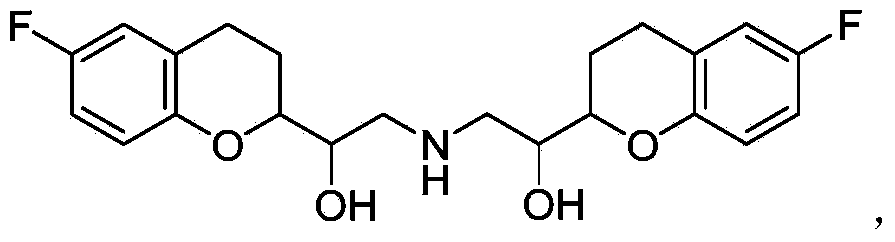
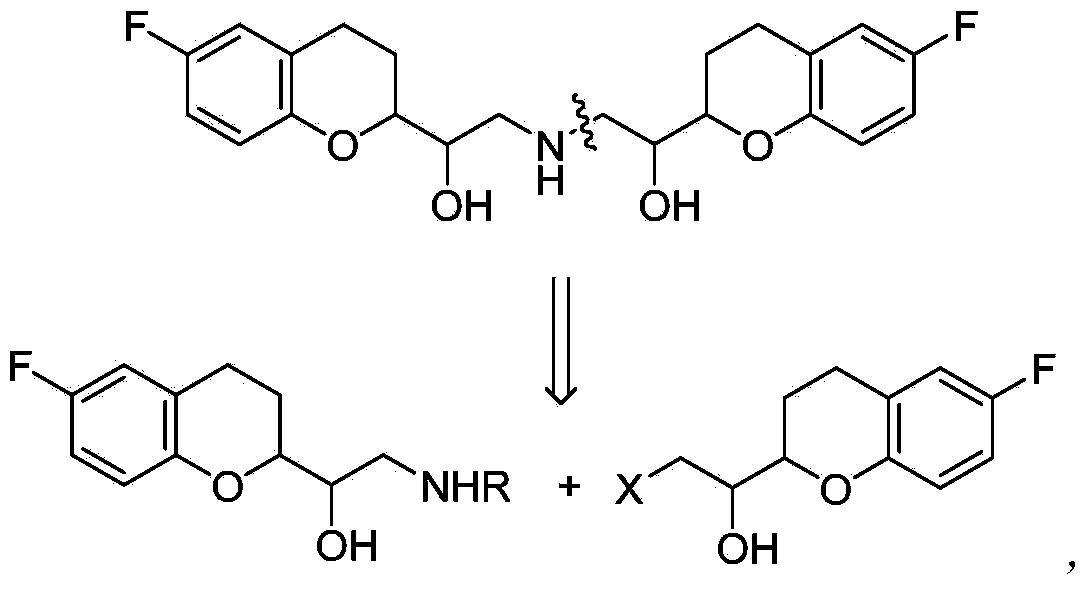
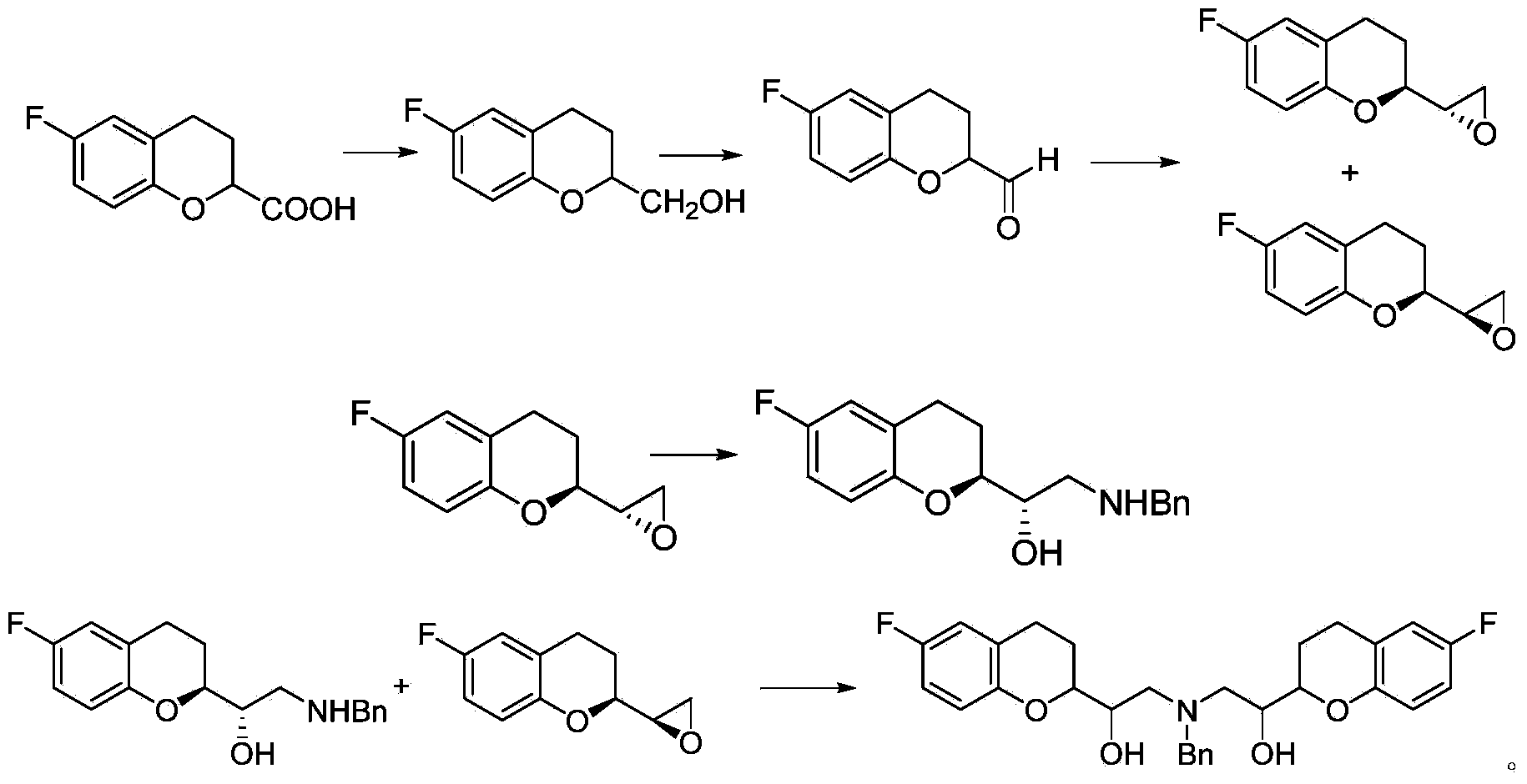
Synthetic method of nebivolol
A synthesis method and mixture technology, applied in the field of chemical synthesis of drugs, can solve the problems of difficult batch production, low yield, long synthesis route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Starting from a mixture of racemic diastereomer A and racemic diastereomer B
[0048] (1) Preparation of racemic diastereomer A and racemic diastereomer B
[0049] 2-Amino-1-(-6-fluoro-2-chromanyl)ethanol diastereomeric mixture (2 g) was recrystallized from ethanol to give crystals (0.6 g, 30%) as pure diastereomer A , The solvent was removed by rotary evaporation of the mother liquor, and the obtained solid was recrystallized several times to obtain diastereomer B.
[0050] Diastereomer A 1 HNMR (CDCl 3 , 400MHz): δ6.78-6.73(m,3H),3.97(ddd,J=10.1Hz,4.6Hz,3.0Hz,1H),3.66(dt,J=7.2Hz,4.5Hz,1H),2.97- 2.73(m,4H),1.98-1.91(m,2H).
[0051] (2) Preparation of Bromide (IIb)
