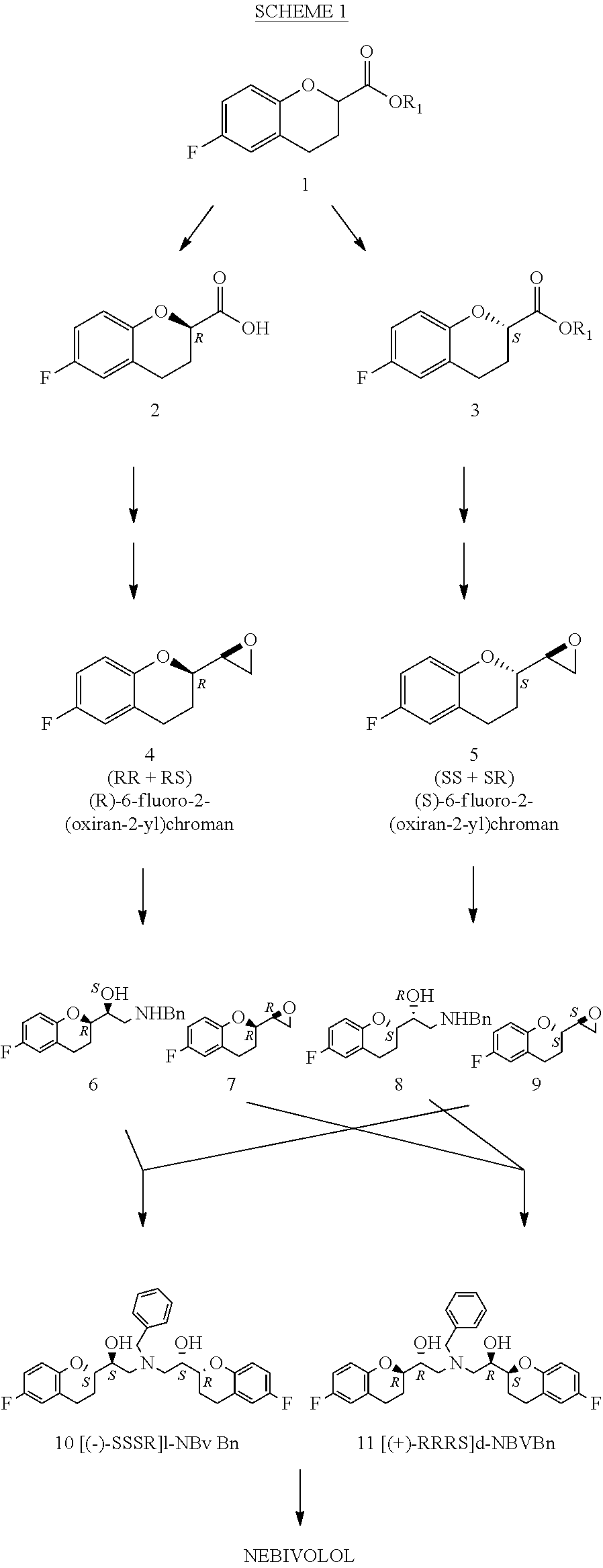
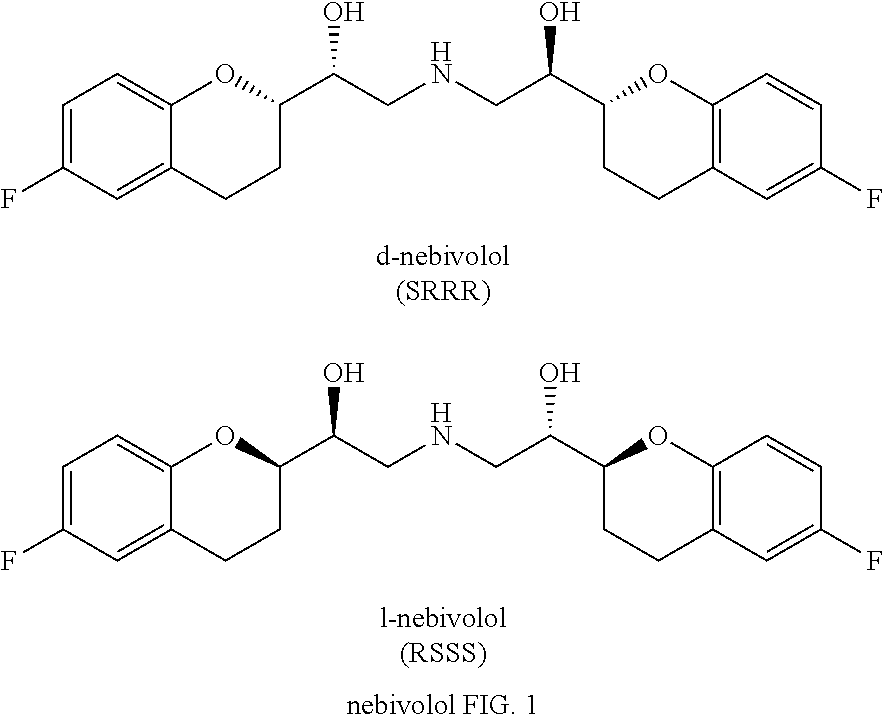
Process for the preparation of nebivolol
a synthesis process and nebivolol technology, applied in the field of new synthesis process of nebivolol, can solve problems such as the increase of the overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0069]As described in EP-0687305, a strain of recombinant E. Coli containing the esterase originally expressed in Ophiostoma novo-ulmi is cultivated according to techniques well-known to a person skilled in the art. A cell fraction is lysed by sonication and the lysate centrifuged to obtain a cell-free supernatant solution. 1.6 mL of solution containing the esterase (lipase) enzyme obtained from Ophiostoma novo-ulmi (6800 units / mL) and a suspension of about 25 g of ethyl 6-fluorochroman-2-carboxylic acid (1) in 25 mL of deionized water with 100 μL of Tween 80, are added to 500 mL of a 0.1N NaHCO3 buffer solution (pH 9.7), optionally adjusting the pH with 2N NaOH to a value of 9.7. The mixture thus obtained is gently stirred.
[0070]pH is automatically maintained at the value of 9.7 with controlled additions of a 2N NaOH solution.
[0071]Evolution of the reaction is controlled by HPLC.
[0072]At the end of the hydrolysis reaction, the mixture is extracted with dichloromethane so as to obta...
example 2
Preparation of Acyl Meldrum Derivative
[0076]
