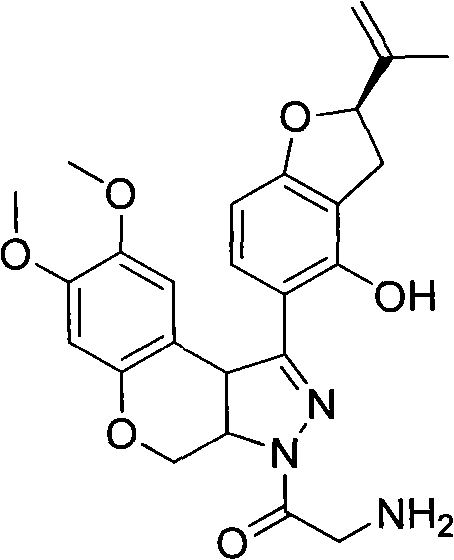
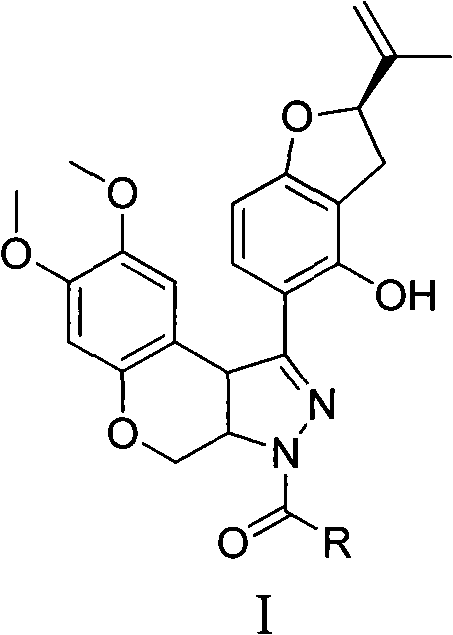
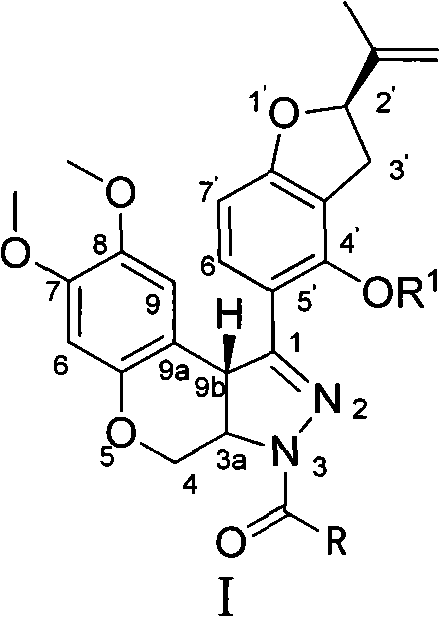
N-acylpyrazole derritol, preparation method thereof, and application thereof
A technology for the reaction of acylpyrazole rotenol, which is applied in the field of N-acylpyrazole rotenol and its preparation and application, can solve the problem that there is no research report on the anti-neuraminidase activity of N-acylpyrazole rotenol, Achieve good anti-neuraminidase activity and high anti-neuraminidase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The preparation of embodiment 1N-acetylpyrazole rotenol
[0017]
[0018] 0.01mol (2R)-5-[7,8-dimethoxy-3,3a,4,9b-tetrahydrobenzopyrano[3,4-c]pyrazol-1-yl]-2- (propen-2-yl)-2,3-dihydrobenzofuran-4-ol, 20mL dichloromethane, 1.5g pyridine, 0.01mol acetyl chloride was added at 25°C, reacted for 15min, and the reaction solution was rotary evaporated to obtain the crude product ; Recrystallized from ethanol to obtain N-acetylpyrazole rotenol with a yield of 54.4%, m.p.210-214°C, 1 H NMR (CDCl 3 , 400MHz) δ: 1.75, 1.78 (2×s, 3H, CH 3 ), 2.37 (s, 3H, COCH 3 ), 2.99(m, 1H, 3'-H), 3.33(m, 1H, 3'-H), 3.66(s, 3H, 8-OCH 3 ), 3.79 (s, 3H, 7-OCH 3 ), 3.94(d, J=12Hz, 1H, 4-H), 4.82(d, J=10Hz, 1H, 3a-H), 4.90~4.93(m, 2H,=CH 2 , 9b-H), 5.06~5.10 (m, 2H, =CH 2 , 4-H), 5.26(q, J=7.6Hz, 1H, 2'-H), 6.46(s, 1H, 6-H), 6.49(d, J=8.4Hz, 1H, 7'-H) , 6.81 (s, 1H, 9-H), 7.49 (d, J=8.4Hz, 1H, 6'-H).
