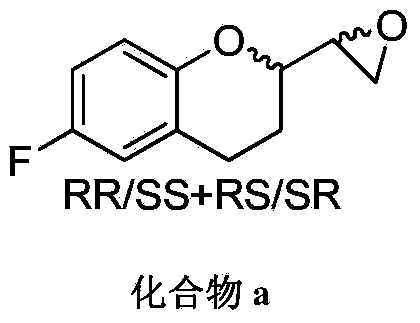
Method for preparing and purifying nebivolol intermediate
A technology for solids and compounds, which is applied in the preparation and purification of nebivolol intermediates, and in the field of improved preparation and purification, can solve the problems of poor purity, uncertain yield and quality, and not too high yield, etc. Simple, green process route, less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
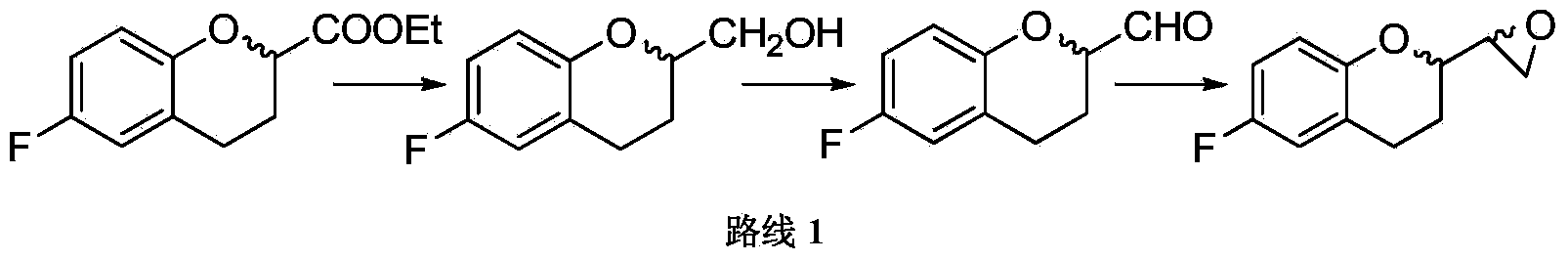
[0042] Preparation Example 1 Preparation of 6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid methyl ester
[0043]In a 500ml reactor equipped with mechanical stirring and condenser, add 6-fluoro-3,4-dihydro-2H-1-chromene-2-carboxylic acid (50g, 255mmol), methanol 394ml and acid methanol (20.3g / 100ml) 6ml, react at about 45°C for 2 hours, cool to room temperature (about 20°C), add NaHCO3 (2.8g, 33.4mmol) and stir for about 1 hour, distill under reduced pressure, and add ethyl acetate to the residue 200ml was dissolved, and the organic phase was washed once with 200ml of 2% sodium bicarbonate and once with 200ml of saturated brine, and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, and the filtrate was concentrated under reduced pressure to dryness to obtain 52.9 g of off-white solid. The purity of the product was determined by HPLC, and the content was 99.46% calculated by the area normalization method.
preparation example 2
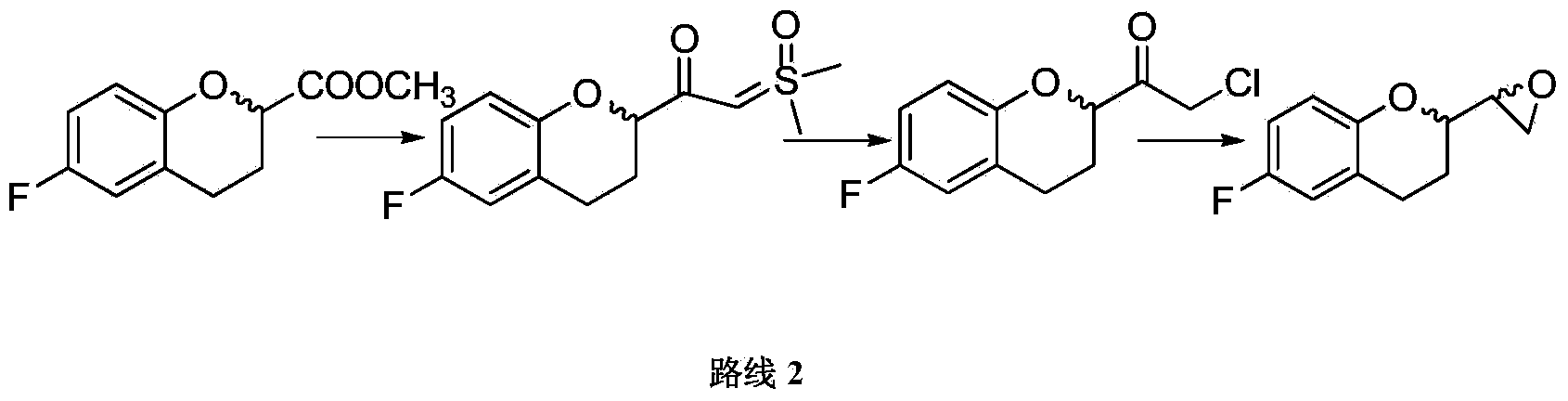
[0044] Preparation Example 2 Preparation of 1,-dimethylsulfoxide methylene-1-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)methanone
[0045] In a 250ml reactor equipped with mechanical stirring, a dropping funnel and a reflux condenser, add potassium tert-butoxide (20g, 179mmol) and 175ml tetrahydrofuran at room temperature (20°C), stir, and add trimethyl iodide at room temperature Sulfoxide (39.3g, 179mmol), heated to about 70°C, reacted for 2h, cooled to the internal temperature of about 0°C, added dropwise 6-fluoro-3,4-dihydro-2H-1-benzopyran- Methyl 2-carboxylate (25g, 119mmol) in 25ml of tetrahydrofuran was added dropwise and stirred at about 0°C for 1h. After the reaction is complete, concentrate under reduced pressure to remove most of the THF, then add 100ml of water, and spin again to remove the remaining THF. Add 250ml of ethyl acetate and 125ml of water to extract the residue at about 25°C, filter, stand to separate layers, and the water phase Extract once more with ...
Embodiment 1
[0046] Example 1 Preparation of 2-chloro-1-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)ethanone
[0047] In a 250ml reactor equipped with mechanical stirring and dropping funnel, add 1,-dimethylsulfoxidemethylene-1-(6-fluoro-3,4-dihydro-2H-1- Benzopyran-2-yl)methanone (20g, 74mmol) and tetrahydrofuran 200ml, add water (0.704g, 39mmol) and thionyl chloride (4.66g, 39mmol) in turn, and keep stirring at about 10°C for 30 minutes, Turn on heating and raise the temperature to about 70°C, react for 2 hours, cool down, concentrate the reaction solution to dryness under reduced pressure (-0.095MPa, water bath 50°C), and then pull it with an oil pump for 10 to 15 minutes after spinning, add 100ml of toluene, 100ml of 2% NaHCO3 Wash once; wash with saturated NaCl100ml*4, add anhydrous Na to toluene 2 SO 4 Let dry for 2 hours. Spin dry the toluene phase (-0.095MPa, water bath 50°C), spin it, and then pull it to a constant weight with an oil pump to obtain 16.16g of oil, add 16ml of iso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com