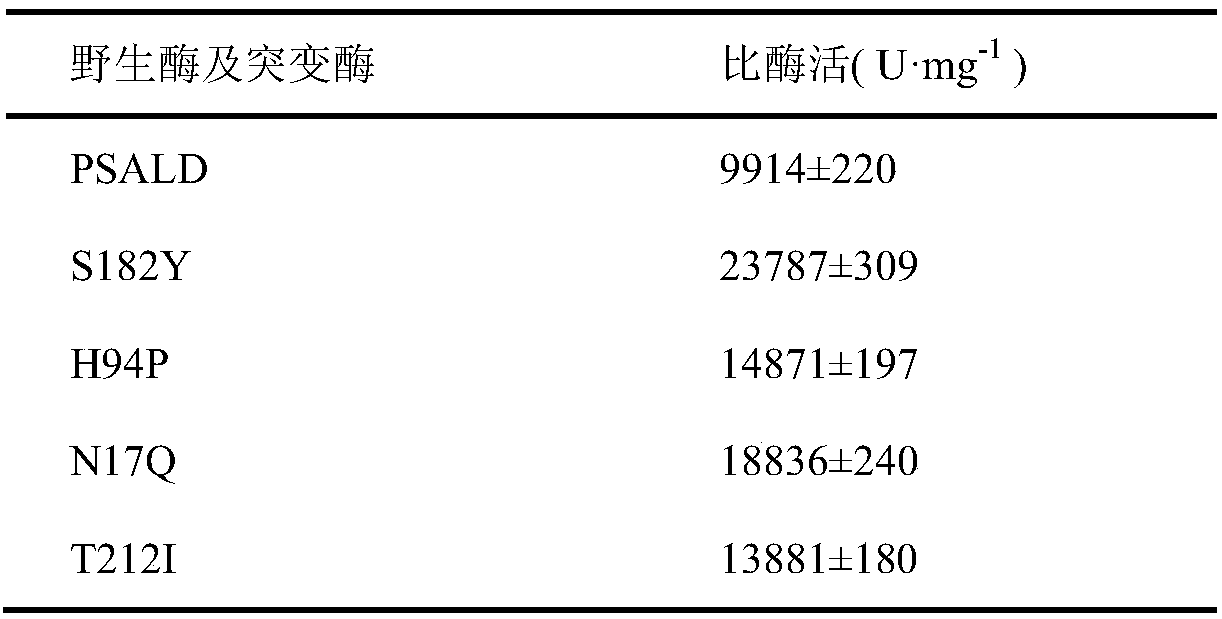
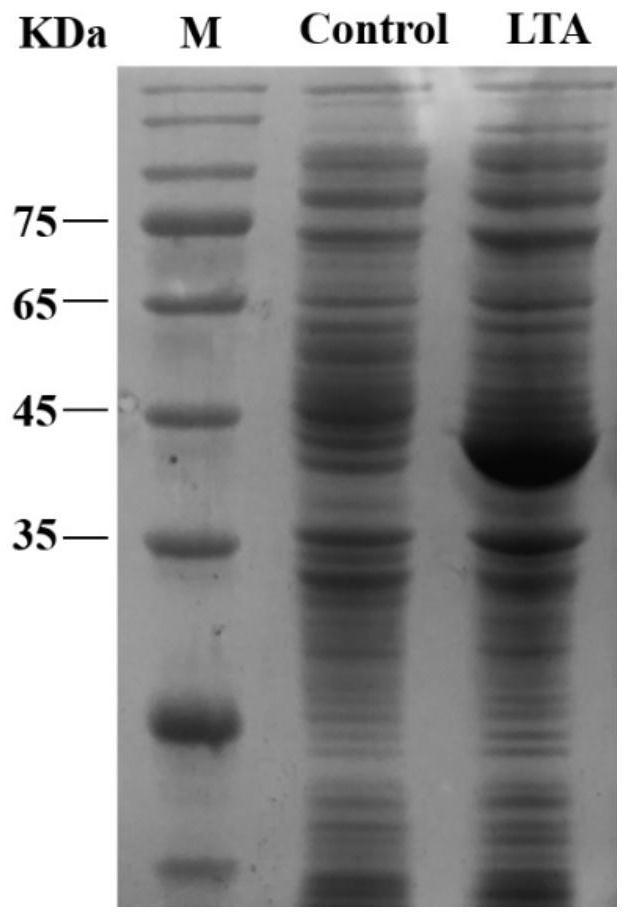
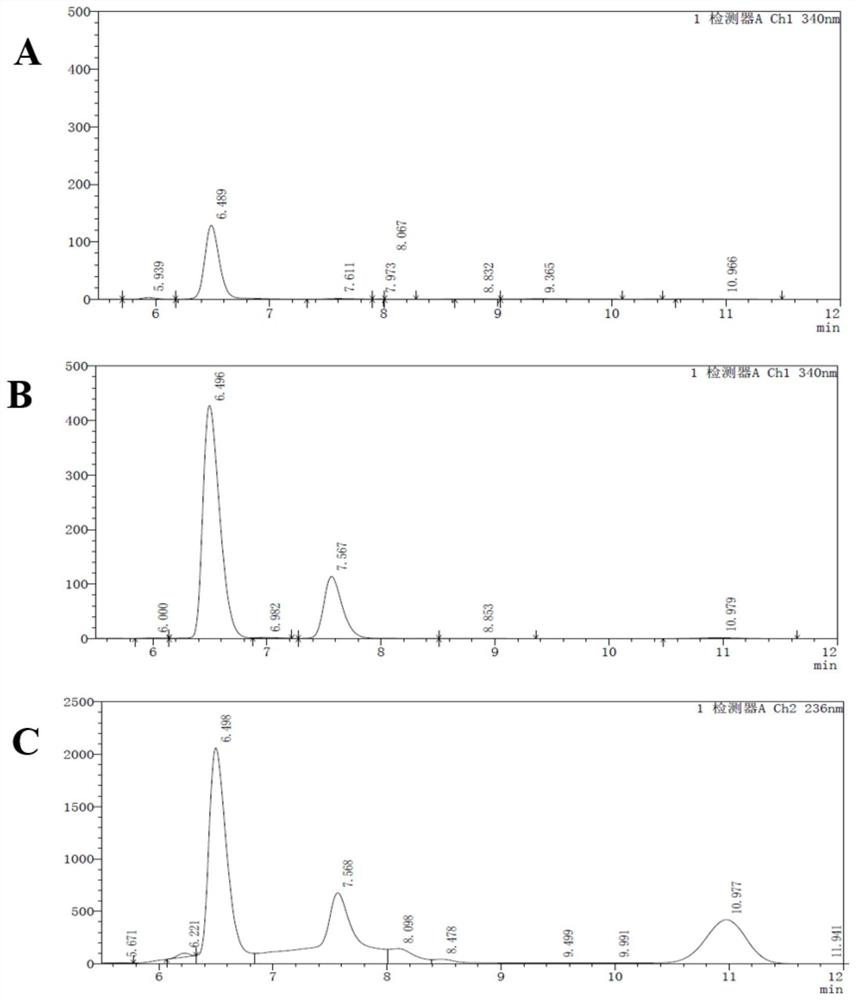
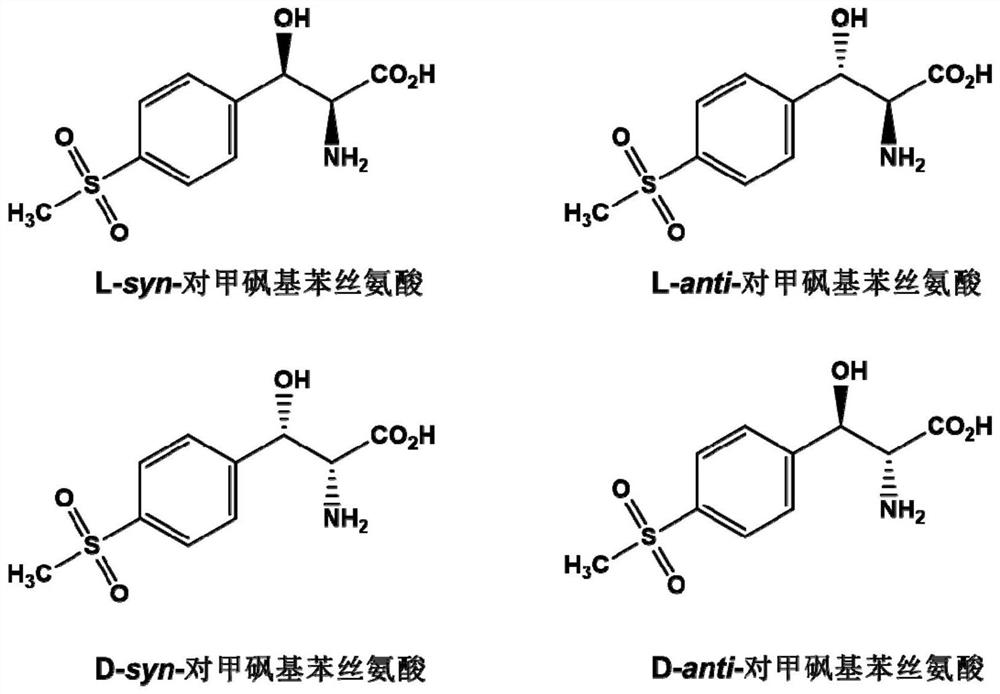
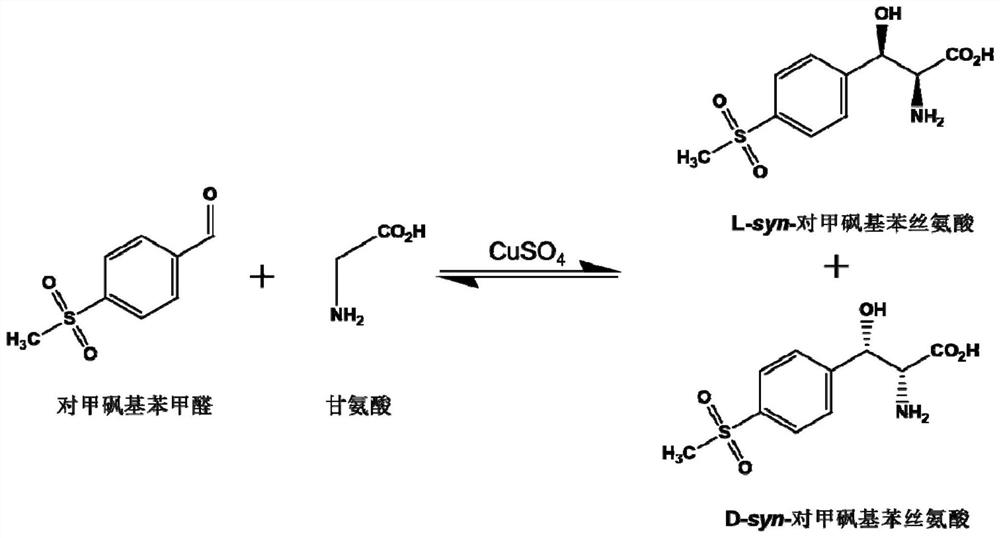
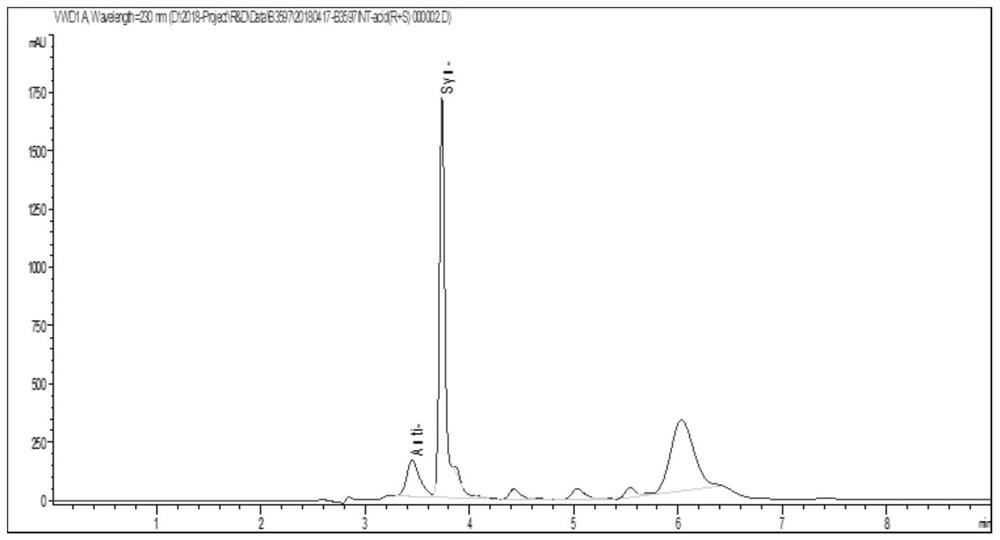
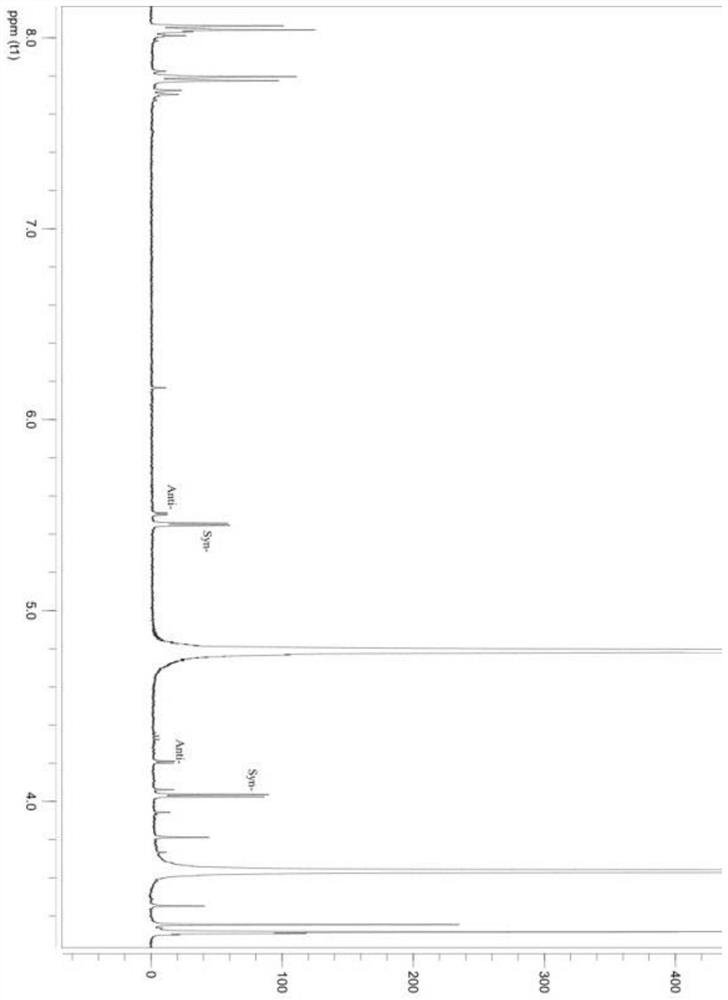
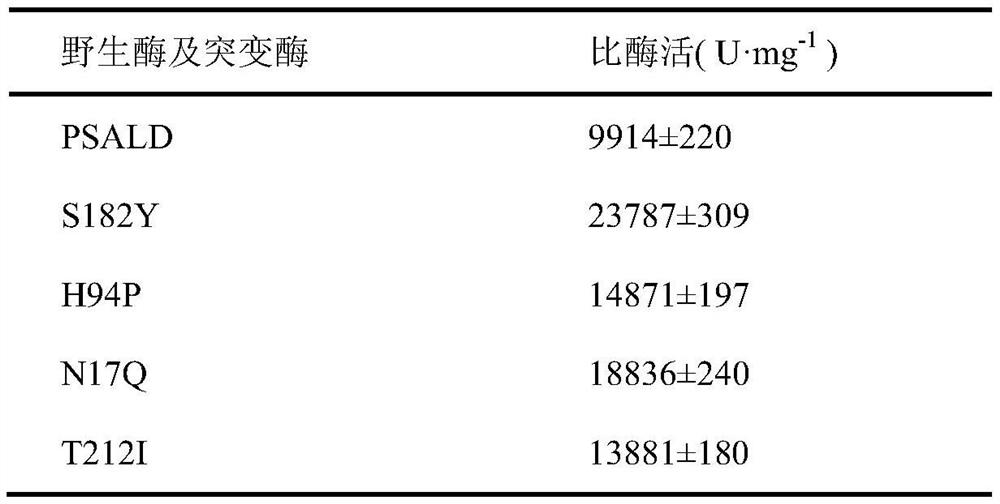
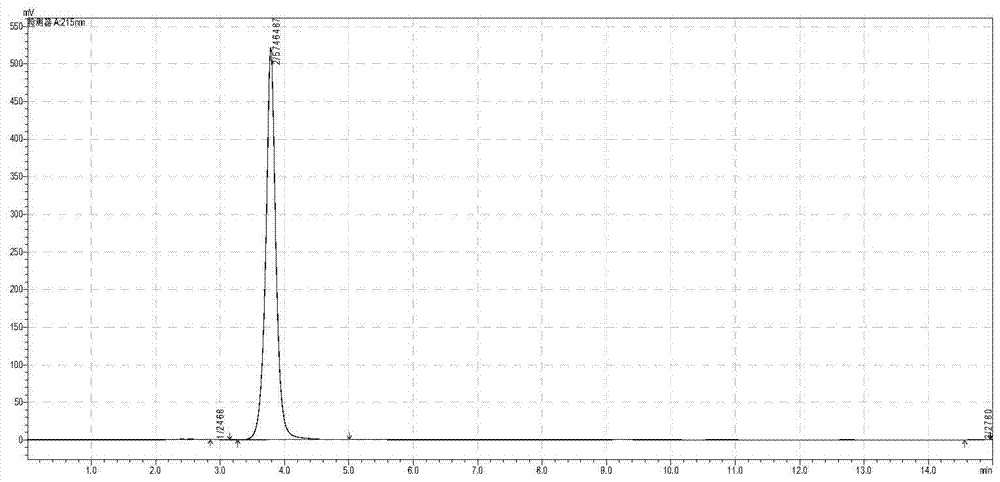
Patents
Literature
39 results about "Threonine aldolase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
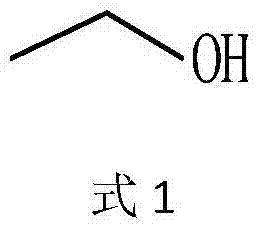
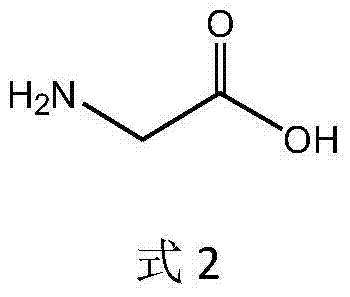
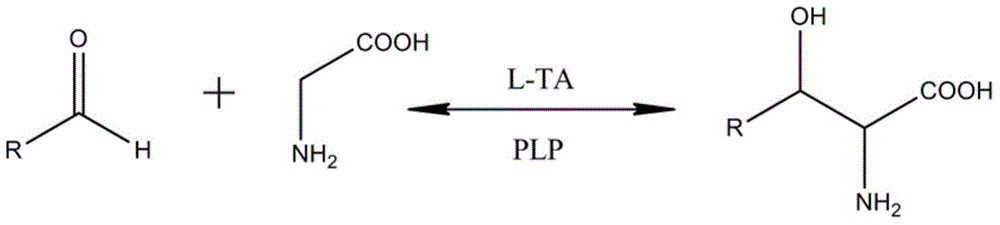
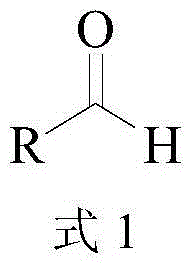
In enzymology, a threonine aldolase (EC 4.1.2.5) is an enzyme that catalyzes the chemical reaction L-threonine ⇌ glycine + acetaldehyde Hence, this enzyme has one substrate, L-threonine, and two products, glycine and acetaldehyde. This enzyme belongs to the family of lyases, specifically the aldehyde-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is L-threonine acetaldehyde-lyase (glycine-forming).
Threonine aldolase, mutant and application of threonine aldolase and mutant to preparation of substituted phenylserine derivatives
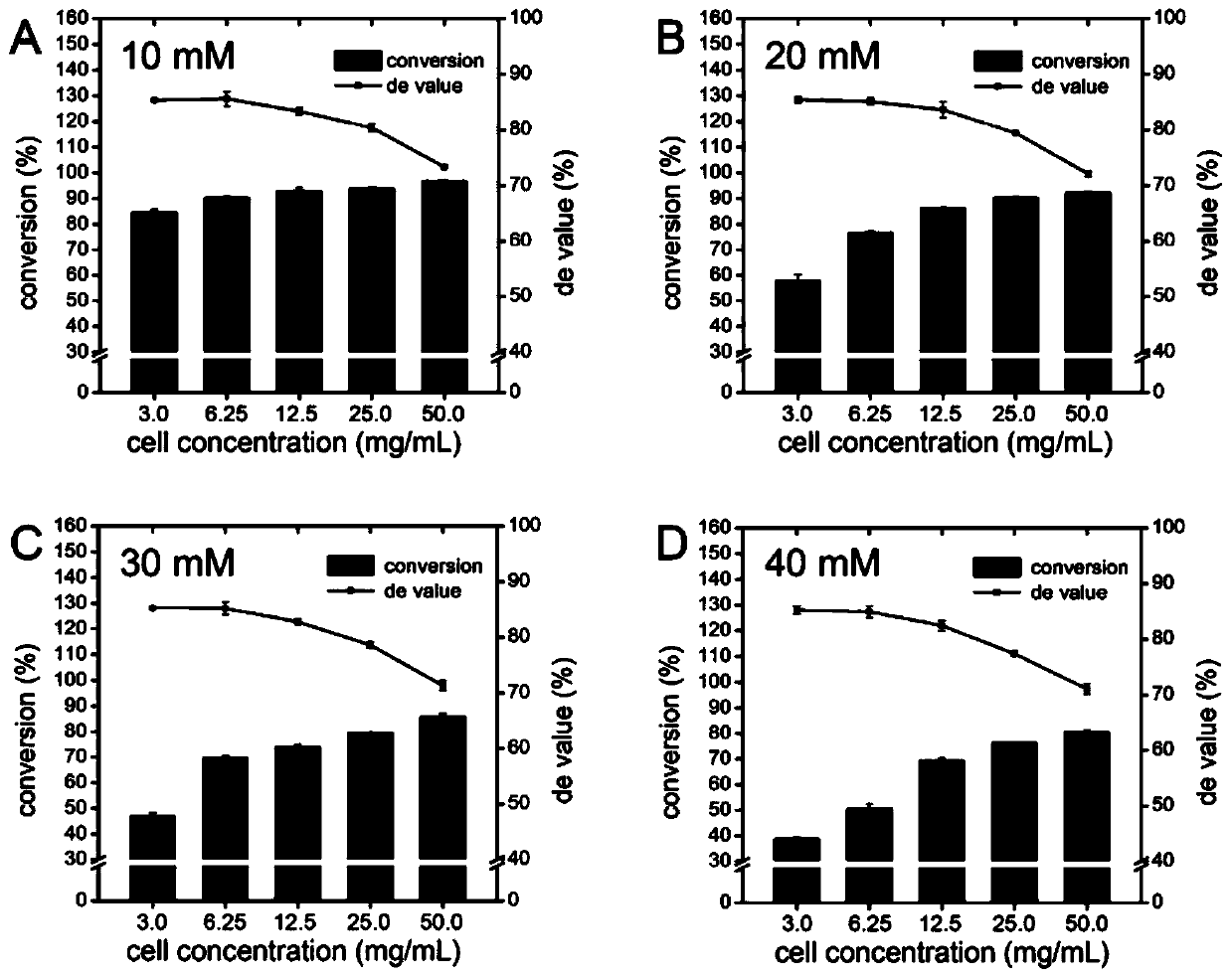
The invention discloses threonine aldolase, a mutant, encoding genes of the threonine aldolase and the mutant, a recombinant vector constructed by the encoding genes, recombinant genetic engineering strains obtained by transforming the recombinant vector and application of the threonine aldolase and the mutant in preparation of 2- / 3- / 4-substituted phenylserine derivatives. The threonine aldolase and the mutant serve as biocatalysts, and 2- / 3- / 4-substituted benzaldehyde serves as a substrate, 5-pyridoxal phosphate serves as coenzyme, glycine / glycine ester serves as an auxiliary substrate, and enzymic catalytic reaction is performed in a medium under appropriate conditions to separate and prepare a series of phenylserine derivatives with different substituents. According to the method, the total yield ranges from 76% to 99%, and the ee value of the product is greater than 99%, and the de value ranges from 70% to 99%.
Owner:王喆明 +1
