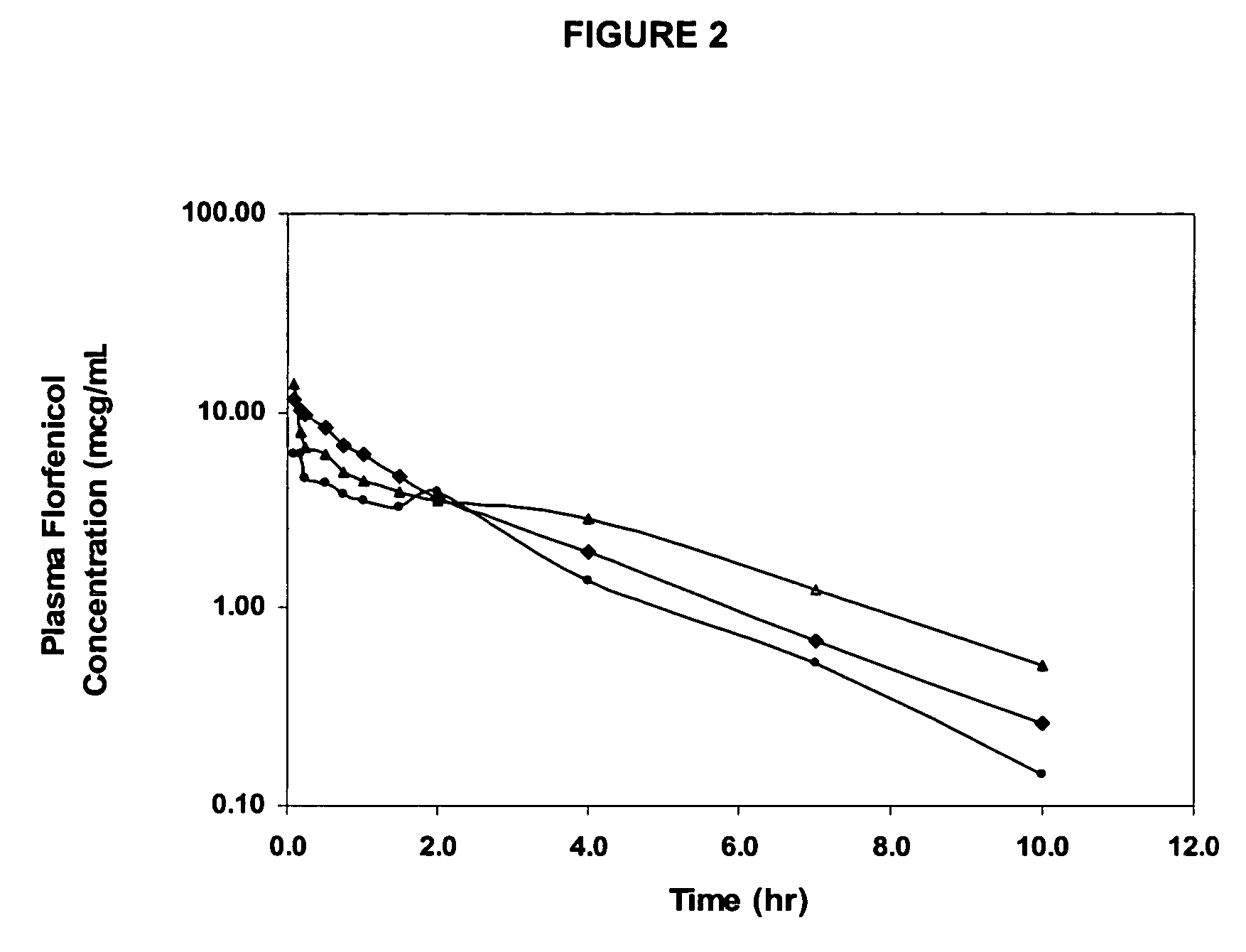
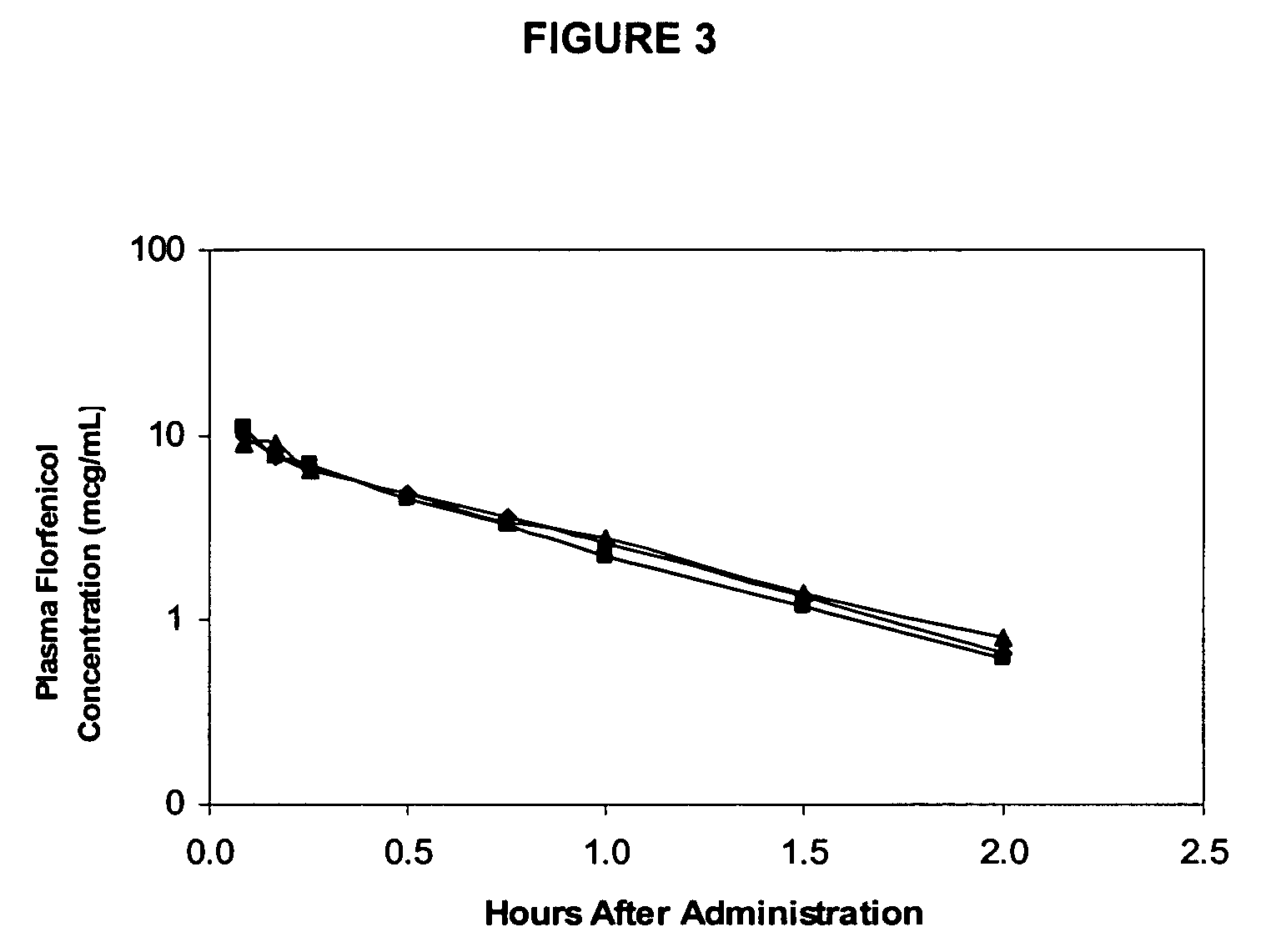
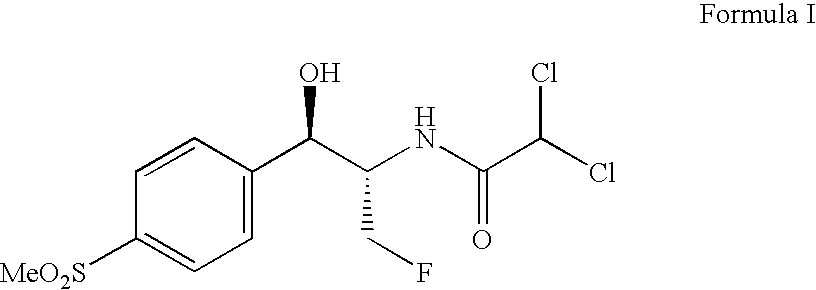
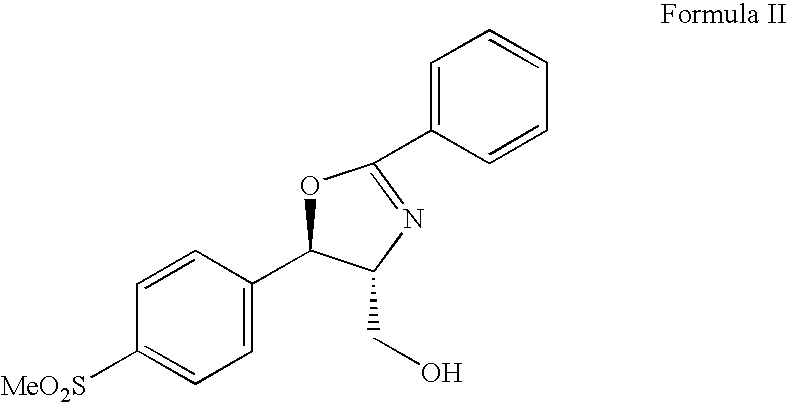
Patents
Literature
627 results about "Florfenicol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
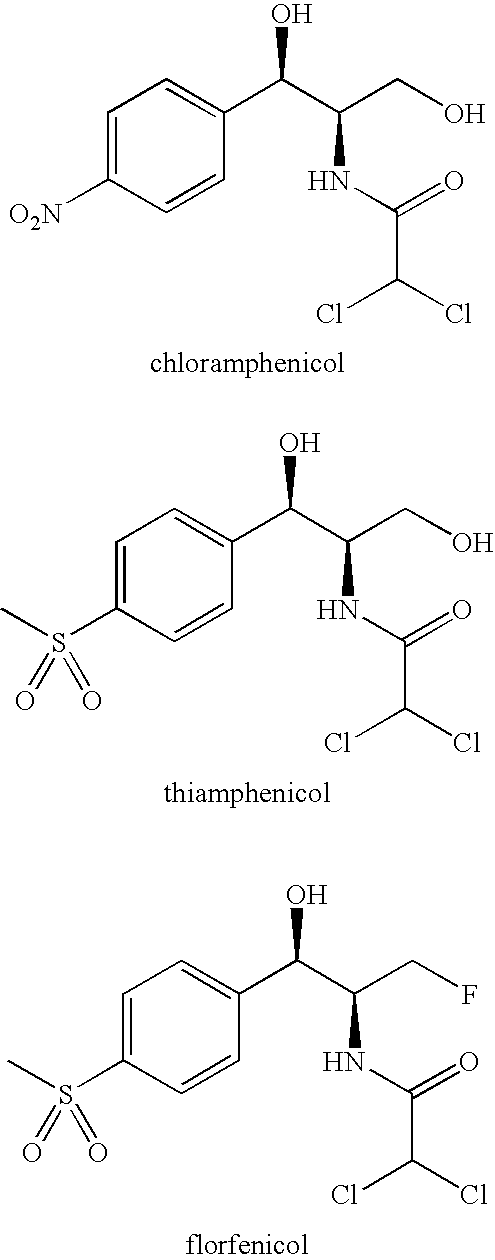
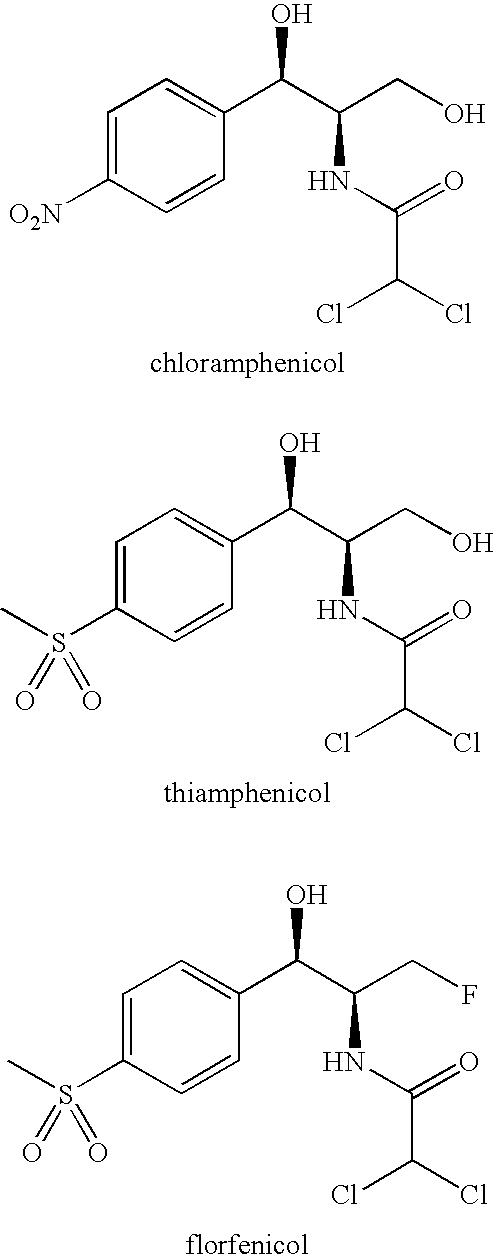
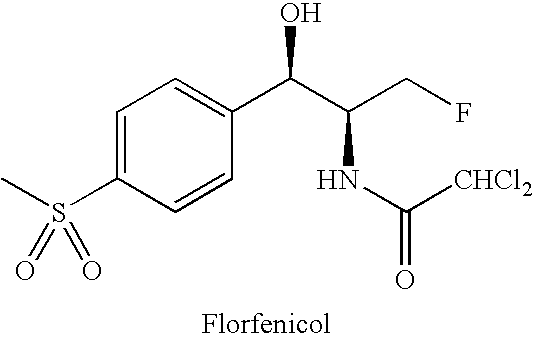
