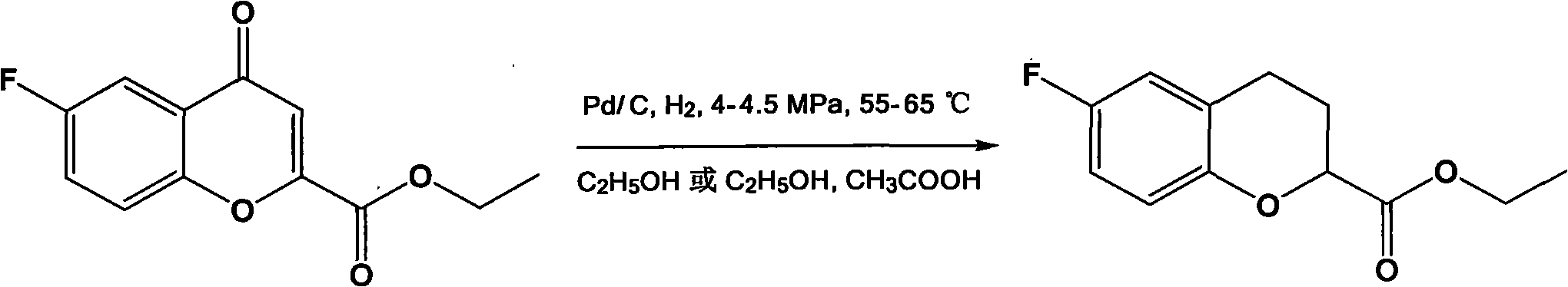
Preparation method for 6-fluorine-3,4-dihydro-2H-1-benzopyran-2-ethyl formate
A technology of benzopyran and ethyl formate, which is applied in the field of preparing nebivolol intermediates, can solve the problems of low yield of starting materials, difficult to obtain, etc., and achieves easy preparation, little environmental pollution, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Using ethanol as a solvent to prepare nebivolol intermediate (6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid ethyl ester)
[0021] In a 500ml hydrogenation kettle, 20g (0.085mol) of nebivolol intermediate (6-fluoro-4-oxo-4H-1-benzopyran-2-carboxylic acid ethyl ester), palladium carbon (7% Pd / C, water content 65%) 6.8g, add in 180ml ethanol solvent. Under the condition of 4.5MPa hydrogen and 62°C, the reaction was mechanically stirred for 24 hours. The reaction solution was filtered to recover palladium carbon, and the ethanol solvent in the filtrate was evaporated under reduced pressure. The distillation residue was dissolved in dichloromethane and neutralized to pH=6-7 with saturated sodium bicarbonate solution. The aqueous phase was back extracted once with dichloromethane, and the dichloromethane phases were combined. The dichloromethane was distilled off under reduced pressure to obtain an oily substance, and after vacuum drying for 24 hours, ...
Embodiment 2
[0022] Example 2: Using ethanol as a solvent to prepare nebivolol intermediate (6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid ethyl ester)
[0023] In a 1200L hydrogenation kettle, 84.5Kg (0.085mol) of nebivolol intermediate (6-fluoro-4-oxo-4H-1-benzopyran-2-carboxylic acid ethyl ester), palladium carbon (7% Pd / C, water content 65%) 44Kg, add in 800L ethanol solvent. Under the condition of 4.2MPa hydrogen and 62°C, the reaction was carried out with mechanical stirring for 96 hours. The reaction solution was filtered to recover palladium carbon, and the ethanol solvent in the filtrate was evaporated under reduced pressure. The distillation residue was dissolved in dichloromethane and neutralized to pH=6-7 with saturated sodium bicarbonate solution. The aqueous phase was back extracted once with dichloromethane, and the dichloromethane phases were combined. Dichloromethane was distilled off under reduced pressure to obtain an oily substance, and after vacuum drying f...
Embodiment 3
[0024] Example 3: Preparation of nebivolol intermediate (6-fluoro-3,4-dihydro-2H-1-benzopyran-2-carboxylic acid ethyl ester) using a mixed solvent of ethanol and acetic acid
[0025] In a 500ml hydrogenation kettle, 30g (0.127mol) of nebivolol intermediate (6-fluoro-4-oxo-4H-1-benzopyran-2-carboxylic acid ethyl ester), palladium carbon (7% Pd / C, water content 65%) 10.2g, add in the mixed solvent of ethanol and acetic acid (ethanol 180ml, acetic acid 1.8ml). Under the condition of 4.5MPa hydrogen and 62°C, the reaction was mechanically stirred for 24 hours. The reaction solution was filtered to recover palladium carbon, and the ethanol solvent in the filtrate was evaporated under reduced pressure. The distillation residue was dissolved in dichloromethane and neutralized to pH=6-7 with saturated sodium bicarbonate solution. The aqueous phase was back extracted once with dichloromethane, and the dichloromethane phases were combined. Dichloromethane was distilled off under red...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com