Method for producing L-2-aminobutyric acid by virtue of biological catalysis
ActiveCN104774881AImprove protectionHigh yieldFermentationL-2-Aminobutyric AcidL-alpha-Aminobutyric Acid
The invention discloses a new method for synthesising L-alpha-aminobutyric acid with a high optical activity. The new method comprehensively applies alcohol dehydrogenase, threonine aldolase, threonine deaminase and L-amino acid dehydrogenase, and ethanol and glycine raw material are directly synthesised into L-alpha-aminobutyric acid by a 'one-pot' method. According to the method disclosed by the invention, L-alpha-aminobutyric acid is synthesised in one pot by virtue of the simple and easily available raw materials; the method has the advantages of being few in operation steps, environment-friendly, good in bio-safety and simple in equipment, and is beneficial to industrial large-scale production for L-alpha-aminobutyric acid with a high optical activity and L-alpha-aminobutyric acid series products.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
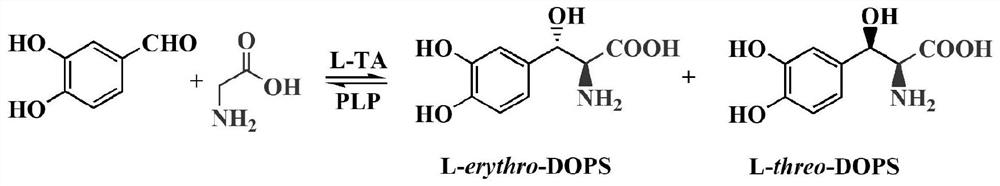
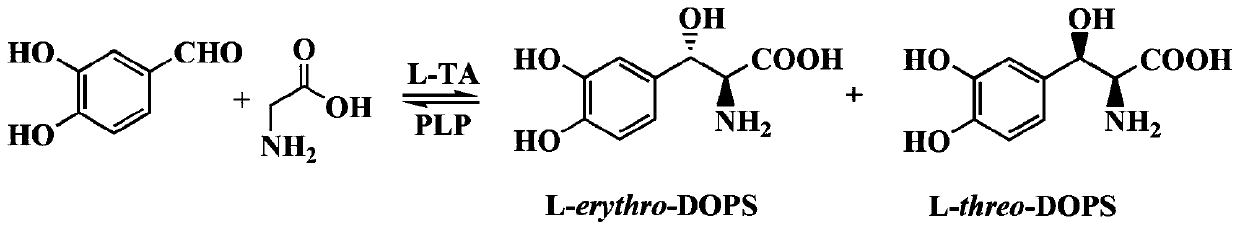
Threonine aldolase, coding gene thereof, and application of threonine aldolase to biosynthesis of L-threo-DOPS
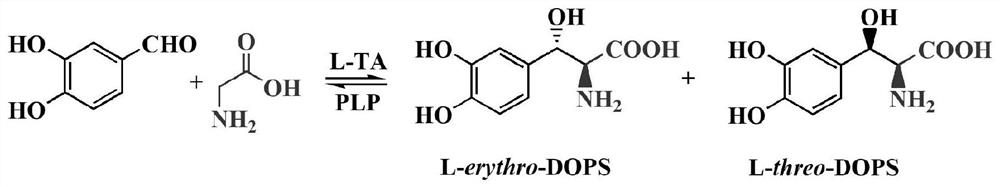
The invention relates to threonine aldolase, a coding gene thereof, and an application of the threonine aldolase to biosynthesis of L-threo-DOPS, and belongs to the field of a biological technology. The threonine aldolase is protein as shown in SEQID NO.1, or protein having the same functions, obtained through replacing and / or deleting and / or adding one or more amino acid residues to an amino acidsequence as shown in SEQID NO.1. The threonine aldolase can use benzaldehyde with replaced 3- / 4- hydroxyl as a substrate, 5-pyridoxal phosphate as a coenzyme and glycine as an assistance substrate. Under the appropriate condition and in an appropriate medium, an enzyme catalysis reaction is performed, and biosynthesis is performed to obtain the L-threo-DOPS (L-threo-DOPS). The non-antipode selectivity of the method can achieve 30%. The threonine aldolase having high selectivity has important significance for biocatalysis of the L-threo-DOPS.