Florfenicol (marketed by Schering-Plough Animal Health under the trade name Nuflor) is a fluorinated synthetic analog of thiamphenicol , mainly used in veterinary medicine. As a generic, it is now available worldwide.
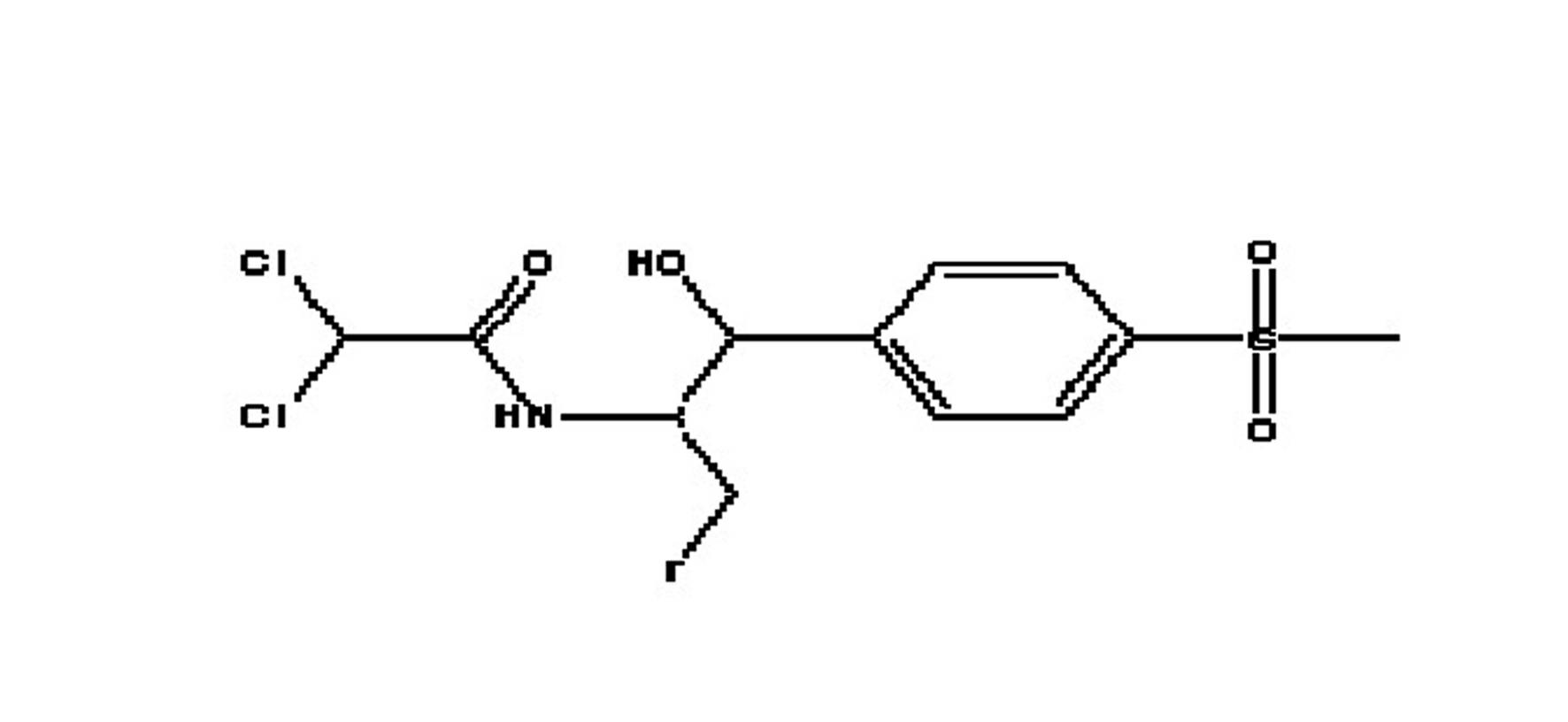
Antibacterial 1-(4-mono- and di-halomethylsulphonylphenyl)-2-acylamino-3-fluoroproponals and preparation thereof
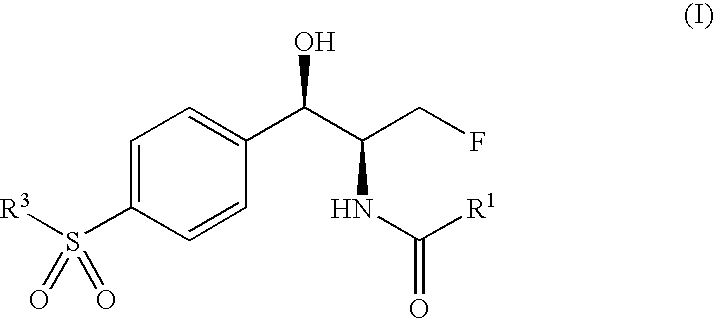
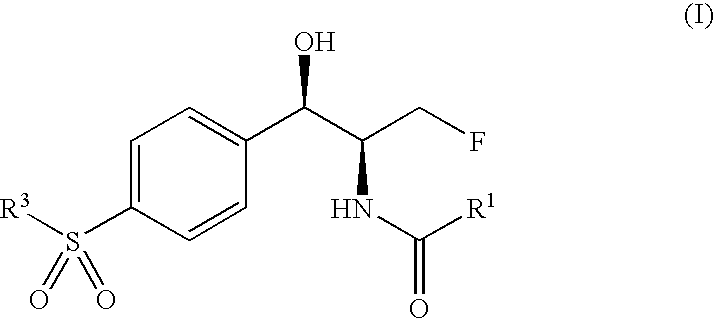
