A kind of l-threonine aldolase and its application in the synthesis of p-thymphenylphenylserine
A technology of thiamphenylphenylserine and threonine aldolase, which is applied in the fields of biotechnology and chemical engineering, can solve the problems of high production cost, heavy environmental pollution, severe reaction conditions, etc., and achieve the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Recombinant expression of L-threonine aldolase
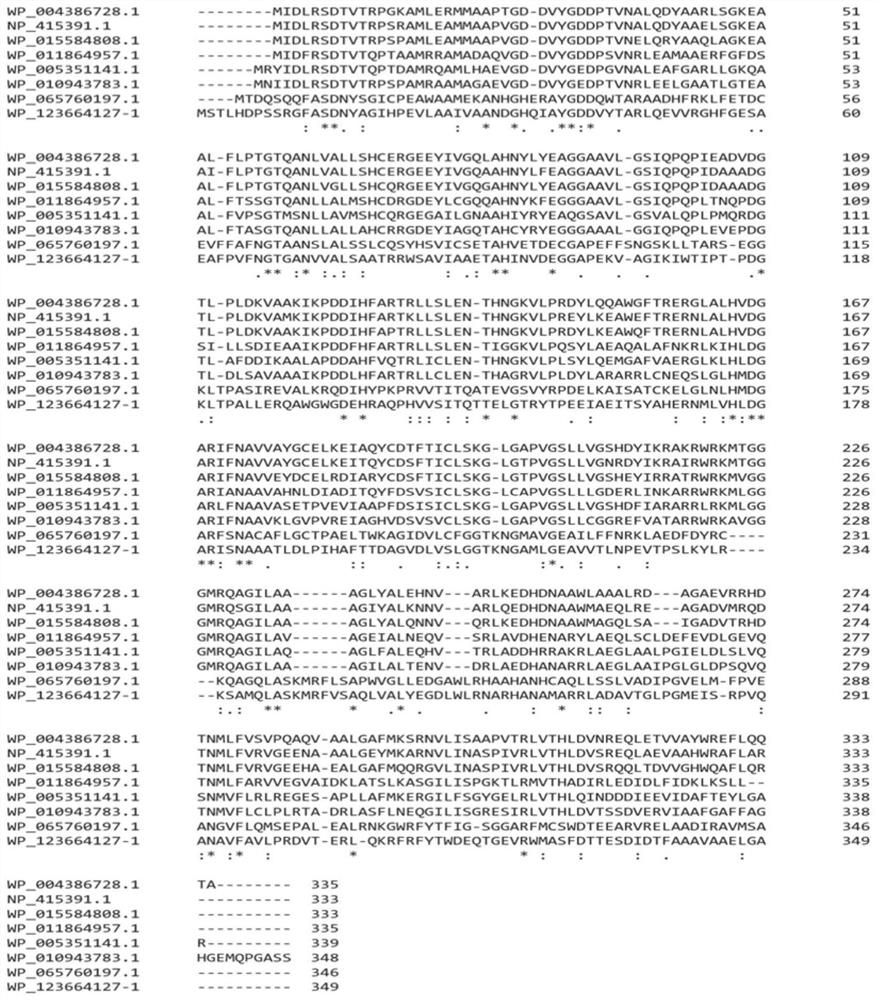
[0039] A novel L-threonine aldolase gene (GenBank No.WP_123664127-1) was screened through gene mining, the amino acid sequence encoded by the gene and the reported L-threonine aldolase (see Table 1) Amino acid sequence similarity is less than 70%, see attached figure 1 The comparison results in .
[0040] Table 1 Information table of L-threonine aldolases reported in the literature
[0041]
[0042]
[0043] The L-threonine aldolase in this example is encoded by the LTA gene (GenBank No. WP_123664127-1) derived from Actinocorallia herbida, and its amino acid sequence is SEQ ID NO:1. The applicant optimized the codon according to the amino acid sequence of L-threonine aldolase, and obtained the optimized base sequence of LTA as SEQ ID NO:2. The optimized LTA gene DNA sequence was artificially synthesized, and the LTA gene was further connected to the plasmid pET28a to construct the recombinant plasmid p...