Owner:CHONGQING UNIV
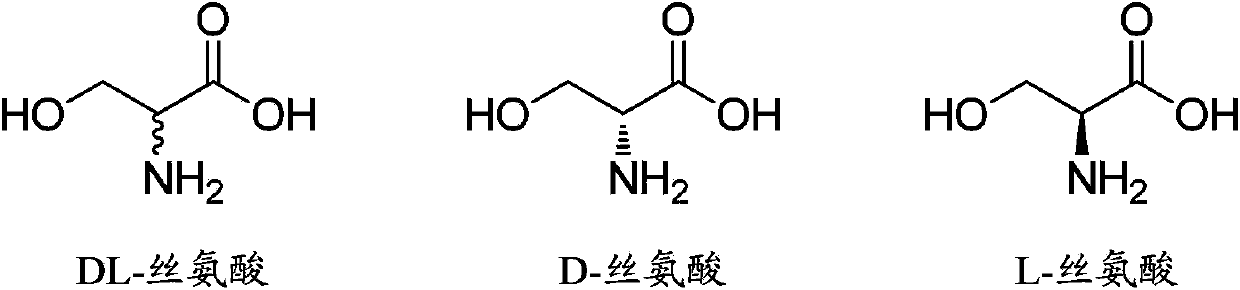
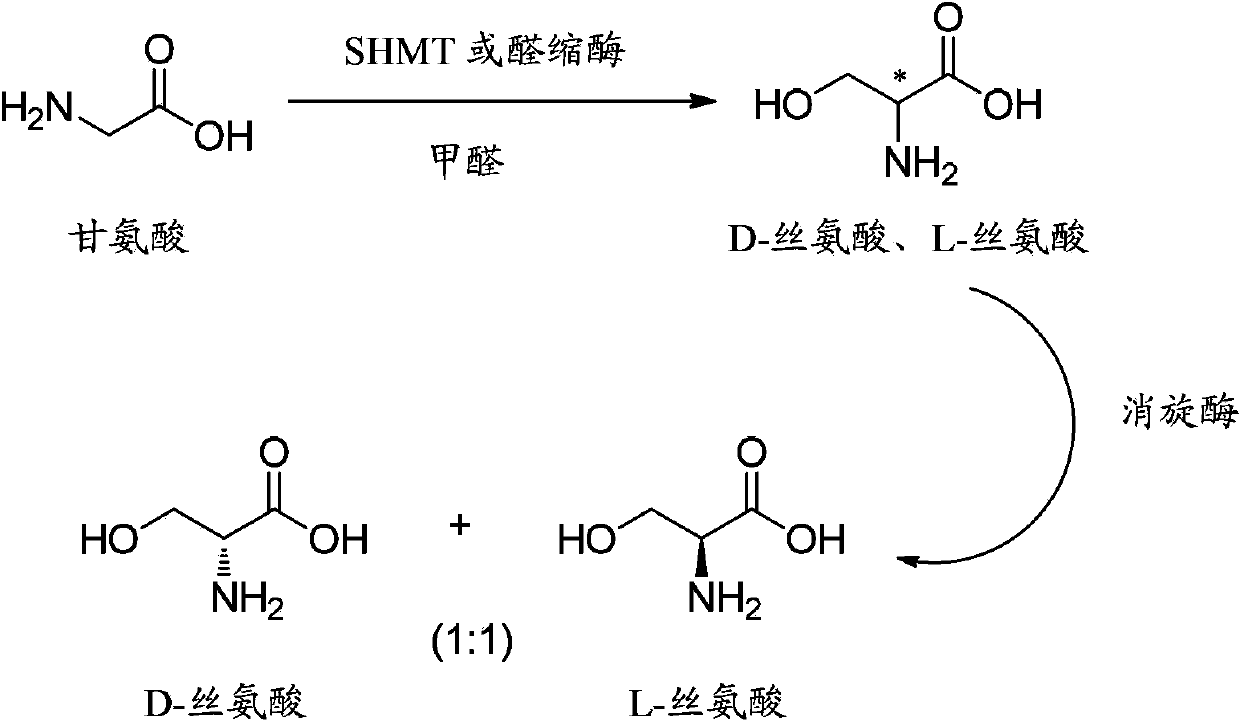
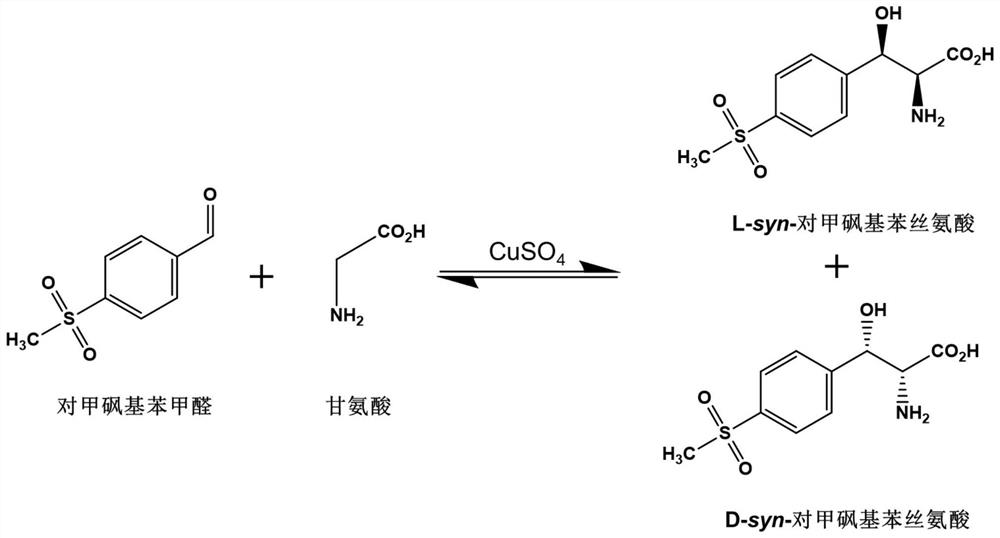
Method for synthesizing DL-serine through enzyme catalysis method
InactiveCN103966276AIngenious designReduce manufacturing costFermentationAmino-acid racemase activityEnzyme catalysis
The present invention relates to a method for synthesizing DL-serine through an enzyme catalysis method. According to the method, formaldehyde and glycine are adopted as substrates, in the presence of coenzyme and under an effect of a catalyst having serine hydroxymethyl transferase activity or D-threonine aldolase activity, one molecule of the formaldehyde and one molecule of the glycine are converted into one molecule of the single configuration serine, and racemization is performed under an effect of a catalyst having amino acid racemase activity to produce DL-serine, wherein preferably the whole enzyme catalysis reaction process is added with the cofactor so as to promote the enzyme catalysis reaction process. The method has advantages of clever design, simple operation, short production cycle, low production cost, high yield, low environmental protection pressure and the like, and is suitable for large-scale industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD +1
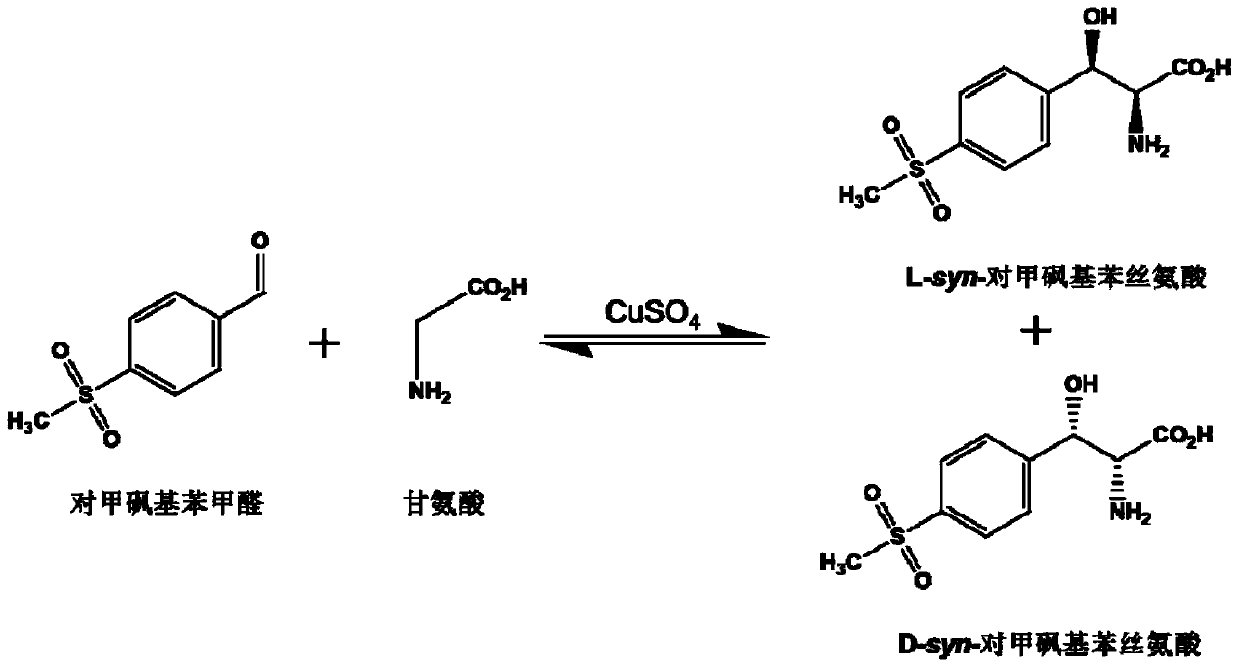
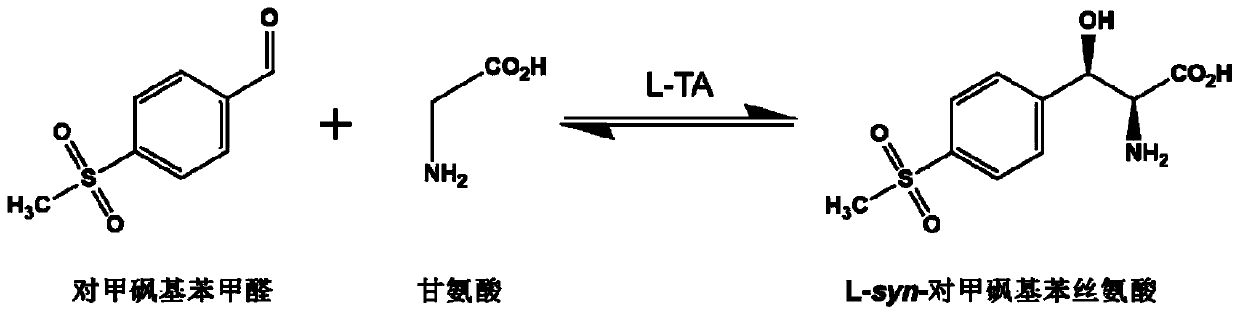
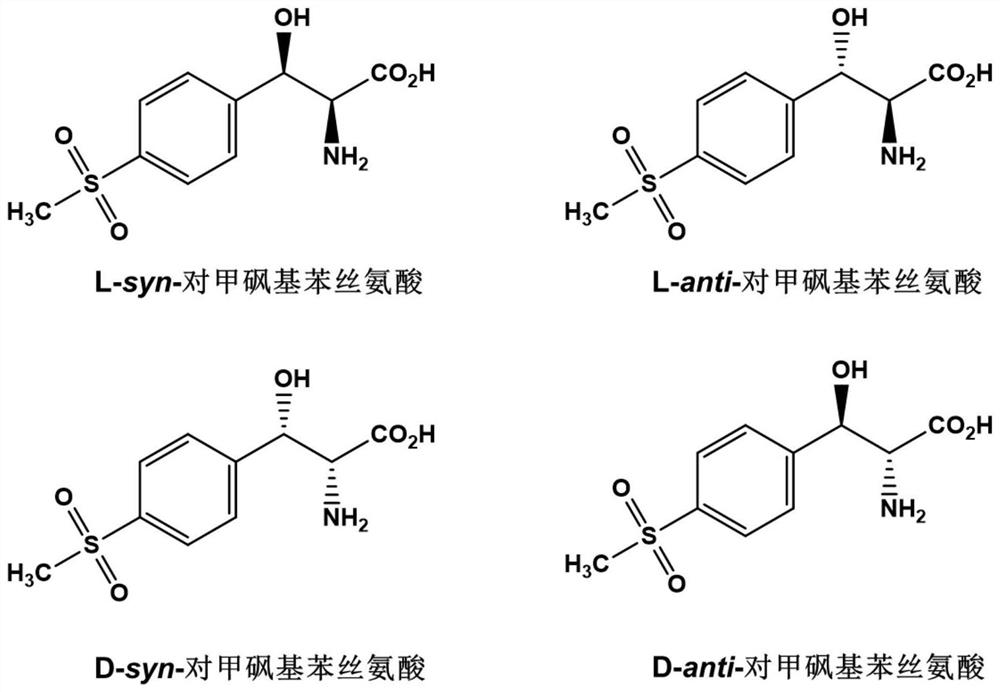
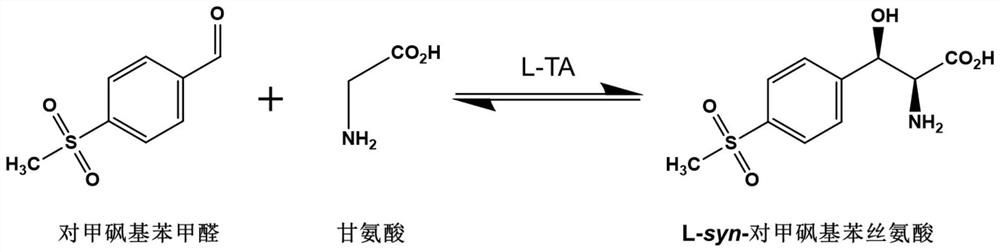
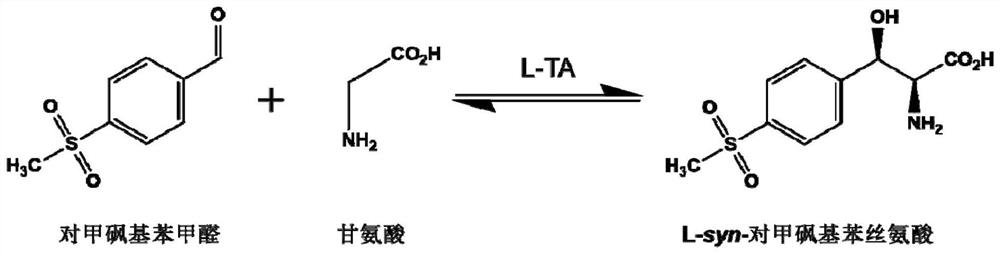
L-threonine aldolase and application of L-threonine aldolase to synthesis of methylsulfonylphenylserine
ActiveCN110577948AShort reaction timeBacteriaMicroorganism based processesEscherichia coliBenzaldehyde
The invention provides L-threonine aldolase. The L-threonine aldolase has the activity of asymmetric catalytic synthesis of (2S,3R)-methylsulfonylphenylserine from methylsulfonyl benzaldehyde and glycine. The L-threonine aldolase is selected from any one of the following groups: (1) polypeptide with the amino acid sequence shown in SEQ ID NO: 1; (2) polypeptide with the amino acid sequence shown in SEQ ID NO: 1, wherein the homology of the amino acid sequence is greater than or equal to 80%; and (3) derived polypeptide formed by substituting, missing or adding of 1-5 amino acid residues of theamino acid sequence shown in SEQ ID NO: 1 and retaining catalytic activity. The invention further provides application of the L-threonine aldolase to synthesis of the methylsulfonylphenylserine, an L-threonine aldolase gene derived from Actinocorallia herbida is mined and screened, the L-threonine aldolase derived from recombinant escherichia coli is expressed through a gene engineering technology, the (2S,3R)-methylsulfonylphenylserine is subjected to asymmetric catalytic synthesis by taking the methylsulfonyl benzaldehyde and the glycine as raw materials and adopting a whole-cell catalysismethod, and the reaction conditions are mild and environmentally friendly.
