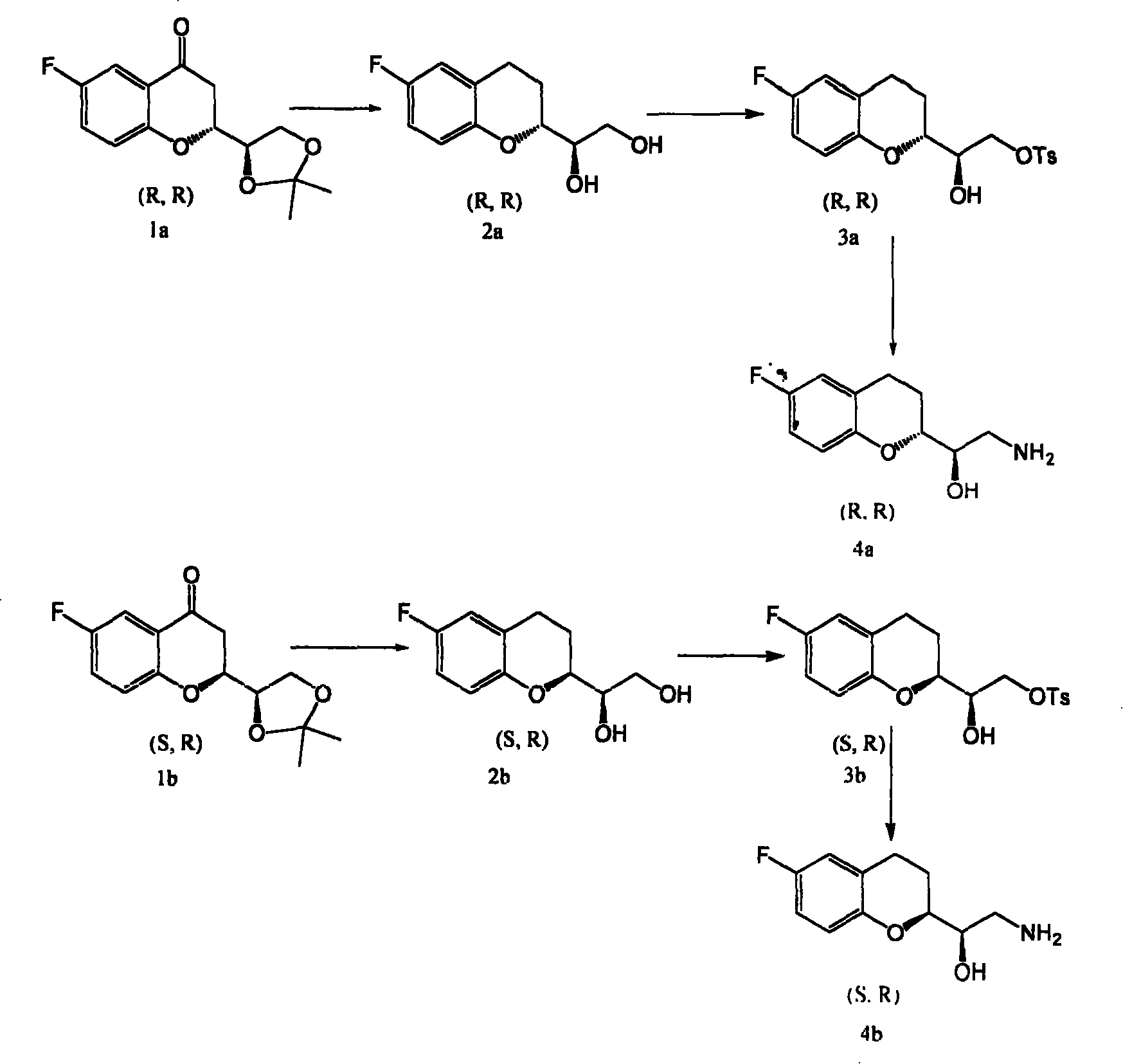
[0022] (1). Synthesis of (R)-1-((R)-6-fluoro-3,4-dihydrochromenyl)ethane-1,2-diol (2a):
[0023] In the reactor, add compound 1a and freshly prepared zinc amalgam (molar ratio, 1: 120), hydrochloric acid (40ml), 48ml ethanol, stir and react for 50 hours, then add hydrochloric acid (20ml), react for 6 hours, and cool , filtered, the filtrate was extracted with ethyl acetate, washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered, and rotary evaporated to obtain viscous liquid (R)-1-((R)-6-fluoro-3, 4-Dihydrochromenyl)ethane-1,2-diol (2a).
[0024] (2).(R)-2-((R)-6-fluoro-3,4-dihydrochromenyl)-2-hydroxyethyl-4-methylbenzenesulfonate (3a) synthesis:
[0025] In the reactor, add (R)-1-((R)-6-fluoro-3,4-dihydrobenzopyranyl)ethane-1,2-diol (2a), add 10ml pyridine to dissolve , stirred, then added p-toluenesulfonyl chloride (mole ratio, 2a: p-toluenesulfonyl chloride=1:1.0), reacted for 48 hours, then, added 10ml of ice water to the reaction syste...
Embodiment 2
[0029] (1). Synthesis of (R)-1-((R)-6-fluoro-3,4-dihydrochromenyl)ethane-1,2-diol (2a):
[0030] In the reactor, add compound 1a, freshly prepared zinc amalgam (molar ratio, 1a:zinc amalgam=1:1.2), 60ml hydrochloric acid, 48ml ethanol, stir and react for 46 hours, then add 30ml hydrochloric acid and react for 6 hours , cooled, filtered, extracted filtrate, washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered, and rotary evaporated to obtain viscous liquid (R)-1-((R)-6-fluoro-3,4- Dihydrochromenyl)ethane-1,2-diol (2a).
[0031] (2).(R)-2-((R)-6-fluoro-3,4-dihydrochromenyl)-2-hydroxyethyl-4-methylbenzenesulfonate (3a) synthesis:
[0032] In the reactor, add (R)-1-((R)-6-fluoro-3,4-dihydrochromenyl)ethane-1,2-diol (2a), add 15ml pyridine to dissolve , stirred, then added p-toluenesulfonyl chloride (molar ratio, 2a: p-toluenesulfonyl chloride=1:1.4), stirred and reacted for 24 hours, added 10ml of ice water to the reaction system, extracted w...
Embodiment 3
[0036] (1). Synthesis of (S)-1-((R)-6-fluoro-3,4-dihydrochromenyl)ethane-1,2-diol (2b):
[0037] In the reactor, add compound and freshly prepared zinc amalgam (molar ratio, 1:110), 90ml hydrochloric acid, 48ml ethanol, stir and react for 50 hours, then add dilute hydrochloric acid, react for 6 hours, cool, filter, and extract the filtrate , washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered, and rotary evaporated to obtain viscous liquid (S)-1-((R)-6-fluoro-3,4-dihydrobenzopyranyl ) Ethane-1,2-diol.
[0038] (2).(R)-2-((S)-6-fluoro-3,4-dihydrochromenyl)-2-hydroxyethyl-4-methylbenzenesulfonate (3b) synthesis:
[0039] In the reactor, add (S)-1-((R)-6-fluoro-3,4-dihydrochromenyl)ethane-1,2-diol (2b), add pyridine to dissolve, Stir, then add p-toluenesulfonyl chloride (molar ratio, 2b: p-toluenesulfonyl chloride=1:1.01), stir for 24 hours, then add 10ml of ice water to the reaction system to terminate the reaction, extract, wash with dilu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com