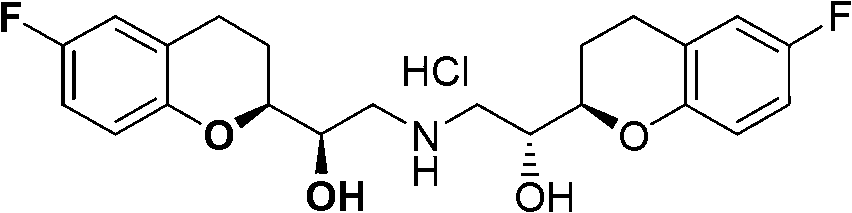
Method for preparing nebivolol racemate hydrochloride
A technology for nebivolol racemate and hydrochloride, applied in the field of preparing nebivolol racemate hydrochloride, can solve the problems of difficult preparation and purification, complex reaction products, difficult purification and the like, and achieves low cost , The effect of easy control of reaction conditions and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
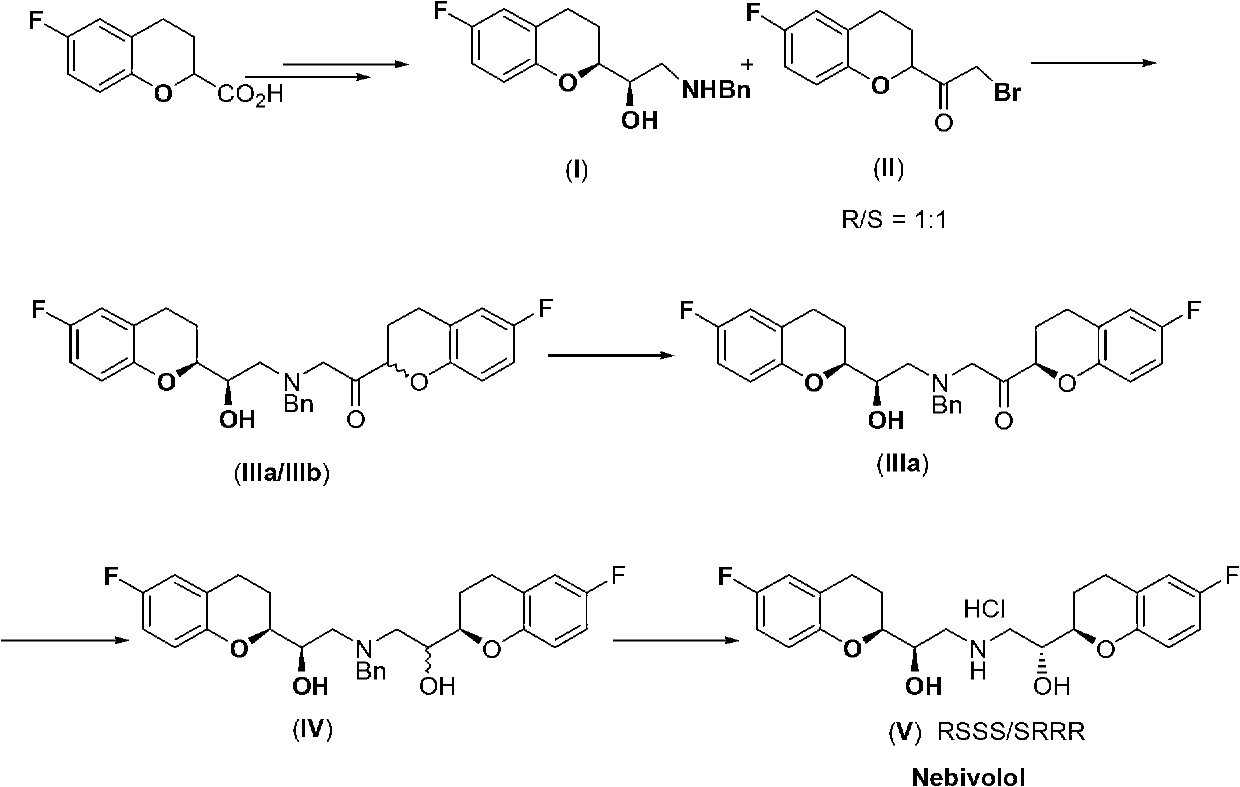
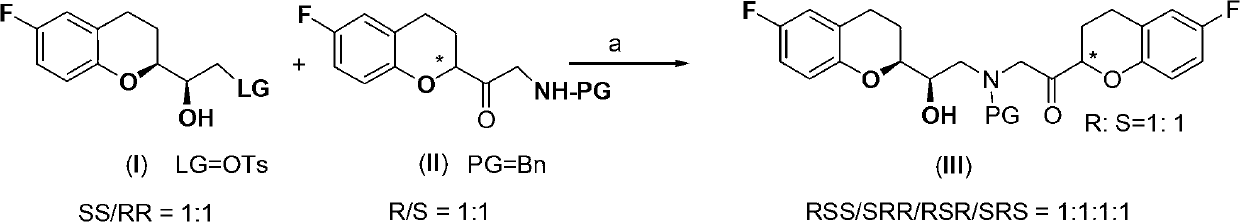
[0048] In a dry 1000mL three-necked flask, the DMF (75mL) solution of the compound of formula I (36.6g; purity 99.6%, HPLC method) was added dropwise to the compound containing formula II (30.0g; purity 99.6%, HPLC method), NaBr ( 1.6 g), NaHCO 3 (12.6g) in DMF (75mL) solution, heat up to 35-40°C under stirring; continue to heat and stir for 3 hours until the reaction is complete; then, use an ice-water bath to drop to 0-5°C, and add Add tertiary methyl ether (400mL) and water (200mL), stir for 15min and then stand to separate the layers. The aqueous layer is extracted with tertiary methyl ether (200mL×2). The organic phases were combined, dried with anhydrous sodium sulfate (25.0g) for 1 hour, filtered, and the filtrate was concentrated under reduced pressure to obtain an amber oil; the oil was dissolved in isopropyl ether and crystallized at room temperature for 2 hours. Crystal for 1 hour. After filtering, the filter cake was dried to obtain a light yellow solid, which wa...
Embodiment 2