Owner:福建昌生生物科技发展有限公司
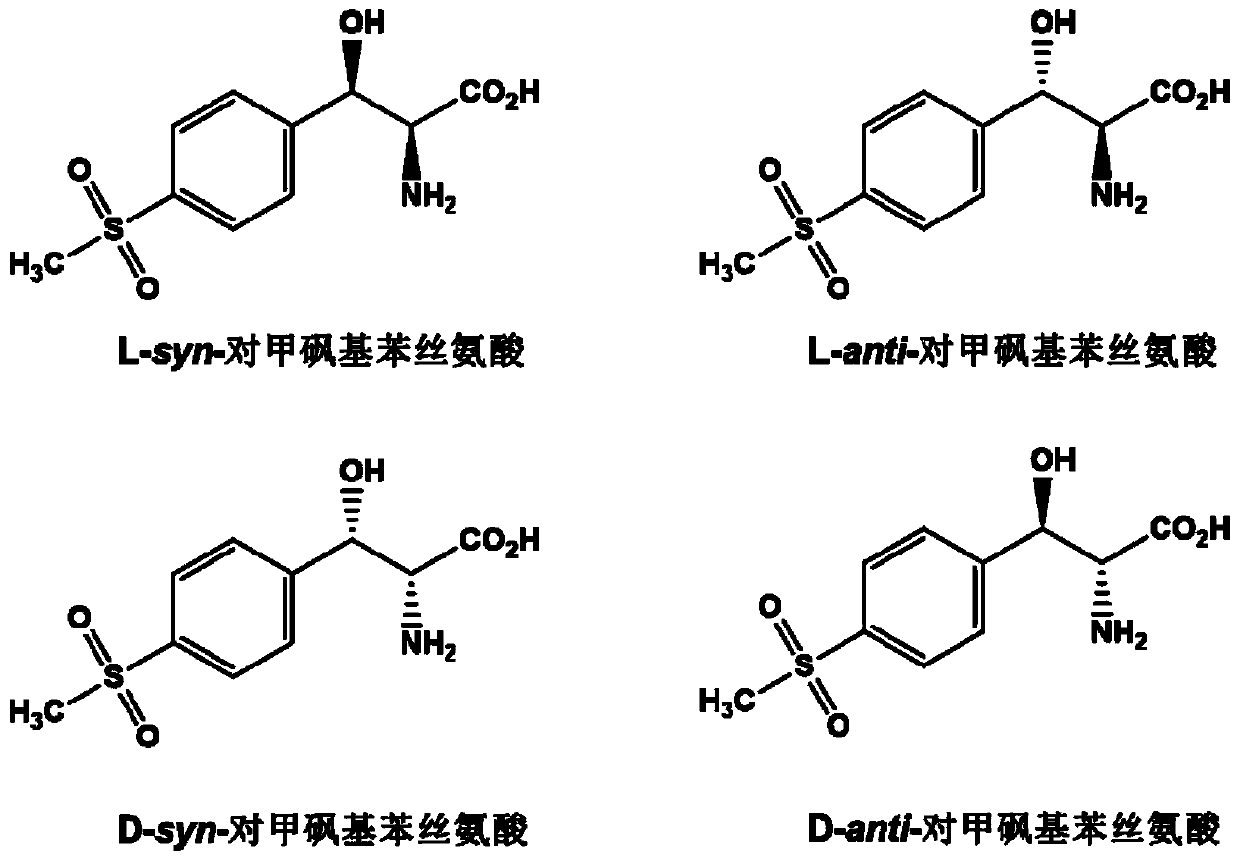
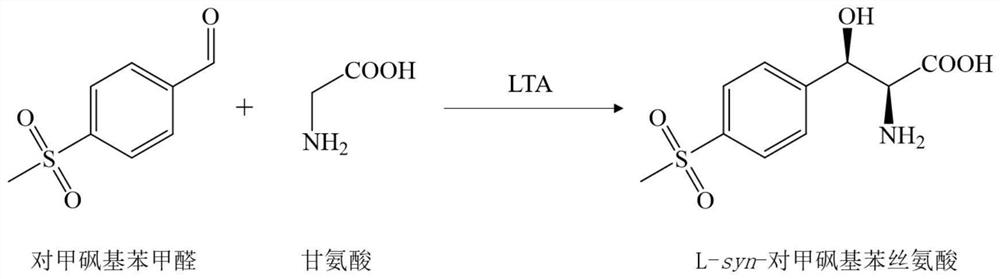
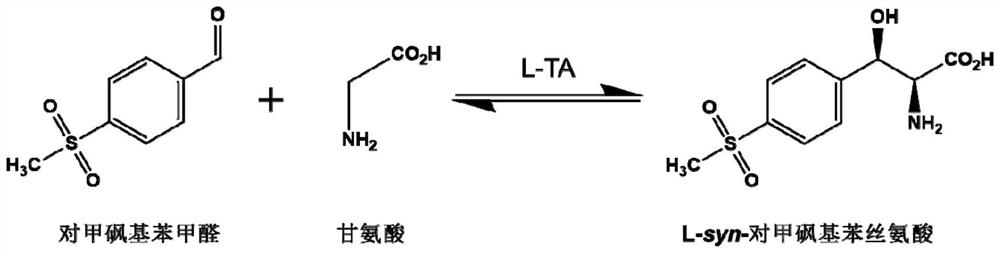
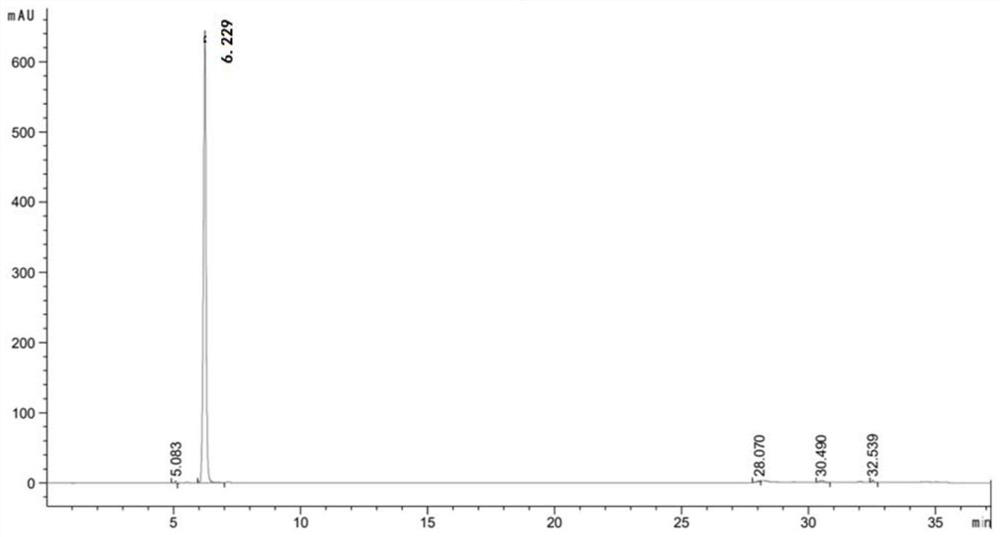
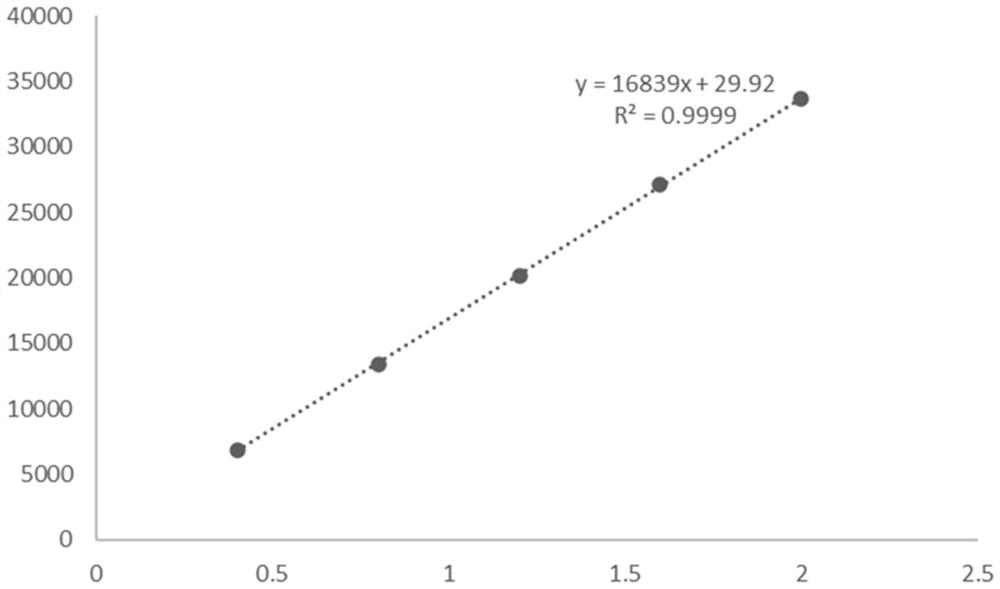
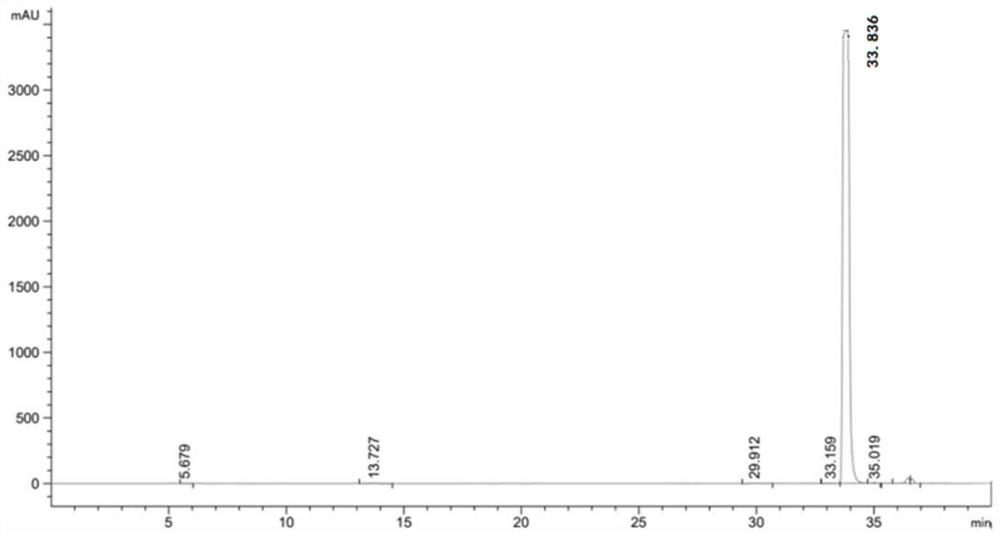
L-threonine aldolase mutant, gene and method for preparing L-syn-p-methylsulfonylphenylserine
ActiveCN111139230AIncrease profitEasy to separate and purifyBacteriaMicroorganism based processesMutantPyridoxine phosphate
The invention discloses an L-threonine aldolase mutant, a gene and a method for preparing L-syn-p-methylsulfonylphenylserine. The L-threonine aldolase mutant is obtained by mutation of wild type L-threonine aldolase; and with glycine and p-methylsulfonylbenzaldehyde as substrates and pyridoxal phosphate as a coenzyme, the L-syn-p-methylsulfonylphenylserine can be generated from the L-threonine aldolase mutant through a catalytic condensation reaction. The L-syn-p-methylsulfonylphenylserine produced by using the L-threonine aldolase mutant disclosed by the invention has the following advantages: 1, the production process is simple, and the reaction conditions are mild; 2, the L-threonine aldolase has high selectivity and high optical purity and does not need to be split; 3, the atom utilization rate is high and theoretically can reach 100%; 4, the production process is environment-friendly and less in pollution, and conforms to the concept of green chemistry; and 5, product separation and purification are simple.
Owner:ZHEJIANG UNIV
Mutant of threonine aldolase and application thereof in preparation of substituted phenylserine derivatives
The invention discloses a mutant of threonine aldolase, a coding gene of the mutant, a recombinant vector constructed by the coding gene, a recombinant genetic engineering bacterium obtained by converting the recombinant vector, and application of the mutant of the threonine aldolase in preparation of 2- / 3- / 4-substituted phenylserine derivatives. According to the invention, the mutant of the threonine aldolase is taken as a biocatalyst, 2- / 3- / 4-substituted benzaldehyde is taken as a substrate, 5-phosphopyridoxal is taken as a coenzyme, glycine / glycine ester is taken as a cosubstrate, the enzyme catalysis reaction is performed under proper conditions and in a proper medium, a series of phenylserine derivatives with different substituent groups are prepared through separation, and the method has the total yield range of 76-99%, the product ee value of over 99%, and the de value range of 71-92%.
Owner:王喆明 +1
Recombinant bacterium expressing D-threonine aldolase and construction method and application of recombinant bacterium
ActiveCN110272856AGood catalyticWide substrate applicabilityBacteriaMicroorganism based processesCatalytic effectThreonine aldolase
The invention discloses a recombinant bacterium expressing a D-threonine aldolase and a construction method and application of the recombinant bacterium, and belongs to the technical field of enzyme engineering. The recombinant bacterium of the present invention expresses the D-threonine aldolase with an amino acid sequence as shown in SEQ ID NO. 2. The invention provides the D-threonine aldolase which can be used as a catalyst for the synthesis of chiral beta-hydroxy-alpha-amino acid, the catalytic efficiency (conversion rate) is high to 65%, the stereoselectivity is high with (e.e.) of 99% and (d.e.) of 95%, and the suitable reaction condition is mild and environment-friendly. According to the present invention, the D-threonine aldolase has good catalytic effect, wide substrate applicability, and good application and development prospect.
