Pharmaceutical composition comprising nebivolol with improved dissolution rate
A composition and drug technology, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of drug efficacy reduction, drug oral absorption effect and bioavailability reduction, etc., to reduce preparation costs , maintain pharmacological effect, constant pharmacological effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
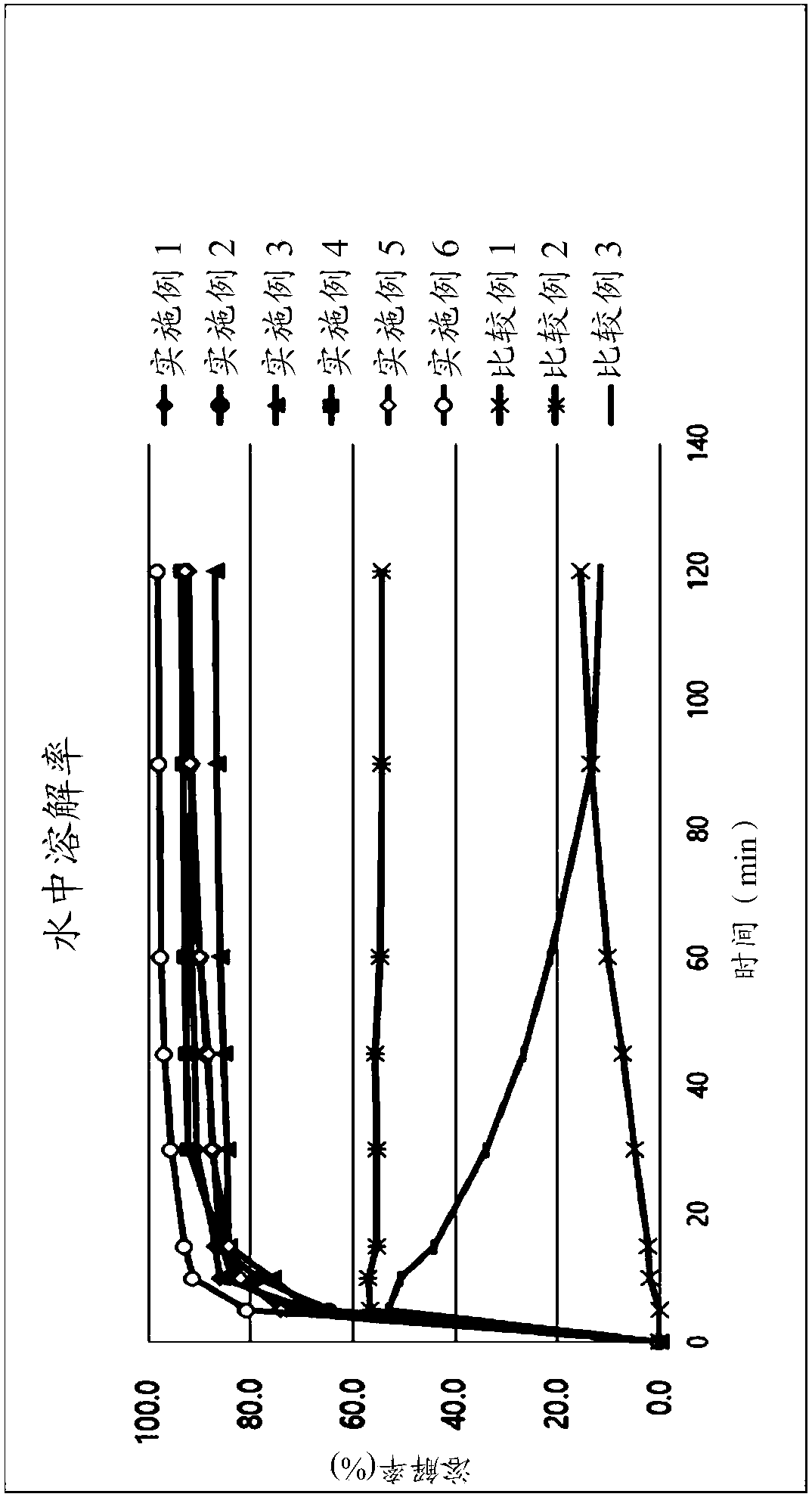
Image
Examples
Embodiment 1~3
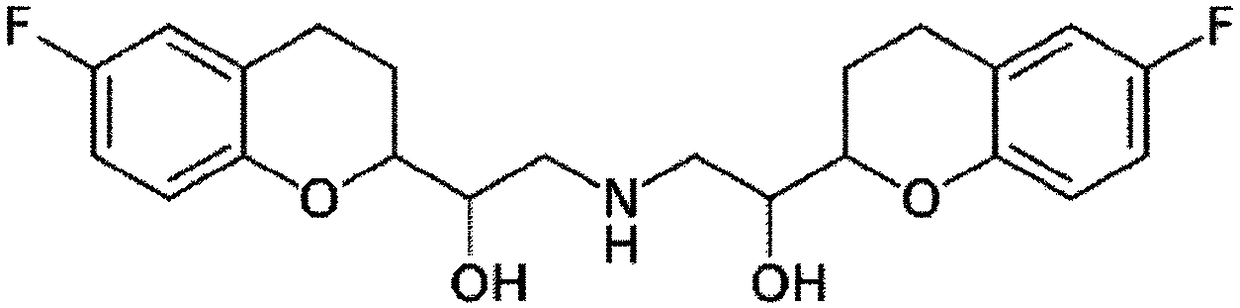
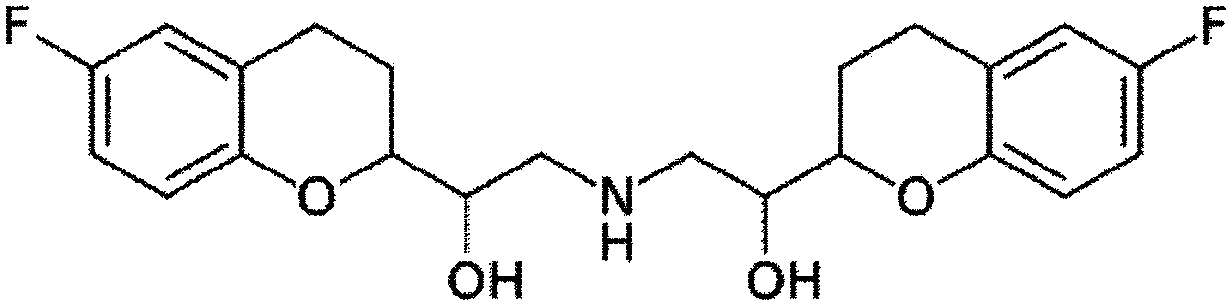
[0053] According to the composition shown in Table 1, Nebivolol hydrochloride was mixed with additives, and purified water with Tween-80 dissolved in it was added. The mixture is used to prepare granules according to the wet granulation method. Subsequently, magnesium oxide, magnesium carbonate or magnesium aluminum silicate and other additives as an alkalizing agent are post-mixed with the particles, and colloidal silica and magnesium stearate as lubricants are added thereto. The resultant was compressed into tablets (239.2 mg / tablet) using a tablet press (STP Machinery Co., Ltd., ZP198 Model) with a circular punch with a diameter of 8.6 mm.
[0054] Table 1 below shows the component content of each tablet.
[0055] [Table 1]
[0056]
Embodiment 4 and 5