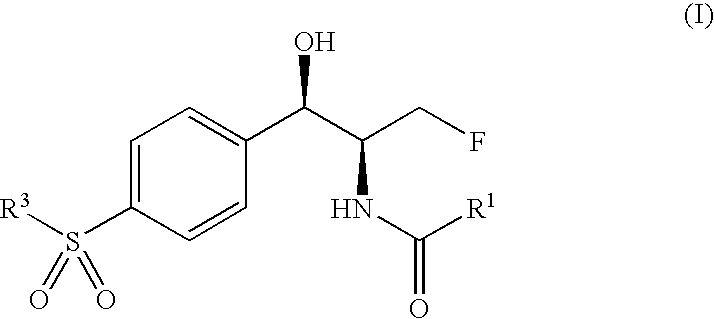
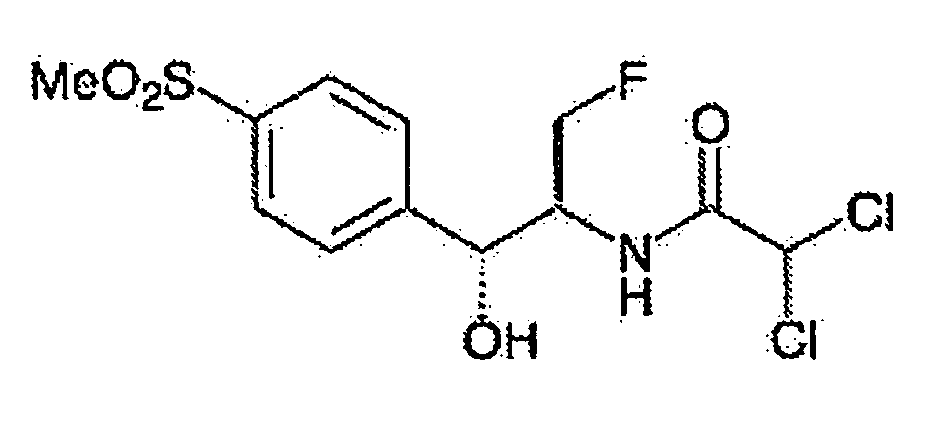
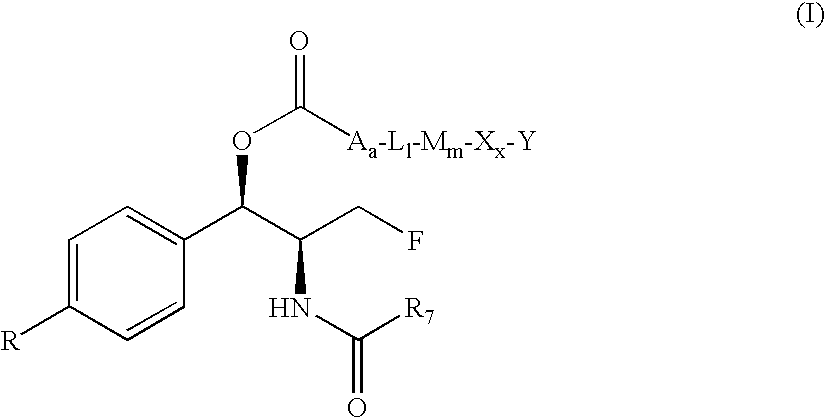
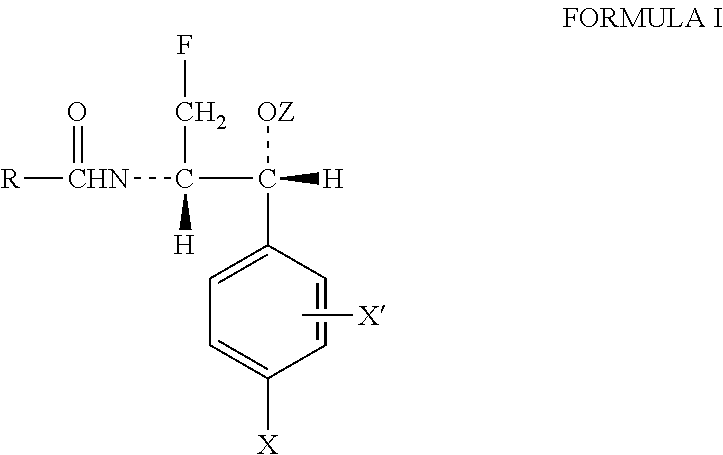
Novel florfenicol compounds having the chemical structure:or a pharmaceutically-acceptable salt thereof or a solvate thereof, or prodrug thereof, wherein R1 is CHCl2, CHClF, CHF2, CHBrCl, CH3, CH2N3, CH2CN, CH(R2)NH2 or CH X1X2; where R2 is H, CH3 or CH2OH, and X1 and X2 are independently selected halogens; and R3 is CH2Cl, CH2F, CHF2, CHCl2 or CH2OH are disclosed. The compounds are useful for the treatment and / or prevention of bacterial infections in a broad range of patients such as, without limitation, birds, fish, shellfish and mammals.
Owner:SCHERING PLOUGH ANIMAL HEALTH +1
Florfenicol prodrug having improved water solubility
Owner:INTERVET INC
Process for preparing florfenicol
The present invention is directed to a new process of preparing highly pure Florfenicol. The invention is further directed to new oxazolidine derivatives useful in making Florfenicol and processes of making these derivatives. Examples of such intermediates include (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine; and (4S,5R)-3-acetyl-2,2-dimethyl-4-fluoromethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine.
Owner:AUROBINDO PHARMA LTD
Florfenicol prodrug having improved water solubility
ActiveUS20050182031A1Good water solubilityRapidly and efficiently convertAntibacterial agentsBiocideSolubilityWater soluble
The present invention discloses phosphate esters of florfenicol (prodrugs) and florfenicol analogs having superior water solubility that are hydrolyzed to florfenicol or the respective florfenicol analog in vivo, upon administration to an animal.