[0052]Mix and stir 2-amino-1-(-6-fluoro-2-chromanyl)ethanol diastereomer B (0.85g) with sulfuric acid (1.25M, 85ml), then add potassium bromide (5.1g) and Diethyl ether (60ml). With cooling and stirring in an ice-water bath, drop NaNO 2 solution (0.45g dissolved in 10ml water). After the dropwise a...
Embodiment 2
[0059] (S,R) and (S,S)-2-amino-1-(-6-fluoro-2-chromanyl)ethanol diastereomeric mixture and (R,S) and (R,R)- 2-Amino-1-(-6-fluoro-2-chromanyl)ethanol diastereomeric mixture as starting material
[0060] (1) Preparation of diastereomer S-A and diastereomer S-B
[0061] (S,R) and (S,S)-2-Amino-1-(-6-fluoro-2-chromanyl)ethanol diastereomeric mixture (2 g) was recrystallized from ethanol to give crystals (0.6 g ) is the pure diastereomer S-A, the mother liquor is rotatably evaporated to remove the solvent, and the obtained solid is recrystallized several times to obtain the diastereomer S-B.
[0062] Diastereomer S-A 1 HNMR (CDCl 3 , 400MHz): δ6.78-6.73(m,3H),3.97(ddd,J=10.1Hz,4.6Hz,3.0Hz,1H),3.66(dt,J=7.2Hz,4.5Hz,1H),2.97- 2.73(m,4H),1.98-1.91(m,2H).
[0063] (2) Preparation of diastereomer R-A and diastereomer R-B.
[0064] The (R,S) and (R,R)-2-amino-1-(-6-fluoro-2-chromanyl)ethanol diastereomeric mixture (2 g) was recrystallized from ethanol to give crystals (0.6 g ) is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com