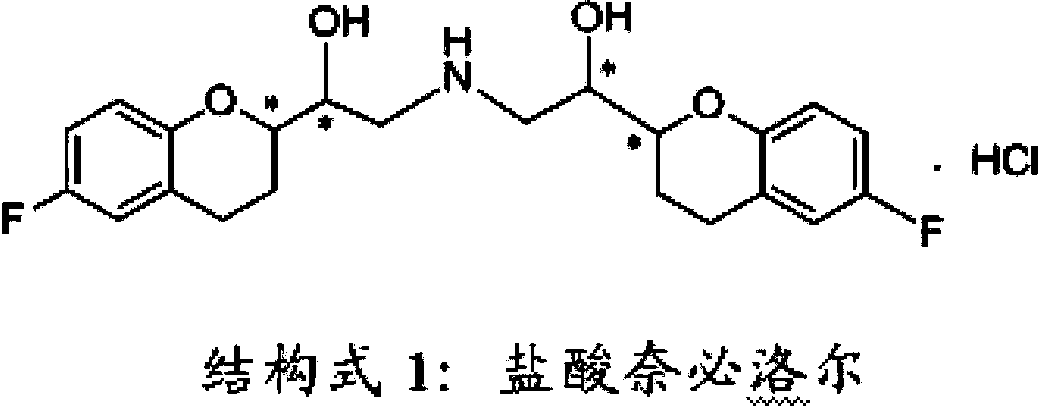
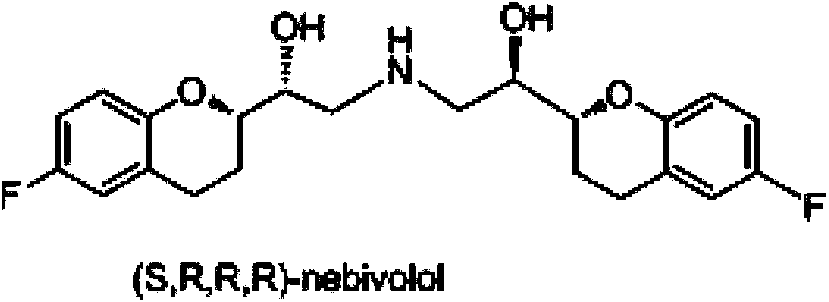
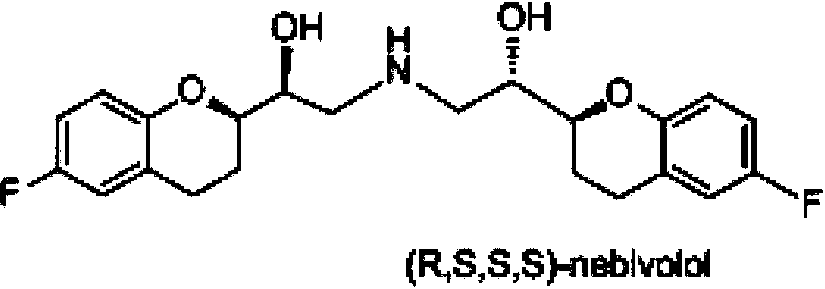
Preparation method of nebivolol and its intermediate compound
A compound and mixed solvent technology, applied in the field of drug synthesis, can solve the problems of high cost, unsuitable for industrial production, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1: Preparation of (R, R)-Compound I and (S, R)-Compound I
[0077] Absolute configuration of 1-[6-fluoro-(2R)-3,4-dihydro-2H-2-benzopyran-4-one]-(1R)-1,2 was obtained by referring to the method disclosed in WO2004041805 -Ethylene glycol, decarbonylation in trifluoroacetic acid / triethylsilane system to give 1-[6-fluoro-(2R)-3,4-dihydro-2H-2-benzopyran]-(1R) -1,2-ethylene glycol. Add 240g of it into 3L of dichloromethane, stir evenly, add 520mL of pyridine, immediately dissolve and clear, cool in an ice-water bath; weigh 236g of p-toluenesulfonyl chloride, add it into a constant pressure funnel, add 800mL of dichloromethane into the constant pressure funnel, It can dissolve completely and form a suspension. Add the suspension dropwise to the reaction bottle under an ice-water bath. After the addition is complete, remove the ice bath and stir at room temperature for 2 days. After the reaction is complete, pour the reaction solution into 3L of water. , stirred and...
Embodiment 2
[0079] Example 2 Preparation of 6-fluoro-(2S)-2-[1-hydroxyl-2-phthalimide-(1R)-ethyl]-3,4-dihydrochroman (S, R) - compound III
[0080] Put 133.2 grams (0.5mol) of S, R-compound I, 166.7 grams (0.9mol) of phthalimide potassium salt (compound II) and 138.2 grams (1.0mol) of anhydrous potassium carbonate into 480ml of DMF, Stir and heat up to 60-70°C, react for 8-10h. Cool to room temperature, add 1200ml of water and stir for 30min, cool to 5°C and filter, rinse the filter cake with 150ml of cold methanol, and dry under reduced pressure to constant weight to obtain compound 146.8g (S,R)-compound III, yield 86%. 1 H-NMR (CDCl 3 ): δ1.86-2.10(m,2H),2.78-2.95(m,2H),3.76-3.82(m,1H),3.86-3.98(m,2H),4.36(m,1H),4.90(d ,1H),6.78(d,1H),6.96-6.98(d,1H),7.01-7.04(d,1H),7.86-7.90(d,2H),7.98-8.02(d,2H),C 19 h 16 NFO 4 HRMS theoretical value 341.3330, measured value 341.3325
Embodiment 3
[0081] Example 3 Preparation of 6-fluoro-(2R)-2-[1-hydroxyl-2-phthalimide-(1R)-ethyl]-3,4-dihydrobenzopyran (R, R) - compound III
[0082] Put 133.2 grams (0.5mol) of R,R-compound I, 166.7 grams (0.9mol) of phthalimide potassium salt (compound II) and 138 grams (1.0mol) of anhydrous potassium carbonate into 480ml of DMF, Stir and heat up to 60-70°C, react for 8-10h. Cool to room temperature, add 1200ml of water and stir for 30min, cool to 5°C and filter, rinse the filter cake with 150ml of cold methanol, and dry under reduced pressure to constant weight to obtain compound 146.5g (R,R)-compound III, yield 85.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com