Owner:JIANGNAN UNIV
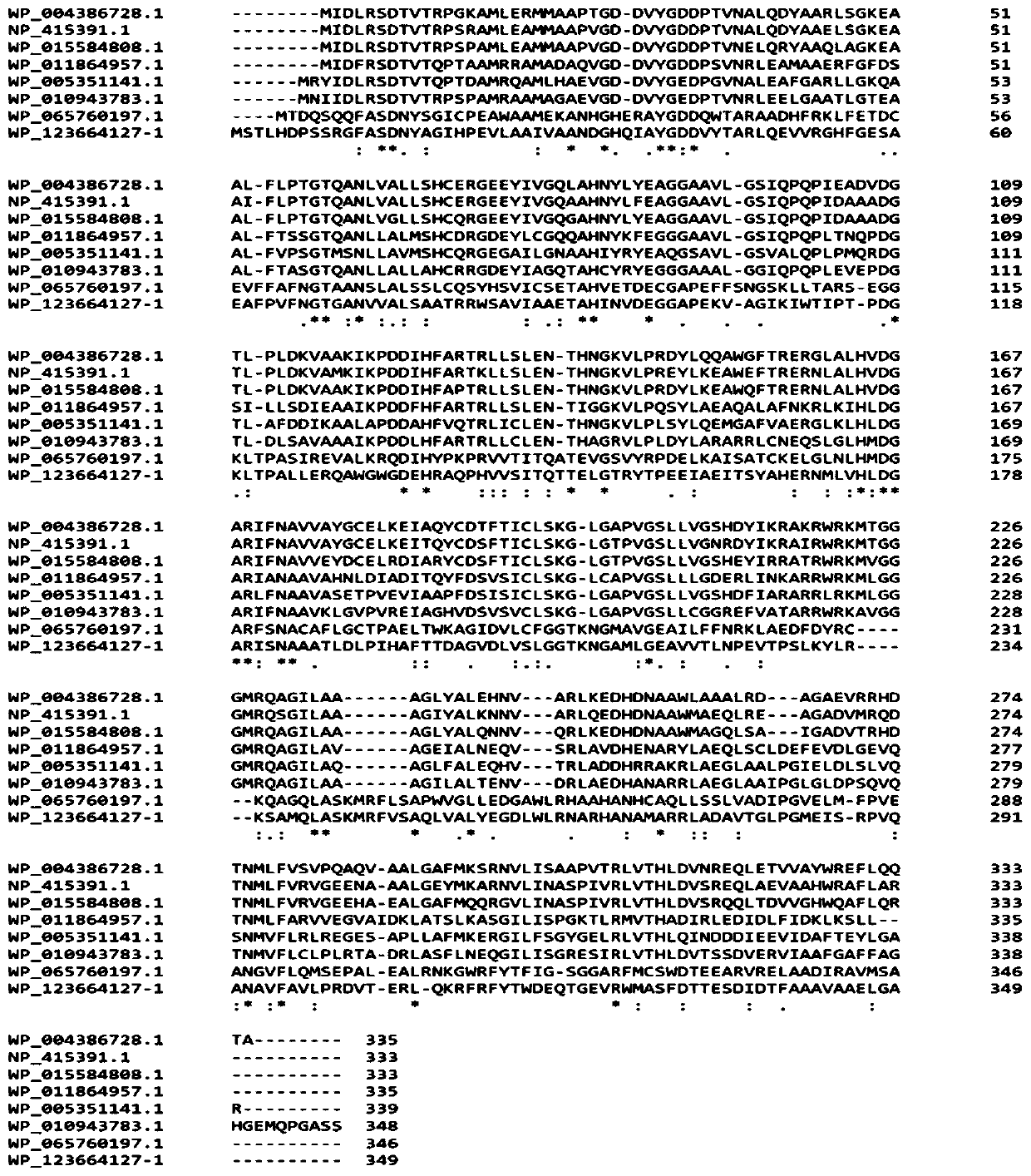
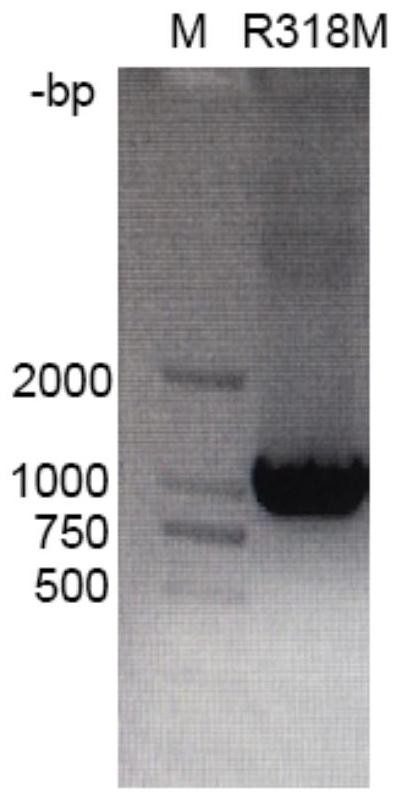
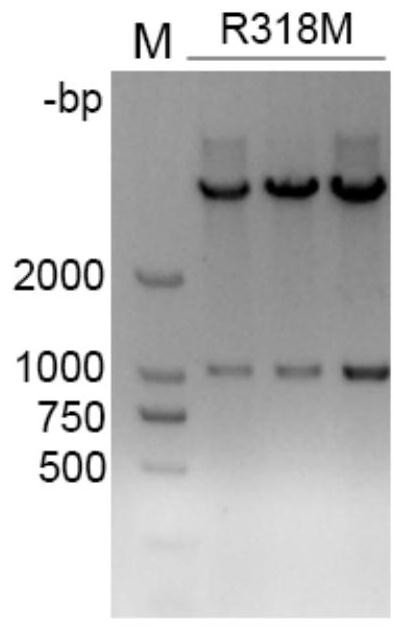
L-threonine aldolase mutant R318M and application thereof
The invention relates to threonine aldolase, in particular to an L-threonine aldolase mutant R318M and application thereof. The amino acid sequence of the L-threonine aldolase mutant is shown as SEQ ID NO: 3, and the L-threonine aldolase mutant is obtained by changing the 318th amino acid of L-threonine aldolase with the amino acid sequence of SEQ ID NO: 1 from Arg to Met. The mutant has diastereoselectivity (de) of 77.1% in a reaction of catalyzing 3, 4-dihydroxy benzaldehyde and glycine to synthesize droxydopa [L-threonine-(3, 4-dihydroxy) phenylserine], the diastereoselectivity (de) is morethan 2.5 times that of a wild type, and the mutant has huge application potential in industrial production of biosynthesis of droxydopa.
Owner:CHONGQING UNIV
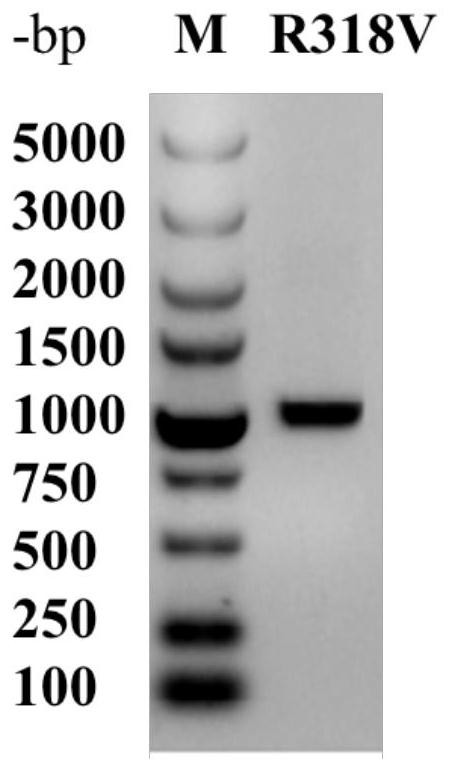
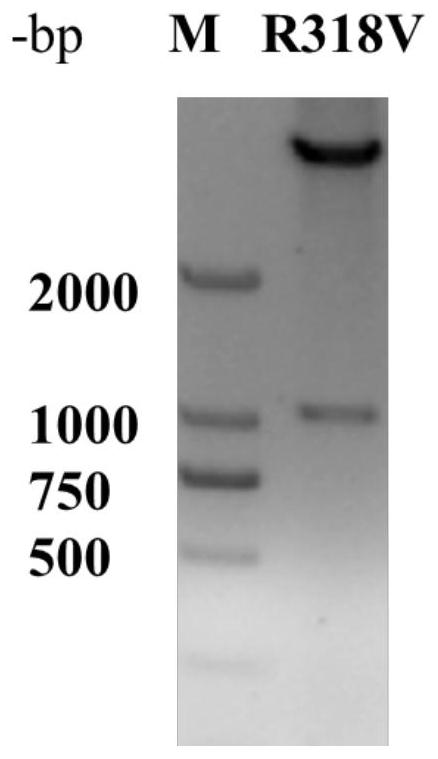
L-threonine aldolase mutant R318V and application thereof
The invention relates to threonine aldolase, in particular to an L-threonine aldolase mutant R318V and application thereof. The amino acid sequence of the L-threonine aldolase mutant is shown as SEQ ID NO: 3, and the L-threonine aldolase mutant is obtained by changing the 318th amino acid of L-threonine aldolase with the amino acid sequence of SEQ ID NO: 1 from Arg to Val. The mutant has 79.9% diastereoselectivity (de) in a reaction of catalyzing 3, 4-dihydroxy benzaldehyde and glycine to synthesize droxydopa [L-threonine-(3, 4-dihydroxy) phenylserine], the diastereoselectivity (de) is more than 2.6 times that of a wild type, and the mutant has huge application potential in industrial production of biosynthesis of droxydopa.
Owner:CHONGQING UNIV
L-threonine aldolase from enterobacter cloacae and application thereof
The invention relates to a nucleotide sequence for coding L-threonine aldolase separated from the enterobacter cloacae. The nucleotide sequence is a nucleotide sequence shown in SEQ ID NO:1, or a fragment, an analogue and a derivative of the nucleotide sequence. The nucleotide sequence for coding L-threonine aldolase is connected with an exogenous regulation sequence; and a carrier for functional expression, a cellular organism containing the carrier and a descendant of the organism are contained. A method for preparing the L-beta-hydroxy alpha-amino acid or D-beta-hydroxy alpha-amino acid from the nucleotide sequence, or polypeptide sequence, or cellular organism containing the carrier and descendant of the organism is provided.
Owner:数谱科技浙江有限公司
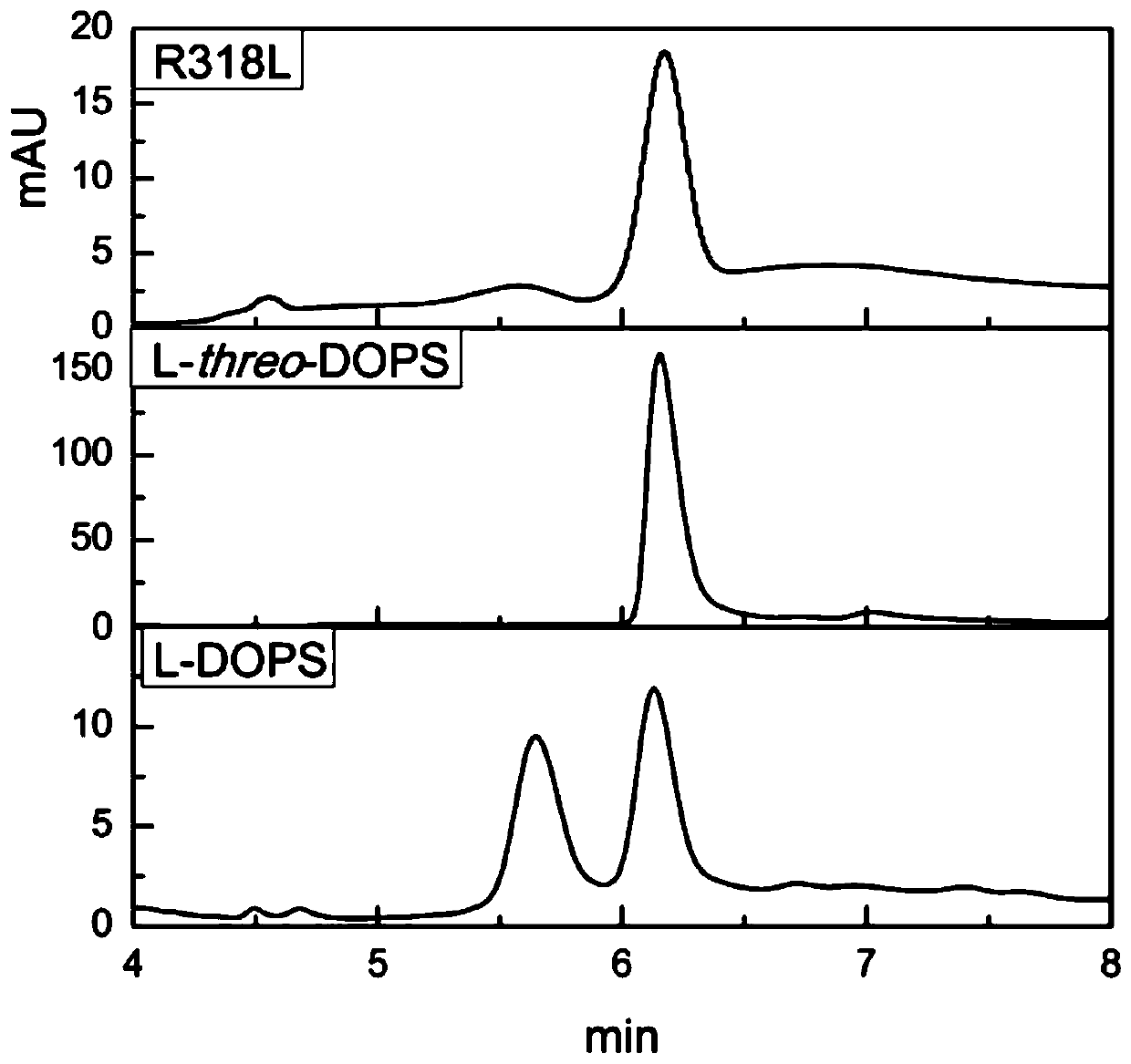
L-threonine aldolase mutant R318L and application thereof
The present invention relates to threonine aldolase and particularly to an L-threonine aldolase mutant R318L and an application thereof. An amino acid sequence of the L-threonine aldolase mutant is shown in SEQ ID NO:3 and the L-threonine aldolase mutant is obtained by changing the 318th site amino acid of L-threonine aldolase with an amino acid sequence of SEQ ID NO:1 from Arg to Leu. The mutanthas a diastereoselectivity of 84.9% in catalyzing synthesis of droxidopa [L-threo-(3,4-dihydroxy)phenylserine] from 3,4-dihydroxybenzaldehyde and glycine, the diastereoselectivity of the mutant is 2.8 times that of the wild type, and the mutant has huge application potential in the biosynthesis of the droxidoca.
Owner:CHONGQING UNIV
Biosynthetic production of acyl amino acids
InactiveCN107075463ABacteriaMicroorganism based processesAcyl Coenzyme A SynthetasesAcyl-CoA synthetase
Owner:EVONIK DEGUSSA GMBH
Application of L-threonine transaldolase to synthesis of florfenicol chiral intermediates
ActiveCN110540977AIncrease added valueSave separation and purificationTransferasesFermentationEscherichia coliBenzaldehyde
The invention provides application of L-threonine transaldolase to synthesis of florfenicol chiral intermediates. The L-threonine transaldolase is selected from any one of the following groups that (1) polypeptide contains an amino acid sequence shown in SEQ ID NO:1; (2) polypeptide contains an amino acid sequence greater than or equal to 90% homology shown in SEQ ID NO:1, and the polypeptide hascatalytic activity; and (3) derived polypeptide is formed by substitution, deletion or addition of 1-5 amino acid residues to the amino acid sequence shown in SEQ ID NO:1 and with the retention of catalytic activity. According to the application, new L-threonine transaldolase genes are screened through gene mining, the L-threonine transaldolase derived from recombinant escherichia coli is expressed by adopting a genetic engineering method, methylsulfonyl benzaldehyde and the L-threonine transaldolase are used as raw materials, (2S,3R)-methylsulfonyl phenylserine is synthesized through a wholecell catalyzed reaction, fluorfenicol key chiral synthesis blocks can be obtained by a one-step reaction under the room temperature and pressure, environmental protection is achieved, and the application is an environment-friendly biosynthetic pathway.
