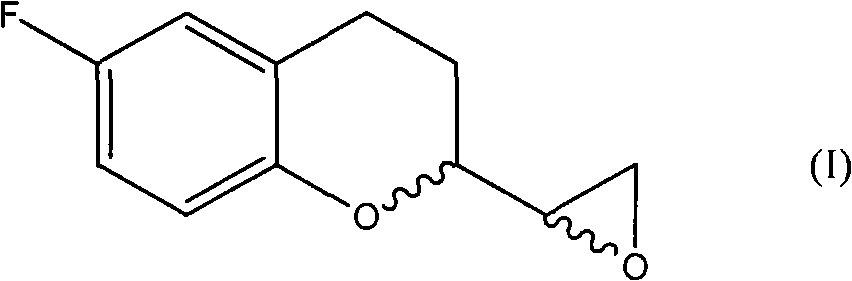
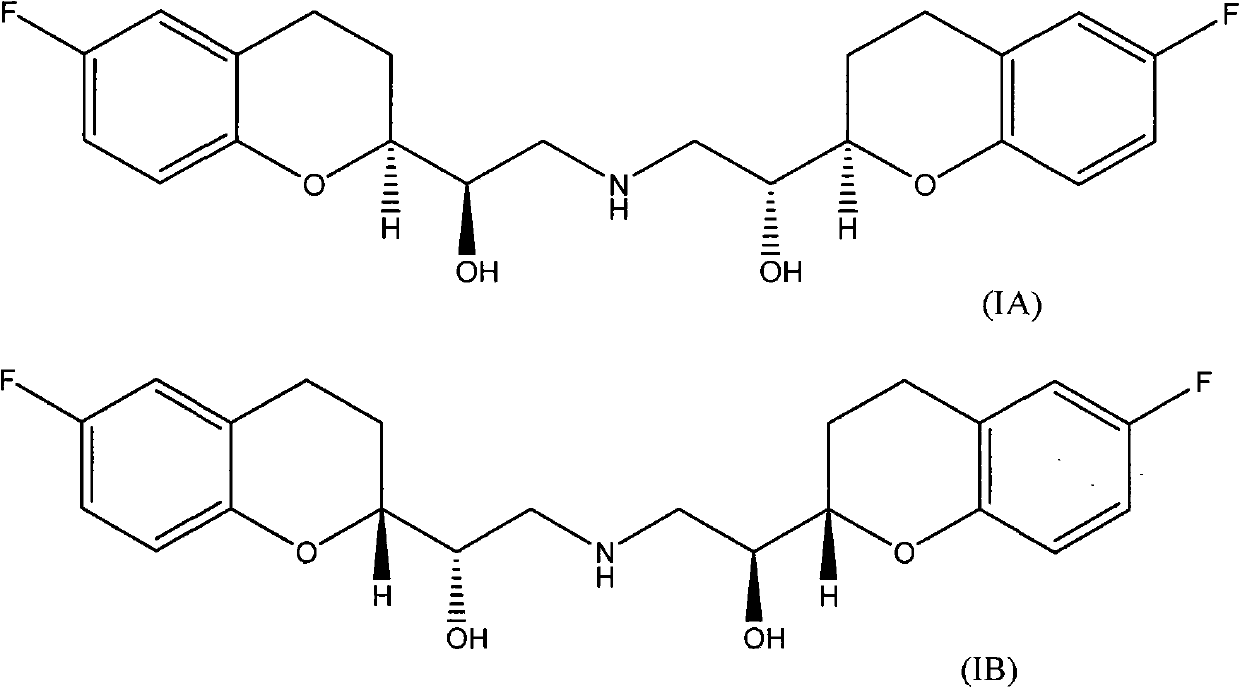
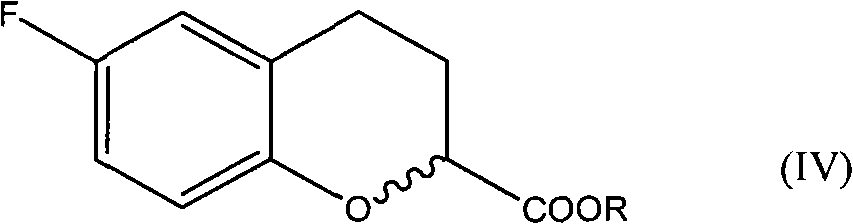
Improvement method for preparing 6-fluorin-3,4-dihydro-2H-1-benzopyranyl-2-epoxy ethane
A technology of benzopyran and ethylene oxide, which is applied in the field of key intermediates for the preparation of nebivolol, can solve the problems of strong corrosion of halogenated hydrogen acids, unfavorable large-scale application, harsh reaction conditions, etc., and achieve environmental friendliness , Conducive to industrial large-scale production, the effect of reaction yield and purity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of 2-chloro-1-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl) ethyl ketone
[0049] In a 500ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a constant pressure dropping funnel, add 150ml of dry tetrahydrofuran, protect it with nitrogen, and add 24.4g of 6-fluoro-3,4-dihydro-2H-benzo Ethyl pyran-2-carboxylate, then add 40.4 g of bromochloromethane, cool down to -50°C, add 130 ml of 2.5 mol / L n-butyllithium n-hexane solution dropwise, after the addition is complete, react for 3 hours, use saturated 100 milliliters of ammonium chloride solution was quenched, after the solvent was distilled off under reduced pressure, 100 milliliters of toluene was added to extract three times, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain 22.1 grams of product with a yield of 89% (HPLC 95% purity).
Embodiment 2
[0051] Preparation of 2-chloro-1-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl) ethyl ketone
[0052] In a 500ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a constant pressure dropping funnel, add 150ml of dry tetrahydrofuran, protect it with nitrogen, and add 24.4g of 6-fluoro-3,4-dihydro-2H-benzo Ethyl pyran-2-carboxylate, then add 40.0 g of iodochloromethane, cool down to -60°C, add 108 ml of 2.5 mol / L n-butyllithium n-hexane solution dropwise, after the dropwise addition, react for 3 hours, use 100 milliliters of saturated ammonium chloride solution was quenched, after the solvent was distilled off under reduced pressure, 100 milliliters of toluene was added to extract three times, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain 23 grams of product, yield 92% ( HPLC purity 96%).
Embodiment 3
[0054] Preparation of 2-bromo-1-(6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl)ethyl ketone
[0055] In a 500ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a constant pressure dropping funnel, add 150ml of dry tetrahydrofuran, protect it with nitrogen, and add 24.4g of 6-fluoro-3,4-dihydro-2H-benzo Ethyl pyran-2-carboxylate, then add 27.6 g of iodobromomethane, cool down to -70°C, add 65 ml of 2.5 mol / L n-butyllithium n-hexane solution dropwise, after the addition is complete, react for 3 hours, use saturated 100 milliliters of ammonium chloride solution was quenched, after the solvent was evaporated under reduced pressure, 100 milliliters of toluene was added to extract three times, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain 26.4 grams of product, yield 89% (HPLC The purity is 96%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com