Embodiment 2
[0046] Embodiment 2 (2S, 3R)-catalyzed synthesis of thiamphenicol phenylserine
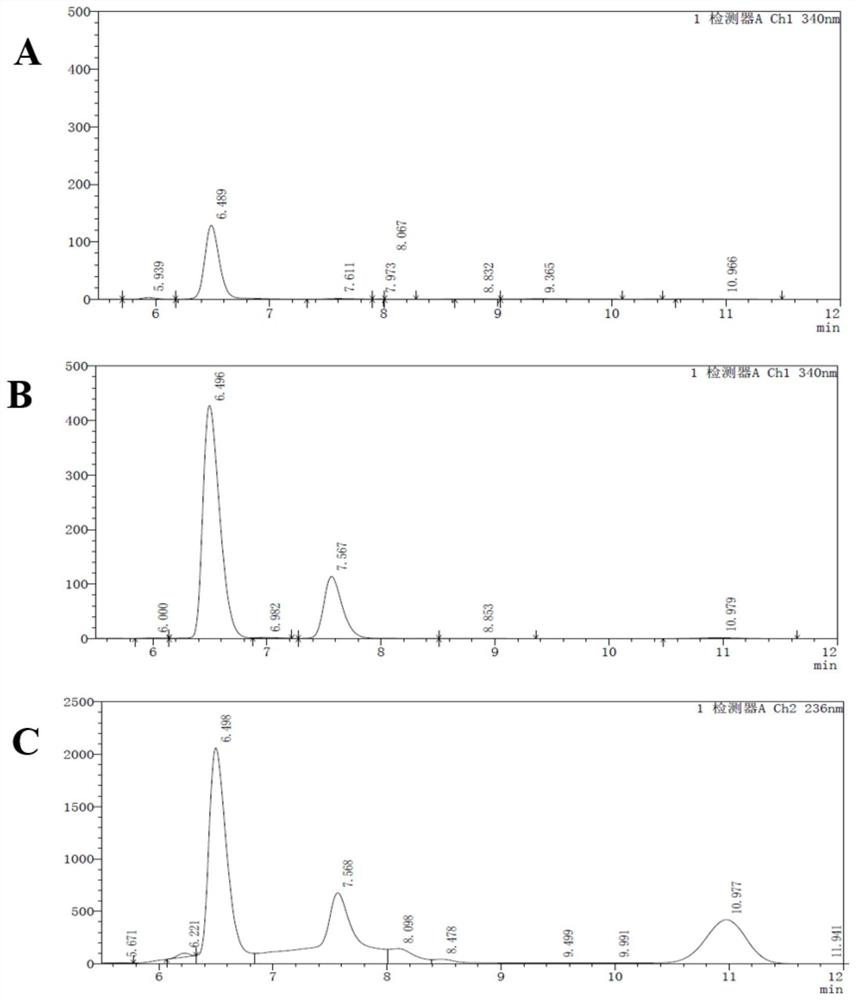
[0047] The whole cells of the recombinant Escherichia coli obtained in Example 1 were used, and a 300mL p-thymphenylphenylserine synthesis reaction system was configured: 27.6g p-thymphenylbenzaldehyde, 37.5g glycine, and 6.7mg pyridoxal phosphate were weighed respectively , into a 1L reaction bottle, and 300 mL of phosphate buffer (200 mM, pH 7) containing 30% acetonitrile (v / v) was pre-added in the bottle. After thorough mixing, 3.75 g (wet weight) of recombinant Escherichia coli cells harvested in Example 1 were weighed, and shaken and reacted at 30° C. for 6 h. After the reaction, the supernatant containing the product was collected by centrifugation at 8000 rpm for 5 min. In other embodiments, the recombinant Escherichia coli harvested in Example 1 can also be resuspended with an appropriate amount of phosphate buffer (200 mM, pH 7.0), and then added to the reaction system.
[0048] The L-t...
experiment example 1
[0057] Experimental example 1 Substrate specificity of aldolase catalyzed by L-threonine aldolase
[0058] In order to explore the catalytic activity of L-TA on different benzene ring substituted substrates, the following experiments were designed. Whole-cell catalytic synthesis of (2S,3R)-phenylserine derivatives using benzaldehyde and glycine with different phenyl ring substituents as substrates. The reaction conditions were: 100 mM benzaldehyde with different benzene ring substituents, 50 mg / mL cell concentration, 30% acetonitrile, 37° C. for 4 h. The experimental results are shown in Table 2. React with benzaldehyde (entry1) as substrate, the conversion rate of substrate is 67%, and the de value of product is 17%; With nitrobenzaldehyde (entries2-4), bromobenzaldehyde (entries5-7), Chlorobenzaldehyde (entries8-10) and fluorobenzaldehyde (entries11-13) are reacted as substrates. When the substitution position of the group on the benzene ring of the substrate is in the orth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com