[0058] According to the composition shown in Table 2, nebivolol hydrochloride was mixed with additives, and purified water was added thereto. The mixture is used to prepare granules according to the wet granulation method. Subsequently, magnesium oxide, magnesium carbonate, or magnesium aluminum silicate as an alkalizing agent and the remaining additives are post-mixed with the particles, and colloidal silica and magnesium stearate as lubricants are added thereto. The resultant was compressed into tablets (350.0 mg / tablet) using a tablet press (STPMachinery Co., Ltd., ZP198 Model) with a circular punch with a diameter of 10.0 mm.
Embodiment 6 and 7
[0060] According to the ingredients shown in Table 2, Nebivolol hydrochloride was mixed with additives, and a mixture of purified water and ethanol in which BHA and Tween-80 were dissolved was added. The mixture is used to prepare granules according to the wet granulation method. Subsequently, the rosuvastatin calcium, the magnesium aluminum silicate as the alkalizing agent and the remaining additives are post-mixed with the particles, and the colloidal silicon dioxide and magnesium stearate as the lubricant are added thereto. Using a tablet press (STPMachinery Co., Ltd., ZP198 Model) with a circular punch with a diameter of 10.0 mm or 7.5 mm, the resultant was compressed into tablets (350.0 mg / tablet: Example 6; 147.0 mg / tablet: implementation Example 7).
[0061] Film-coated tablets were prepared by spraying Opadry 03B28796 (Colorcon, Inc.) dissolved in 80% ethanol water to the tablets obtained above.
[0062] Table 2 below shows the component content of each tablet.
[0063] [T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com