Owner:福建昌生生物科技发展有限公司
Byosynthetic Production of Acyl Amino Acids
InactiveUS20170130248A1PurityYield andLigasesAcyltransferasesAcyl Coenzyme A SynthetasesAcyl-CoA synthetase
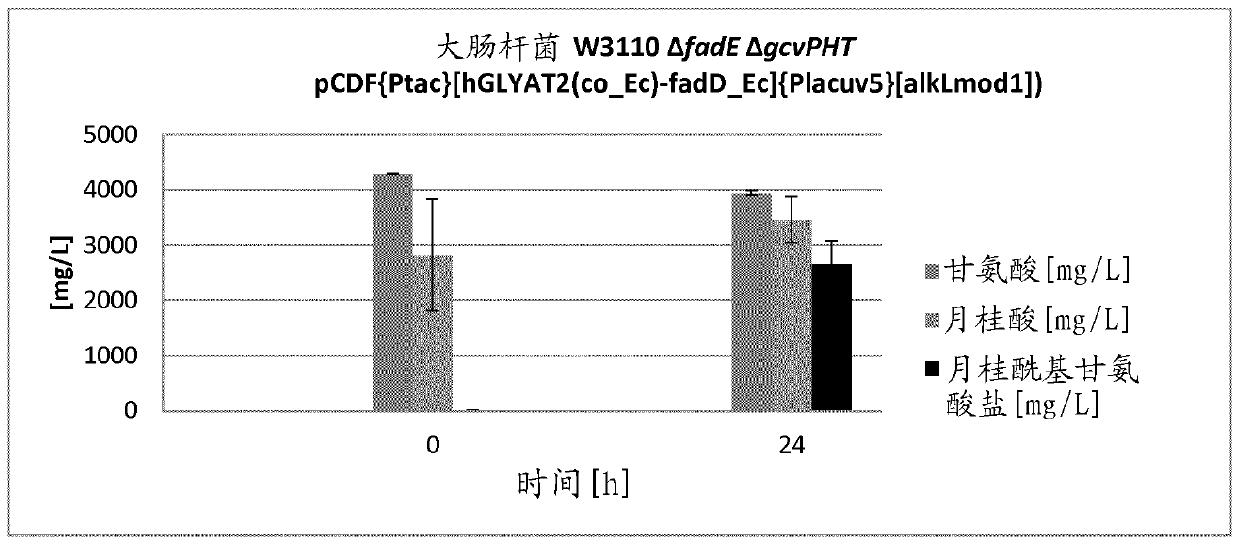
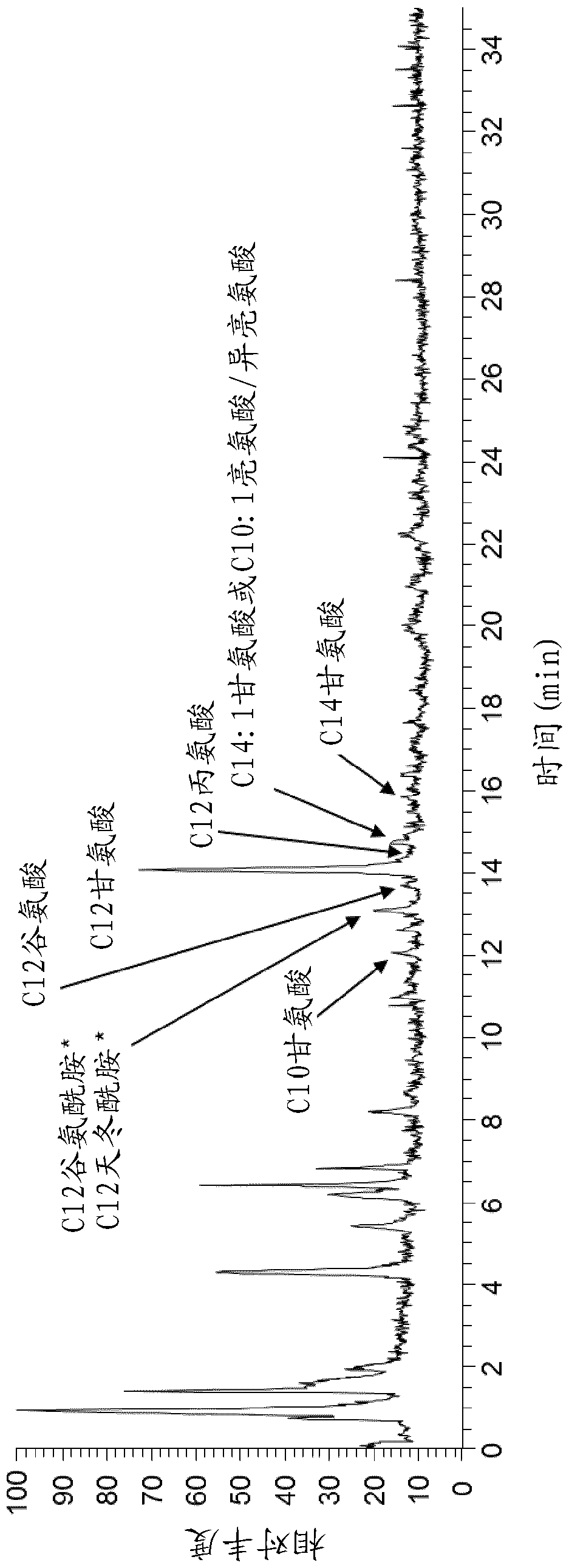
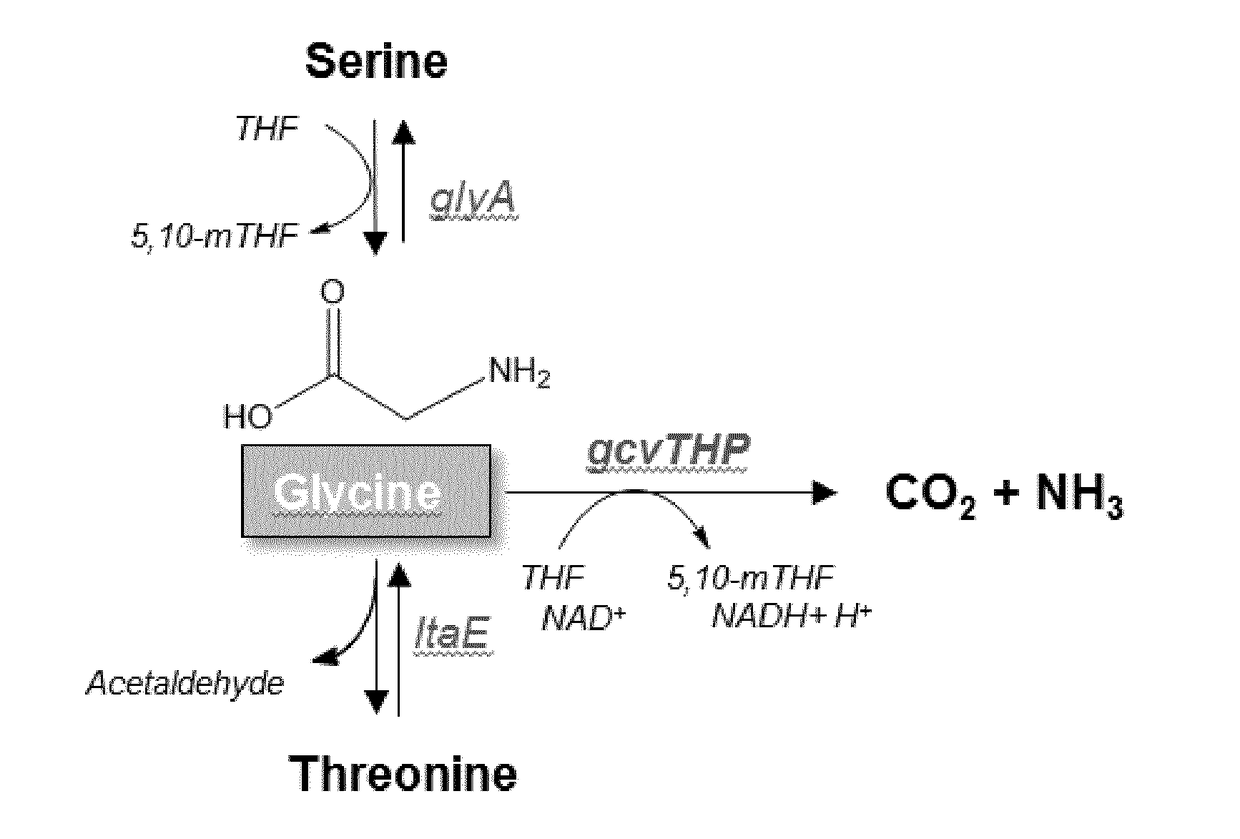
The present invention relates to a cell for producing acyl glycinates wherein the cell is genetically modified to compriseat least a first genetic mutation that increases the expression relative to the wild type cell of an amino acid-N-acyl-transferase,at least a second genetic mutation that increases the expression relative to the wild type cell of an acyl-CoA synthetase, andat least a third genetic mutation that decreases the expression relative to the wild type cell of at least one enzyme selected from the group consisting of an enzyme of the glycine cleavage system, glycine hydroxymethyltransferase (GlyA) and threonine aldolase (LtaE).
Owner:EVONIK DEGUSSA GMBH
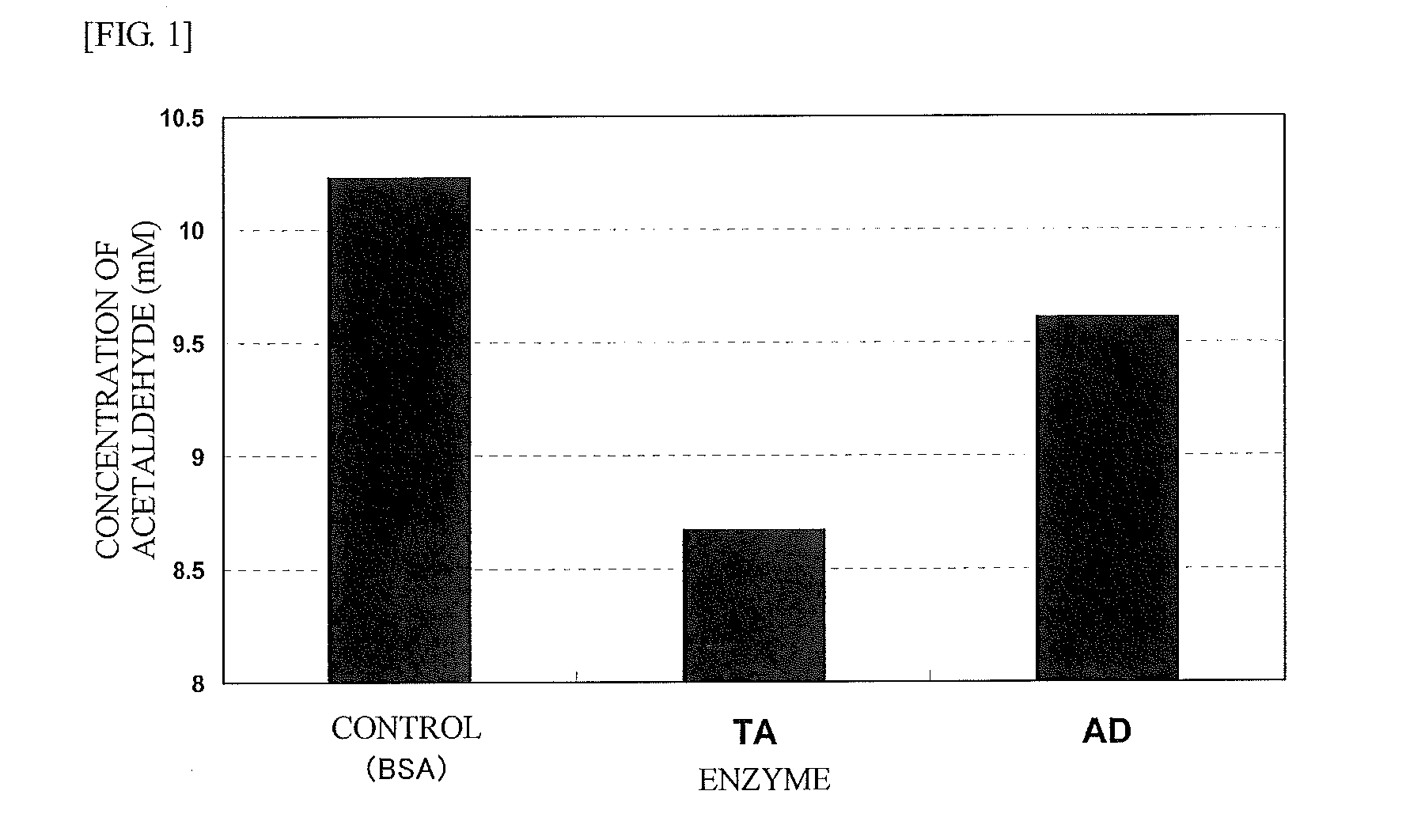
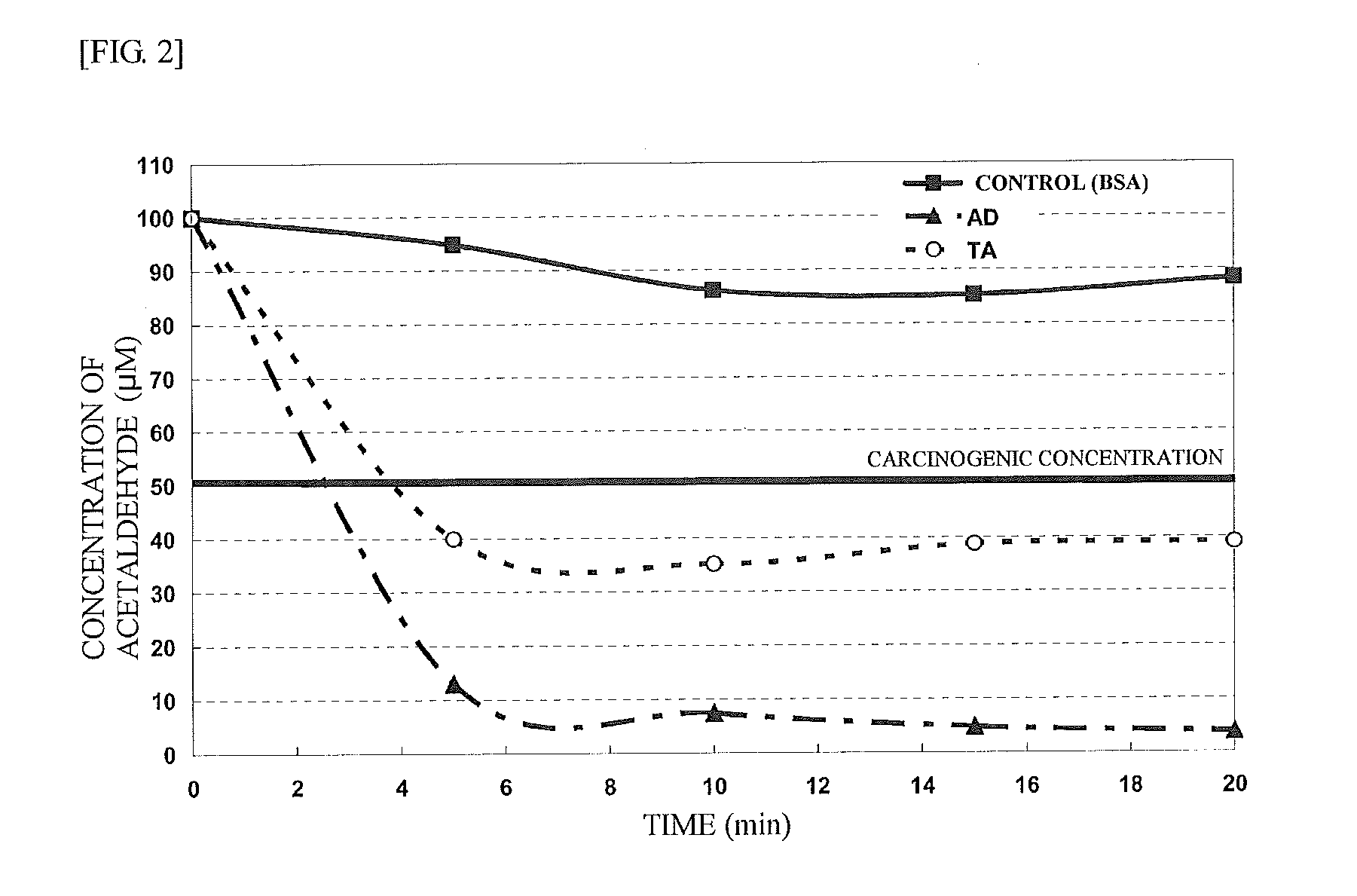
Agent for reducing acetaldehyde in oral cavity
InactiveUS20130089535A1Reduction in acetaldehydeEfficient reductionCosmetic preparationsPeptide/protein ingredientsMicroorganismEscherichia coli
Disclosed herein is a novel enzymatic agent effective in reducing acetaldehyde in the oral cavity. It has been found that an aldehyde dehydrogenase derived from a microorganism belonging to the genus Saccharomyces and a threonine aldolase derived from Escherichia coli are effective in reducing low concentrations of acetaldehyde. Therefore, an agent for reducing acetaldehyde in the oral cavity is provided, which contains these enzymes as active ingredients.