Embodiment 2
[0019] The preparation of embodiment 2N-propionyl pyrazole rotenol
[0020]
[0021] 0.01mol (2R)-5-[7,8-dimethoxy-3,3a,4,9b-tetrahydrobenzopyrano[3,4-c]pyrazol-1-yl]-2- (propen-2-yl)-2,3-dihydrobenzofuran-4-ol, 20mL dichloromethane, 1.5g pyridine, 0.01mol propionyl chloride was added at 25°C, reacted for 35min, and the reaction solution was rotary evaporated to obtain the crude product ; Recrystallized from ethanol to obtain N-propionylpyrazole rotenol with a yield of 36.6%, m.p.205-208°C, 1 H NMR (CDCl 3 , 400MHz) δ: 1.21(t, J=7.2Hz, 3H, CH 3 ), 1.74, 1.77 (2×s, 3H, CH 3 ), 2.71 (q, J=7.2Hz, 2H, COCH 2 ), 3.01(m, 1H, 3'-H), 3.32(m, 1H, 3'-H), 3.65(s, 3H, 8-OCH 3 ), 3.79 (s, 3H, 7-OCH 3 ), 3.95(dd, J=2.4Hz, J=12Hz, 1H, 4-H), 4.81(d, J=10.4Hz, 1H, 3a-H), 4.89, 4.93(2×s, 1H, 9b- H), 4.93(s, 1H, =CH 2 ), 5.09 (s, 1H, =CH 2 ), 5.09(dd, J=2.4Hz, J=12Hz, 1H, 4-H), 5.26(t, J=8.8Hz, 1H, 2'-H), 6.46(s, 1H, 6-H), 6.49 (d, J=8.0 Hz, 1H, 7'-H), 6.81 (s, 1H, 9-H), 7.49 (d, ...
Embodiment 3
[0022] The preparation of embodiment 3N-isobutyryl pyrazole rotenol
[0023]
[0024] 0.01mol (2R)-5-[7,8-dimethoxy-3,3a,4,9b-tetrahydrobenzopyrano[3,4-c]pyrazol-1-yl]-2- (propen-2-yl)-2,3-dihydrobenzofuran-4-ol, 20mL dichloromethane, 1.5g pyridine, 0.01mol isobutyryl chloride (CH 3 ) 2 CHCOCl, reacted for 35 minutes, and the reaction solution was rotary evaporated to obtain a crude product; recrystallized from ethanol to obtain N-isobutyrylpyrazole rotenol, the yield was 62.6%, m.p.185~203°C, 1 H NMR (CDCl 3 , 400MHz) δ: 1.19 (d, J=7.2Hz, 3H, CH 3 ), 1.26 (d, J=7.2Hz, 3H, CH 3 ), 1.75, 1.78 (2×s, 3H, CH 3 ), 2.99(m, 1H, 3'-H), 3.23(m, 1H, COCH), 3.34(m, 1H, 3'-H), 3.66(s, 3H, 8-OCH 3 ), 3.79 (s, 3H, 7-OCH 3 ), 3.94(dd, J=2.4Hz, J=12Hz, 1H, 4-H), 4.82(dd, J=2.4Hz, J=10.4Hz, 1H, 3a-H), 4.88~4.93(m, 2H , 9b-H, =CH 2 ), 5.02~5.09 (m, 2H, =CH 2 , 4-H), 5.09 (s, 1H, =CH 2 ), 5.27(q, J=8.0Hz, 1H, 2'-H), 6.46(s, 1H, 6-H), 6.49(d, J=8.4Hz, 1H, 7'-H), 6.82(s , 1H, 9-H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com