Synthesis method of (R) or (S)-6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid
A technology of benzopyran and synthesis method, which is applied in the direction of organic chemistry, can solve the problem of low resolution and other problems, and achieve the effect of less reaction steps and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Below in conjunction with specific embodiment further illustrate how the present invention is realized:
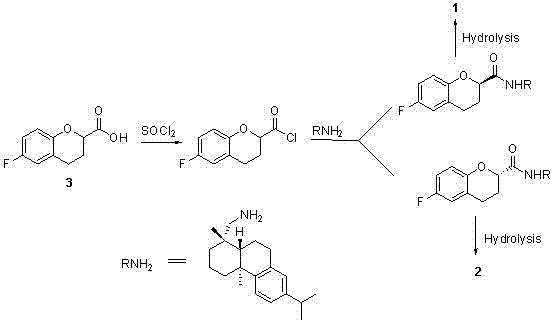
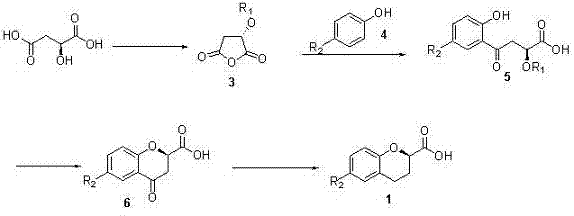
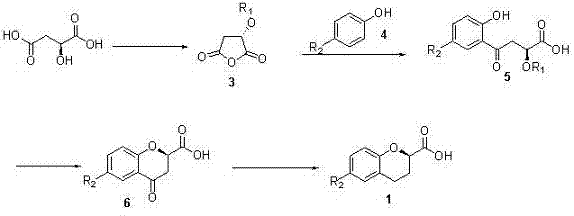
[0025] Such as figure 2 Shown in the reaction formula of the present invention, step A:
[0026] In a 100 ml single-necked bottle, add (L)-malic acid 13.4 g and methanesulfonyl chloride 40 ml, heat up to 45 o C was reacted for about 4 hours, and then the excess methanesulfonyl chloride was removed under reduced pressure to obtain 19.3 g of product 3, and the yield was quantitative.
[0027] Step B:
[0028] 5.6 g of p-fluorophenol and 11.6 g of the product 3 of the previous step were mixed and dissolved in 250 ml of 1,2-dichloroethane, 9.9 g of aluminum trichloride was added, and the temperature was raised to reflux overnight. After cooling in an ice-water bath, 10 % hydrochloric acid to quench the reaction, separate the liquids, extract the aqueous phase with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com