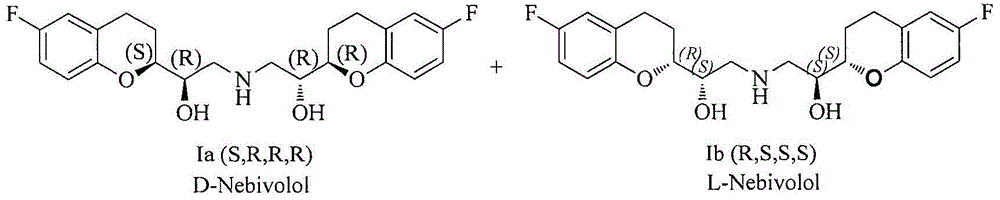
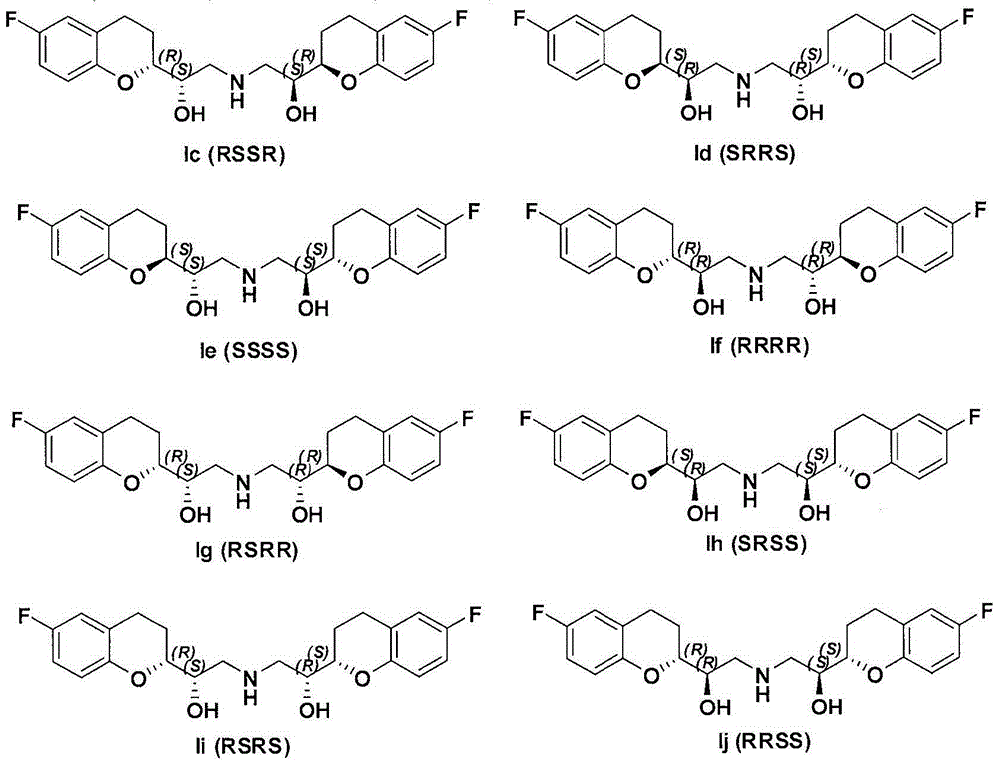
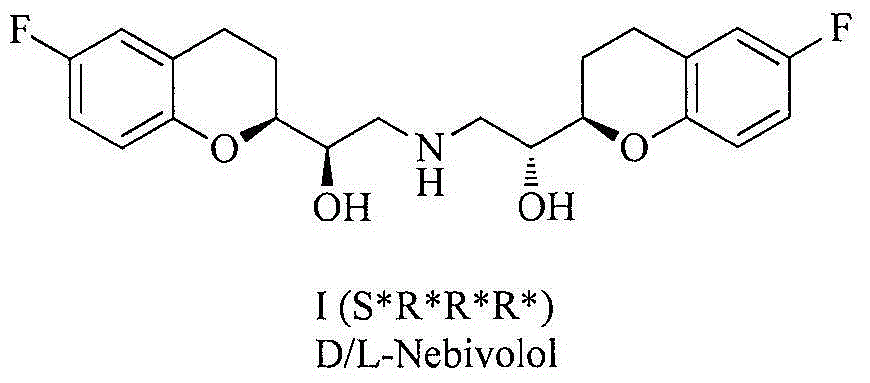Synthesis method and intermediate compound of nebivolol
A technology of compounds and intermediates, applied in the field of synthesizing nebivolol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0355] Example 1: Preparation of 1-benzyloxy-2-bromomethyl-4-fluorobenzene (compound XIV, wherein R is benzyl)
[0356]
[0357] The raw material 2-benzyloxy-5-fluorobenzyl alcohol used in this embodiment can be obtained from the references of known compound 2-hydroxyl-5-fluorobenzyl alcohol (Medicinal Chemistry letters, 2010, vol.1, #7p.321-325 , Bioorganic & Medicinal Chemistry, 2006, vol.14, #6p.2022-2031) method preparation.