[0077]28 g of resolved (R) acid are solubilized in 250 mL anhydrous dichloromethane; to the resulting solution, 1.4 equivalents of oxalyl chloride and DMF dropwise are added. The solution is maintained under stirring at room temperature and under N2; after 1.5 hours solvent is evaporated, obtaining an oil that is redissolved into 200 mL anhydrous dichloromethane. Separately, Meldrum's acid (1.05 equivalents) and pyridine (2 equivalents) are dissolved in anhydrous dichloromethane (150 mL) and left under stirring at 0° C. for 15 min. To this solution the previously formed acid chloride is added. At the end of the adding the mixture is left under stirring at 0° C. for 1 hour, and other 45 min at room temperature. Then, it is diluted with other 500 mL dichloromethane and the organic phase is washed with H2O (2×200 mL), 2N HCl (100 mL), water, and brine, and dried on Na2SO4. An oil is obtained which is taken up with 20 volumes of diisopropylete...
example 3
Preparation of β-Keto Ester
[0078]
[0079]40 grams of crude acyl Meldrum derivative (R) are placed under stirring with 110 mL tert-butanol; the resulting mixture is heated to 80° C. for 1 h until a control by HPLC highlights the disappearance of the starting product. At the end of the reaction, tert-butanol is evaporated under reduced pressure; it is taken up with 500 mL ethyl acetate and the organic phase is washed with a saturated NaHCO3 solution, H2O to neutrality, brine and it is dried on Na2SO4. Then the solvent is evaporated, obtaining 28 g of crude β-keto ester (HPLC purity=69%, λ=280 nm) as an oil, which is used in the subsequent reaction without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com