Owner:AMANO ENZYME INC
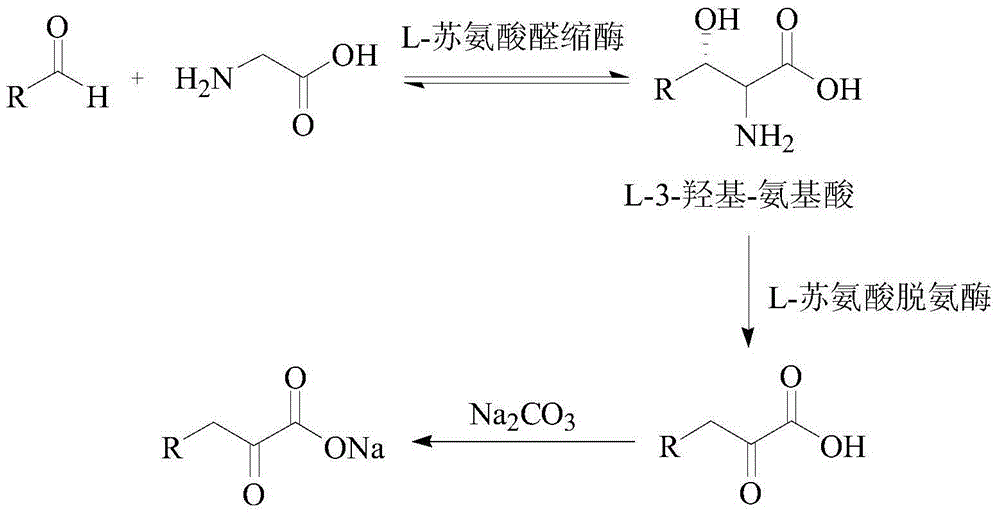
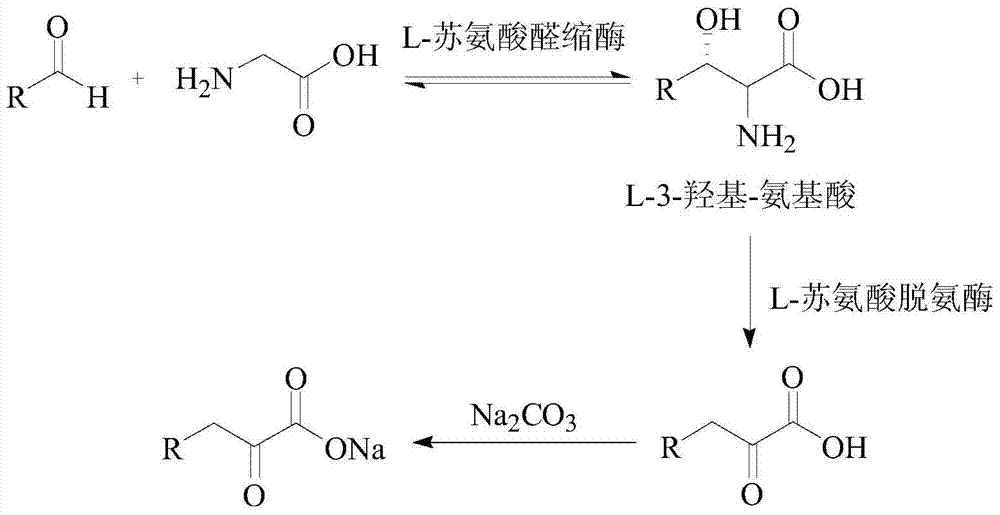
Biosynthetic method of high purity L-alpha-amino acid
The invention discloses a biosynthetic method of high purity L-alpha-amino acid. The method comprises the following steps: in a reaction liquid, carrying out enzymic catalytic reaction on an aldehyde compound and a glycine raw material under the catalytic effect of L-threonine aldolase and L-threonine dehydratase compound enzymes to obtain a 2-ketonic acid product; and carrying out enzymic catalytic reaction on the 2-ketonic acid product under catalysis of a coenzyme consisting of leucine dehydrogenase and hydrogenlyase or glucose dehydrogenase, and sequentially carrying out electrodialysis desalting, active carbon decoloring, concentration and crystallization and vacuum drying on the reaction product so as to obtain high purity L-alpha-amino acid crystals with a high yield. The method which uses an aqueous phase as a reaction system is environment-friendly and cheap, low in equipment requirement and low in cost, and the enzyme can be repeatedly used, so that the method can be used for satisfying industrial production.
Owner:HUNAN BAOLISHI BIOTECH
Method for synthesizing high-purity 2-ketonate
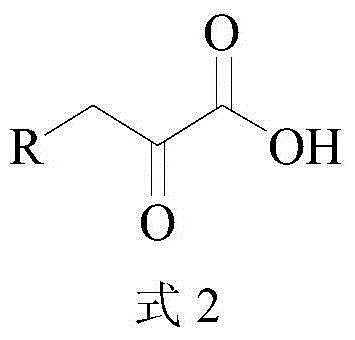
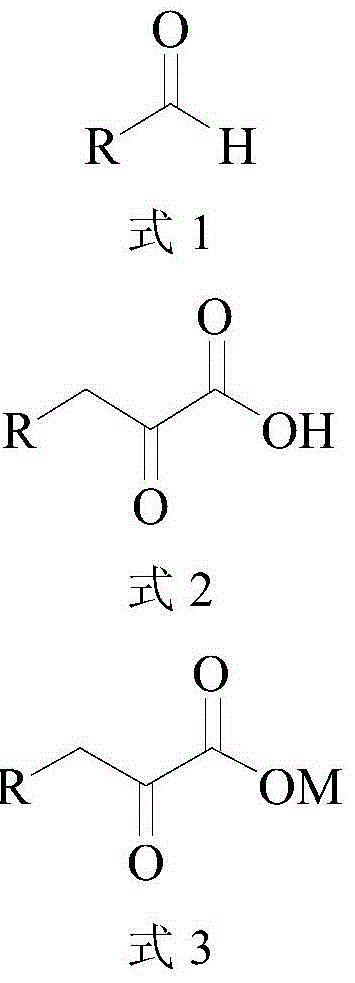
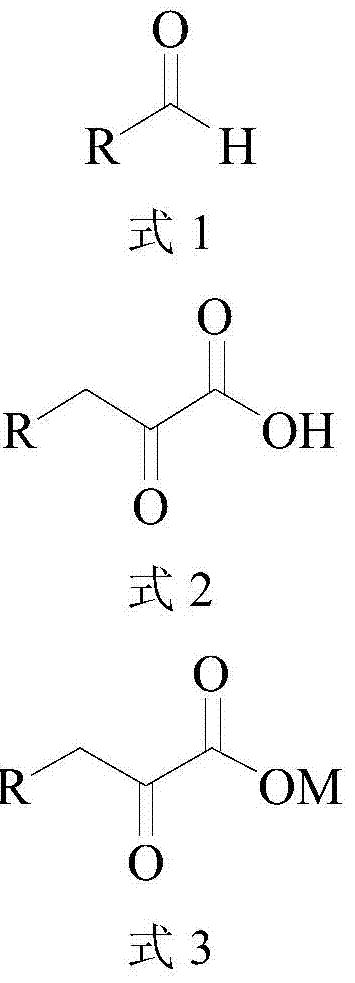
ActiveCN104372035AHigh recovery rateImprove conversion rateFermentationCarboxylic compound separation/purificationKetonic acidsCatalytic effect
The invention discloses a method for synthesizing high-purity 2-ketonate. The method comprises the following steps: carrying out an enzyme catalysis reaction on an aldehyde compound and a glycine raw material in the catalysis of L-threonine aldolase and L-threonine deaminase compound enzyme to generate a 2-ketonic acid product; carrying out separation purification on the 2-ketonic acid product by chromatography, adjusting the pH to a proper value by adopting alkali carbonate or alkali hydrocarbonate, and sequentially carrying out actived carbon decoloration, concentration crystallization and vacuum drying to obtain 2-ketonate. The preparation method has the advantages of simplicity in operation and mild reaction condition, does not cause pollution by adopting an aqueous phase system, and has low production cost; prepared 2-ketonate has high yield and high purity.
Owner:HUNAN BAOLISHI BIOTECH
L-threonine aldolase mutant and method for preparing L-syn-p-methylsulfonyl phenyl serine
ActiveCN113249365ASimple production processMild reaction conditionsBacteriaMicroorganism based processesBenzaldehydeEnzyme catalysis
Owner:ZHEJIANG UNIV
High-temperature-resistant L-threonine aldolase and application thereof to synthesis of p-methylsulfonyl phenyl serine
ActiveCN113322248AHigh catalytic activityCatalytic activity retentionBacteriaMicroorganism based processesEscherichia coliBenzaldehyde
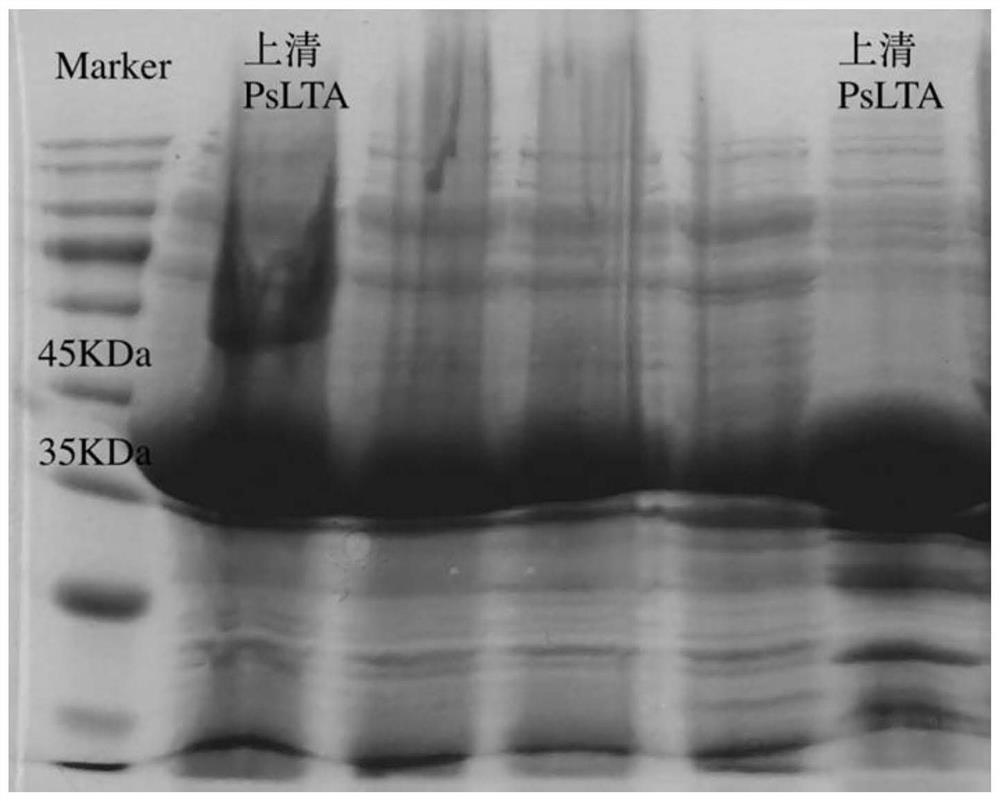
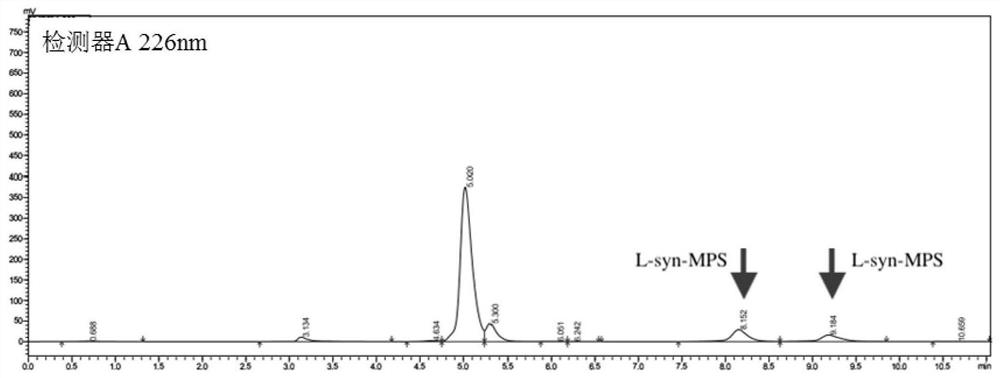
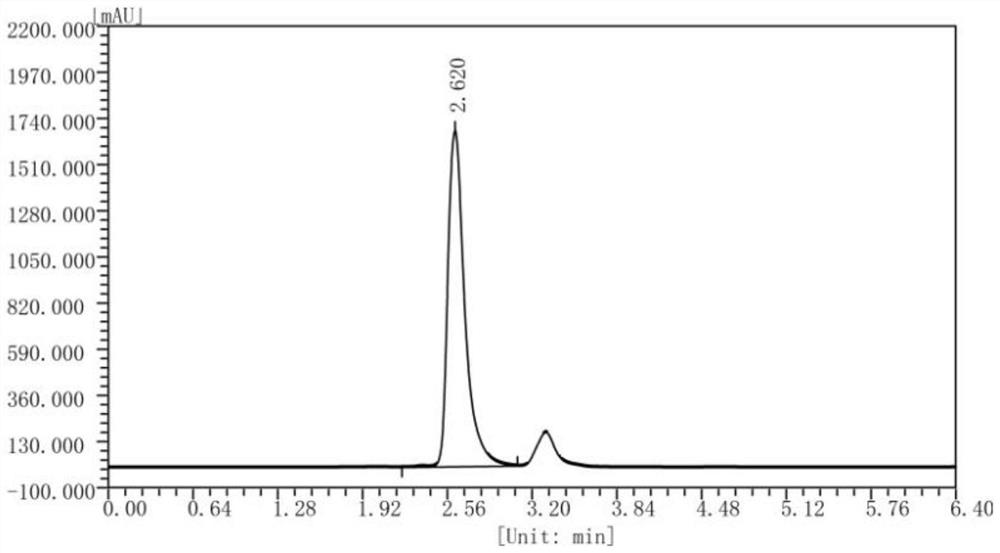
The invention relates to the technical field of enzyme engineering, and discloses a high-temperature-resistant L-threonine aldolase and application thereof to synthesis of p-methylsulfonyl phenyl serine. After the L-threonine aldolase, to which the invention relates, of a L-threonine aldolase (LTA) gene derived from Pelosinus sp. is expressed in escherichia coli, glycine and p-methylsulfonyl benzaldehyde are catalyzed to synthesize L-p-methylsulfonyl phenyl serine under the condition that a pH value is 7-10, the ee value is greater than 99%, and the process has the advantages of being mild in reaction condition, simple in step and the like. Moreover, the L-threonine aldolase PsLTA has relatively high catalytic activity at the temperature of 65 DEG C, still keeps 90% of the catalytic activity after the L-threonine aldolase PsLTA is incubated for 2h at the temperature of 55 DEG C, and has high thermal stability and catalytic activity and has industrial significance.