[0358] Dissolve 5.14g (22mmol) of 2-benzyloxy-5-fluorobenzyl alcohol in 180mL of anhydrous ether, and drop into PBr at 0°C 3 (2.3mL, 24.4mmol) in 20mL of anhydrous diethyl ether solution was raised to room temperature for 2h, and TLC showed that the reaction was complete.
[0359] Post-processing: add 50mL of water, separate the organic layer, extract the aqueous layer with (50mL*3) DCM, combine the organic phase, wash with saturated sodium bicarbonate, wash with water, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, filte...
Embodiment 2
[0361] Example 2: Preparation of 4-[(2-benzyloxy-5-fluorophenyl)-butyn-1-yl]trimethylsilane (compound XV, wherein R is benzyl)
[0362]
[0363] Add 2.4mL, 16.1mmol trimethylsilylpropyne to 40mL anhydrous THF, cool to -23°C, add 2.5M n-BuLi 7.7mL (19.3mmol) dropwise, and stir at this temperature for 2h until the reaction liquid After cooling down to -100°C, 3.5g (11.9mmol) of compound XIV (wherein R is benzyl) in 5mL of anhydrous THF solution was added dropwise, and the reaction was completed at this temperature for 1h, and TLC showed that it was complete.
[0364] Post-processing: stop the reaction with 10% saturated ammonium chloride, separate several layers, extract the aqueous layer (100mL*2) with ether, combine the organic phases, wash with saturated ammonium chloride, dry with anhydrous sodium sulfate, filter, concentrate, and column chromatography (PE / Et 2 O=100:1) to obtain 3.79 g of pure product with a yield of 97.6%.
[0365] 1 H-NMR (400MHz, CDCl 3 )δ7.38~7.4...
Embodiment 3
[0366] Example 3: Preparation of 1-(benzyloxy)-2-(butyn-3-yl)-4-fluorobenzene (compound XVI, wherein R is benzyl)
[0367]
[0368] Dissolve 1.15g (3.52mmol) of compound XV (wherein R is benzyl) in 20ml of MeOH, add 0.5g (3.6mmol) of K 2 CO 3 , stirred at room temperature for 3h, the solvent was evaporated under reduced pressure, the residue was extracted with EtOAc, washed with water, washed with saturated NaCl, anhydrous NaCl 2 SO 4 , dried, filtered, and the filtrate was evaporated to dryness to obtain 0.87 g of a colorless oil. Filter through a short column of silica gel and elute with PL / EtOAc (100 / 2) to obtain 0.85 g of a colorless oil.
[0369] 1 H-NMR (400MHz, CDCl 3 )δ7.33~7.42(m, 5H), 6.93~6.96(dd, J=9.6, 2.8Hz, 1H), 6.81~6.86(m, 2H), 5.05(s, 2H), 2.86~2.90(t, J=7.2Hz, 2H), 2.47~2.51(t, J=7.2Hz, 2H), 1.96(s, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com