Medical composition of levamlodipine or pharmaceutically acceptable salt thereof and beta-blocker and application thereof
A technology of levamlodipine and levammonium chloride, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problem of limited contribution of drug dosage, reduce the incidence rate, improve the quality of life, The effect of reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
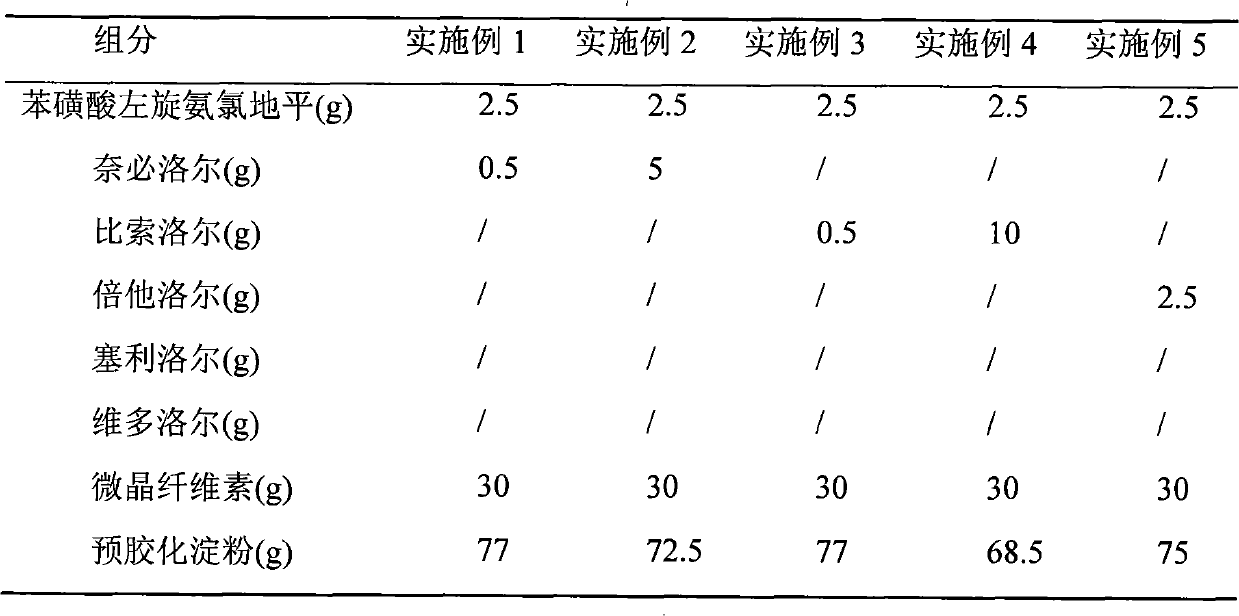
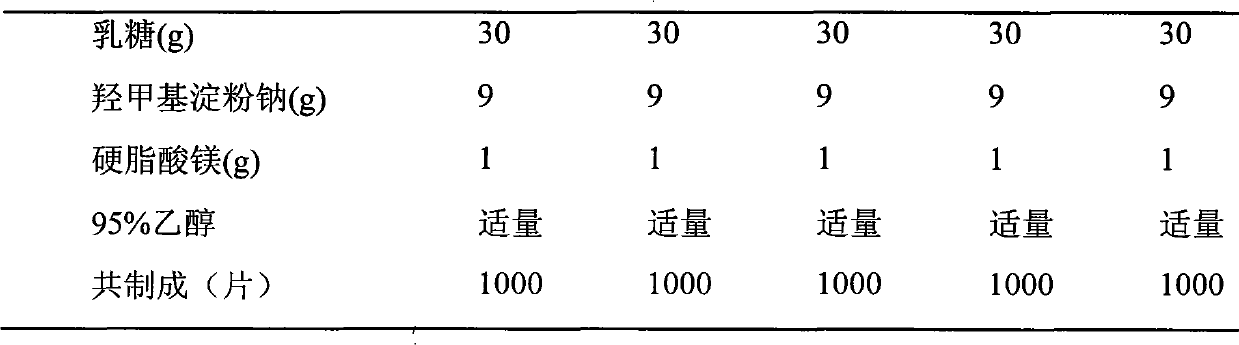
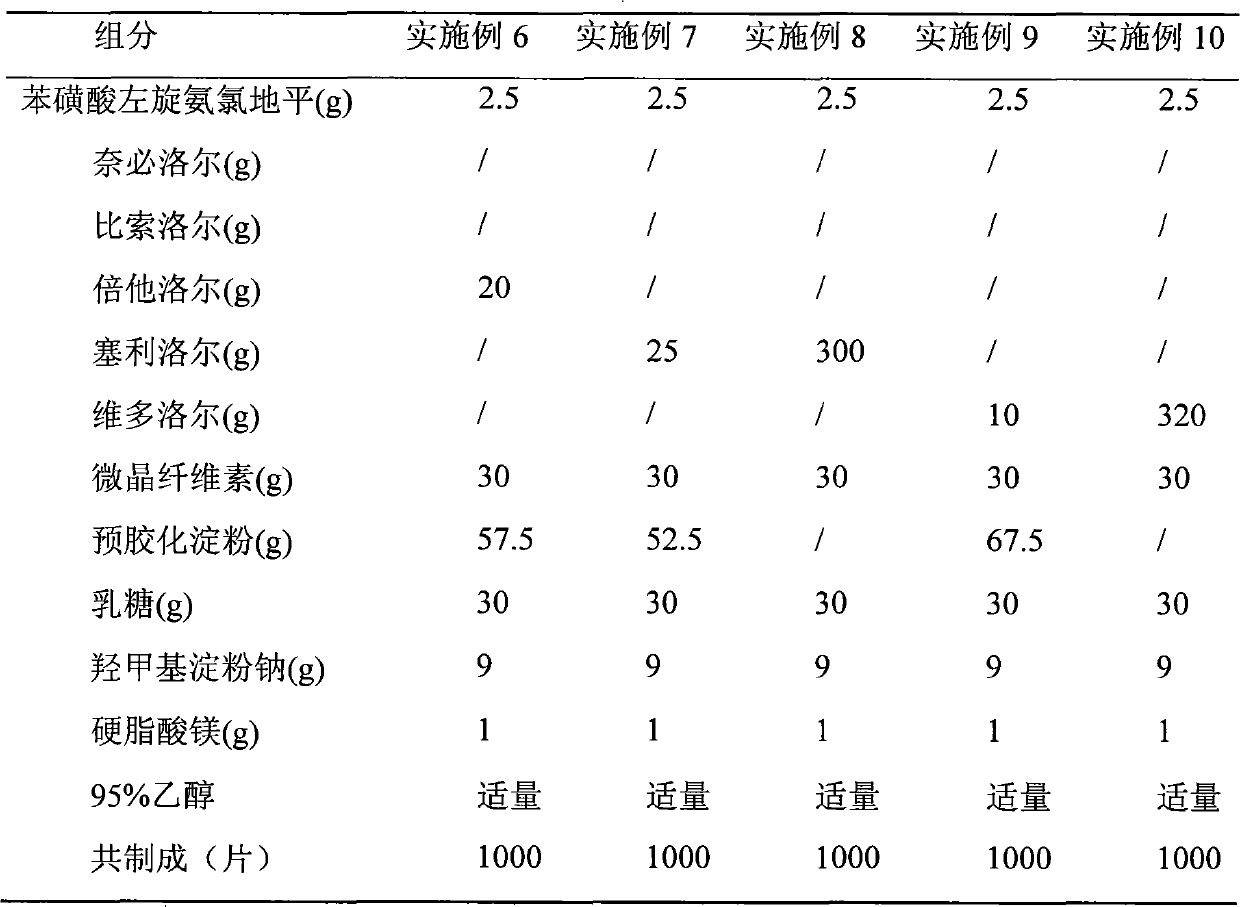
[0024] The preparation of embodiment 1-10 compound tablet
[0025]
[0026]
[0027]
[0028] Preparation process: put levamlodipine, β receptor blocker, microcrystalline cellulose, pregelatinized starch, lactose, and hydroxymethyl starch sodium into a mortar, grind and mix evenly, pass through a 20-mesh sieve, and add an appropriate amount of 95% Ethanol is made into a soft material, granulated through a 20-mesh sieve, ventilated and dried at 40°C, the dry granules are sized with a 16-mesh sieve, magnesium stearate is added, mixed evenly, and then compressed into tablets.
[0029] Dosage: Take orally, once a day, 1-2 tablets each time, in the morning.
[0030] The preparation of embodiment 11-15 compound capsule
[0031]
[0032]
[0033] Preparation process: put levamlodipine, β-receptor blocker, and microcrystalline cellulose into a mortar, grind and mix evenly, pass through a 20-mesh sieve, add an appropriate amount of 0.5% PVP-k30 ethanol solution to make ...
example 16
[0036] The antihypertensive test of example 16 rats
[0037] Experimental method: Take 120 healthy spontaneously hypertensive SHR rats, half male and half female, weighing 200-240g, male and female rats are divided into 12 groups according to the balance of blood pressure (see Table 1 for the specific grouping method), and the drug is administered by intragastric administration For blood pressure, rat electronic sphygmomanometers were used to indirectly measure the systolic blood pressure of the rats when they were awake and quiet by the tail volume method.
[0038] Table 1: Grouping methods
[0039]
[0040]
[0041] Experimental results: single drug 1 group, single drug 2 groups, single drug 3 groups, single drug 4 groups, single drug 5 groups, single drug 6 groups, compound 1 group, compound 2 groups, compound 3 groups, compound 4 groups, compound Compared with the model group, group 5, group 6, group 7, group 8, group 9, and group 10 had a significant antihypertensive...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com