[0050] In a dry 250 mL three-neck flask, the compound of formula III (30.0 g, IIIa / IIIb=50.2 / 49.8) was added to anhydrous acetonitrile (100 mL, water content: 0.01% KF). Slowly raise the temperature to an internal temperature of 70°C with stirring until the solid is completely dissolved and continue stirring for 15 minutes. Slowly cool down to 60°C, add DBU (2.5g), keep warm and stir for 2 hours; slowly cool down to 50°C, keep warm and stir for 3 hours; continue to slowly cool down to 40°C, keep warm and stir for 5 hours; continue to slowly cool down to 30°C, keep warm Stir for 1 hour; dropwise add glacial acetic acid (0.98g) to neutralize the reaction system to neutrality, then cool down to 25°C, filter, soak the filter cake in anhydrous acetonitrile (25mL×2), drain it, and dry it under reduced pressure to obtain Colorless to pale yellow solid, the compound of formula IIIa. A total of 18.8g (purity 99.2%, HPLC method; IIIa / IIIb=99.4 / 0.6).
Embodiment 3
[0052] N 2 Under protection, in a dry 500mL three-neck flask, NaBH 4 (2.24g) was added to anhydrous THF (100mL), cooled to -15~-20°C; and Ti(OPr-i) was slowly added dropwise 4 (33.0g), maintain the temperature at -10~-15°C, and continue to insulate and stir for 30min after dropping; then, slowly add anhydrous THF solution (90mL) containing the compound of formula IIIa (28.0g) dropwise to the above reaction system, drop After completion, keep the temperature at -10~-15°C for 2 hours; after the reaction is complete, keep the temperature at -10~-15°C and add saturated NH 4 Cl (200mL) solution until the reaction system is clear. The layers were separated, and the aqueous layer was washed with CH 2 Cl 2 (100mL×2) extraction, recover THF and CH respectively 2 Cl 2 , to obtain a colorless to light yellow foam, about 30 g, and heated to 60 ° C with ethanol / water (150 mL, v / v=3 / 1) to dissolve until clear, slowly lowered to room temperature, and stirred for 6 hours to crystallize....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com