Owner:ZHEJIANG UNIV OF TECH
L-threonine aldolase mutant and application of L-threonine aldolase mutant in synthesis of L-synn-p-methylsulfonylphenylserine
ActiveCN114134134ASimple production processMild reaction conditionsBacteriaMicroorganism based processesEnzyme catalysisThreonine aldolase
The invention discloses an L-threonine aldolase mutant and an application of the L-threonine aldolase mutant in synthesis of L-syn-p-methylsulfonyl phenyl serine. The L-threonine aldolase mutant is obtained by mutating wild type L-threonine aldolase, and L-syn-p-methylsulfonyl phenyl serine can be generated by a catalytic condensation reaction by taking glycine and p-methylsulfonyl benzaldehyde as substrates and taking pyridoxal phosphate as a coenzyme. When the L-threonine aldolase mutant is used for producing L-synn-p-methylsulfonylphenyl serine, the L-threonine aldolase mutant has the following advantages: 1, the production process is simple, and the reaction conditions are mild; 2, the selectivity of the L-threonine aldolase is high, the optical purity of the product is high, and resolution is not needed; 3, the atom utilization rate is high and can reach 100% theoretically; the production process is environmentally friendly, pollution is small, and the green chemistry concept is met; 5, the product is simple to separate and purify.
Owner:宁波泓森生物科技有限公司
Method for preparing DL-serine by one-pot enzyme method
ActiveCN110373440ARealize resource reuseReduce "three wastes" emissionsFermentationAlanine racemaseD-threonine aldolase
Owner:CHANGXING PHARMA
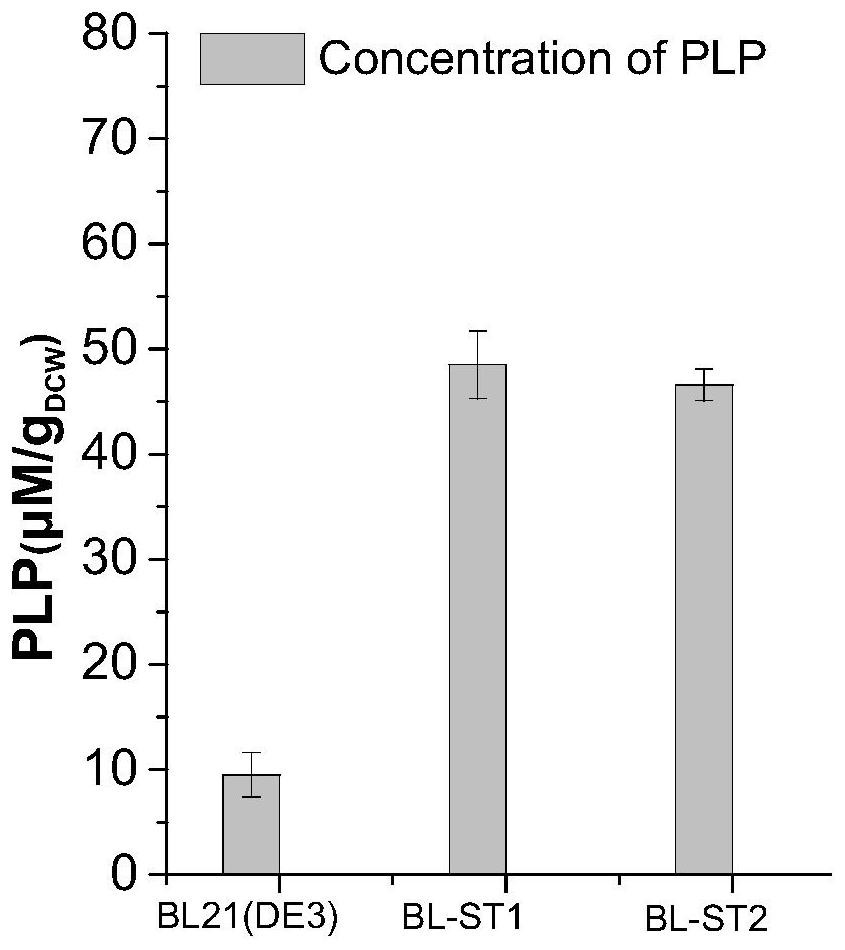
Engineering bacterium for co-expressing L-threonine aldolase and PLP synthase and application
ActiveCN112175892AReduce usageIncrease synthesisCarbon-nitrogen lyasesBacteriaThreonine aldolaseSynexpression
The invention discloses an engineering bacterium for co-expressing L-threonine aldolase and PLP synthase and an application. The invention relates to an engineering bacterium for co-expressing L-threonine aldolase and PLP synthase. The engineering bacterium is obtained by transferring an L-threonine aldolase gene and a PLP synthase gene into an expression strain. The L-threonine aldolase gene andthe PLP synthase gene are introduced into the engineering bacterium in an exogenous manner, the two enzymes are co-expressed, the synthesis amount of endogenous coenzyme PLP of the strain can be increased through over-expression of the PLP synthase, and the co-expressed engineering bacterium is crushed to prepare crude enzyme liquid. According to the co-immobilized enzyme prepared by co-immobilizing the L-threonine aldolase and coenzyme PLP, the use amount of exogenous PLP in the production process of the L-syn-p-methyl sulfonyl phenylserine can be remarkably reduced, the use efficiency of theL-threonine aldolase and the PLP is improved, and the production cost is reduced. The method has the advantages of mild reaction conditions, environmental protection, simple production process, highreutilization efficiency and low cost, and has a wide application prospect in the production of L-syn-p-methyl sulfonyl phenylserine.
Owner:ZHEJIANG UNIV
A kind of l-threonine aldolase and its application in the synthesis of p-thymphenylphenylserine
ActiveCN110577948BShort reaction timeBacteriaMicroorganism based processesGenetic engineeringBiology
The invention provides L-threonine aldolase. The L-threonine aldolase has the activity of asymmetric catalytic synthesis of (2S,3R)-methylsulfonylphenylserine from methylsulfonyl benzaldehyde and glycine. The L-threonine aldolase is selected from any one of the following groups: (1) polypeptide with the amino acid sequence shown in SEQ ID NO: 1; (2) polypeptide with the amino acid sequence shown in SEQ ID NO: 1, wherein the homology of the amino acid sequence is greater than or equal to 80%; and (3) derived polypeptide formed by substituting, missing or adding of 1-5 amino acid residues of theamino acid sequence shown in SEQ ID NO: 1 and retaining catalytic activity. The invention further provides application of the L-threonine aldolase to synthesis of the methylsulfonylphenylserine, an L-threonine aldolase gene derived from Actinocorallia herbida is mined and screened, the L-threonine aldolase derived from recombinant escherichia coli is expressed through a gene engineering technology, the (2S,3R)-methylsulfonylphenylserine is subjected to asymmetric catalytic synthesis by taking the methylsulfonyl benzaldehyde and the glycine as raw materials and adopting a whole-cell catalysismethod, and the reaction conditions are mild and environmentally friendly.
Owner:福建昌生生物科技发展有限公司
A kind of l-threonine aldolase mutant, gene and method for preparing l-syn-p-thymphenylphenylserine
ActiveCN111139230BSimple production processMild reaction conditionsBacteriaMicroorganism based processesEnzyme catalysisMutant
The invention discloses an L-threonine aldolase mutant, a gene and a method for preparing L-syn-p-methylsulfonylphenylserine. The L-threonine aldolase mutant is obtained by mutation of wild-type L-threonine aldolase, and can use glycine and p-methylsulfonylbenzaldehyde as substrates and pyridoxal phosphate as coenzyme to catalyze condensation The reaction produces L-syn-p-methylsulfonylphenylserine. Using the L-threonine aldolase mutant of the present invention to produce L-syn-p-methylsulfonylphenylserine has the following advantages: 1. the production process is simple and the reaction conditions are mild; 2. the selection of L-threonine aldolase High performance, high optical purity of the product, no need for splitting; 3. High atomic utilization rate, theoretically up to 100%; 4. Environmental protection in the production process, less pollution, in line with the concept of green chemistry; 5. Simple product separation and purification.
Owner:ZHEJIANG UNIV
Method for biosynthesizing droxidopa based on L-threonine aldolase
PendingCN112941123AImprove friendlinessMild conditionsBacteriaMicroorganism based processesEscherichia coliPseudomonas putida
The invention discloses a method for biosynthesizing droxidopa based on L-threonine aldolase. The method comprises the following steps: taking BL21DE3 as an original strain; and on the basis of an original strain, an ItaE gene for coding L-threonine aldolase and derived form Pseudomonas aeruginosa, a P-TA gene for coding L-threonine aldolase in Pseudomonas putida, an LTA gene for coding L-threonine aldolase in Escherichia coli, or an STA gene for coding L-threonine aldolase in Streptomyces are overexpressed. Through the above operations to get the engineering bacteria, through the centrifugation of wet cells to collect the reaction to get the yield of droxydopa is 3.3 -4.15g / L. Compared with the prior art, the biosynthesis method disclosed by the invention has the advantages of greenness, high yield, mild conditions and the like, and particularly has few byproducts and high environmental friendliness.
Owner:NANJING UNIV OF TECH
Threonine aldolase, mutants and their application in the preparation of substituted phenylserine derivatives
ActiveCN109402098BImprove stabilityHigh chiral selectivityFungiBacteriaEnzyme catalysisEngineered genetic
The invention discloses threonine aldolase, mutants, threonine aldolase and mutant coding genes, recombinant vectors constructed by coding genes, recombinant genetically engineered bacteria obtained by transformation of recombinant vectors, and threonine aldolase and the application of mutants in the preparation of 2- / 3- / 4-position substituted phenylserine derivatives. The present invention uses threonine aldolase and mutants as biocatalysts, 2- / 3- / 4-position substituted benzaldehyde as substrate, pyridoxal 5-phosphate as coenzyme, glycine / glycine ester as Auxiliary substrate, carry out enzyme-catalyzed reaction in appropriate conditions and media, separate and prepare a series of phenylserine derivatives with different substituents, the total yield of this method is in the range of 76-99%, the ee value of the product is greater than 99%, and the de value is greater than 99%. The range is 70‑99%.
Owner:王喆明 +1
Method for synthesizing high-purity 2-keto acid salt
ActiveCN104372035BHigh recovery rateImprove conversion rateFermentationCarboxylic compound separation/purificationKetonic acidsSynthesis methods
The invention discloses a method for synthesizing high-purity 2-ketonate. The method comprises the following steps: carrying out an enzyme catalysis reaction on an aldehyde compound and a glycine raw material in the catalysis of L-threonine aldolase and L-threonine deaminase compound enzyme to generate a 2-ketonic acid product; carrying out separation purification on the 2-ketonic acid product by chromatography, adjusting the pH to a proper value by adopting alkali carbonate or alkali hydrocarbonate, and sequentially carrying out actived carbon decoloration, concentration crystallization and vacuum drying to obtain 2-ketonate. The preparation method has the advantages of simplicity in operation and mild reaction condition, does not cause pollution by adopting an aqueous phase system, and has low production cost; prepared 2-ketonate has high yield and high purity.
Owner:HUNAN BAOLISHI BIOTECH
Method for synthesizing noradrenaline through double-enzyme coupling
PendingCN112961873ARaw materials are easy to getReduce manufacturing costNucleic acid vectorFermentationAldehyde formationBacilli
The invention discloses a method for synthesizing noradrenaline through double-enzyme coupling, which comprises the following steps: by taking BL21DE3 as an original strain, respectively overexpressing an S-TA gene for coding L-threonine aldolase from black bear and a TDC gene for coding tyrosine decarboxylase from lactobacillus brevis on the basis of the original strain, so as to obtain two engineering bacteria, performing the reaction of a crude enzyme liquid obtained by cell disruption with 3, 4-dihydroxybenzaldehyde as a substrate, glycine as an auxiliary substrate and pyridoxal 5-phosphate as a cofactor to obtain noradrenaline of which the yield is 6.3 g / L. Compared with the prior art, the biosynthesis method disclosed by the invention has the advantages of greenness, high yield, mild conditions and the like, particularly has few byproducts and high environmental friendliness, and the cost of required production equipment is greatly reduced.
Owner:NANJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com