Owner:INTERVET INC
Antibacterial 1-(4-mono- and di-halomethylsulphonylphenyl)-2-acylamino-3-fluoroproponals and preparation thereof
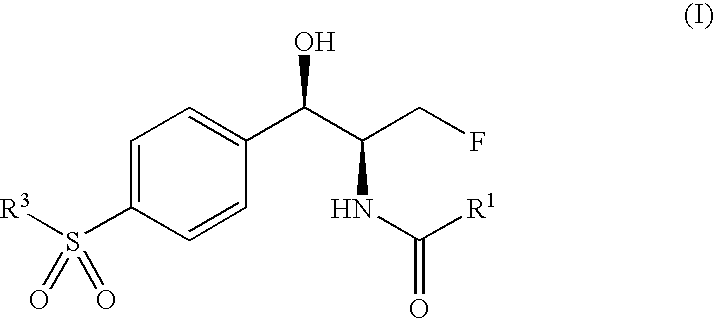
Novel florfenicol compounds having the chemical structure: or a pharmaceutically-acceptable salt thereof or a solvate thereof,or prodrug thereof, wherein R1 is CHCl2, CHClF, CHF2, CHBrCl, CH3, CH2N3, CH2CN, CH(R2)NH2 or CH X1X2; where R2 is H, CH3 or CH2OH, and X1 and X2 are independently selected halogens; and R3 is CH2Cl, CH2F, CHF2, CHCl2 or CH2OH are disclosed. The compounds are useful for the treatment and / or prevention of bacterial infections in a broad range of patients such as, without limitation, birds, fish, shellfish and mammals.
Owner:SCHERING PLOUGH ANIMAL HEALTH +1
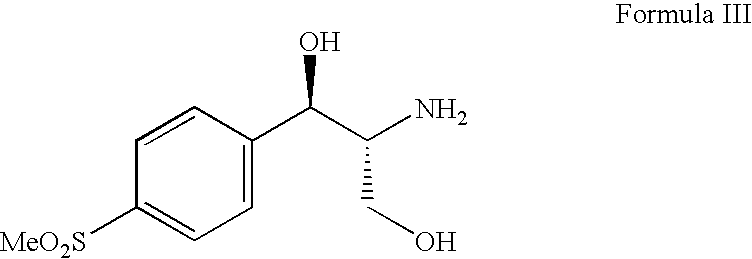
Method for analyzing (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol as intermediate of florfenicol
ActiveCN101941927AEasy to manufactureHigh yieldOrganic chemistryOrganic compound preparationPtru catalystBenzaldehyde
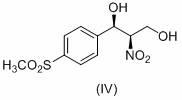
The invention relates to a method for analyzing (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol as an important intermediate of florfenicol, which is chloramphenicol spectrum antibiotic special for animals. The method comprises the following steps of: performing a reaction of methylsulfonyl benzaldehyde and nitro alcohol at 0-40 DEG C in the presence of a chiral catalyst to obtain (1R, 2R)-2-nitro-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol; and reducing the (1R, 2R)-2-nitro-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol through hydrogen to obtain the intermediate. In the invention, the compound (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol with a high e.e. value is obtained by adopting the chiral catalyst, and the compound has the advantages of strong reaction selectivity, high productivity, simple process, low cost, easy preparation of raw materials and low price, is suitable for industrial production, avoids chiral separation frequently used in industry at present and saves the raw materials and the cost. The compound (1R, 2R)-2-amino-1-(4-(methylsulfonyl)-phenyl)-1,3-propylene glycol is used for preparing florfenicol and can reduce the preparation cost.
Owner:MASTEAM BIO TECH
Method for synthesizing florfenicol
InactiveCN101265220AEasy marketEasy to operateAntibacterial agentsOrganic chemistrySodium methoxideSynthesis methods
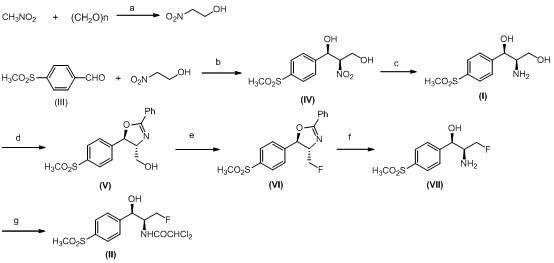
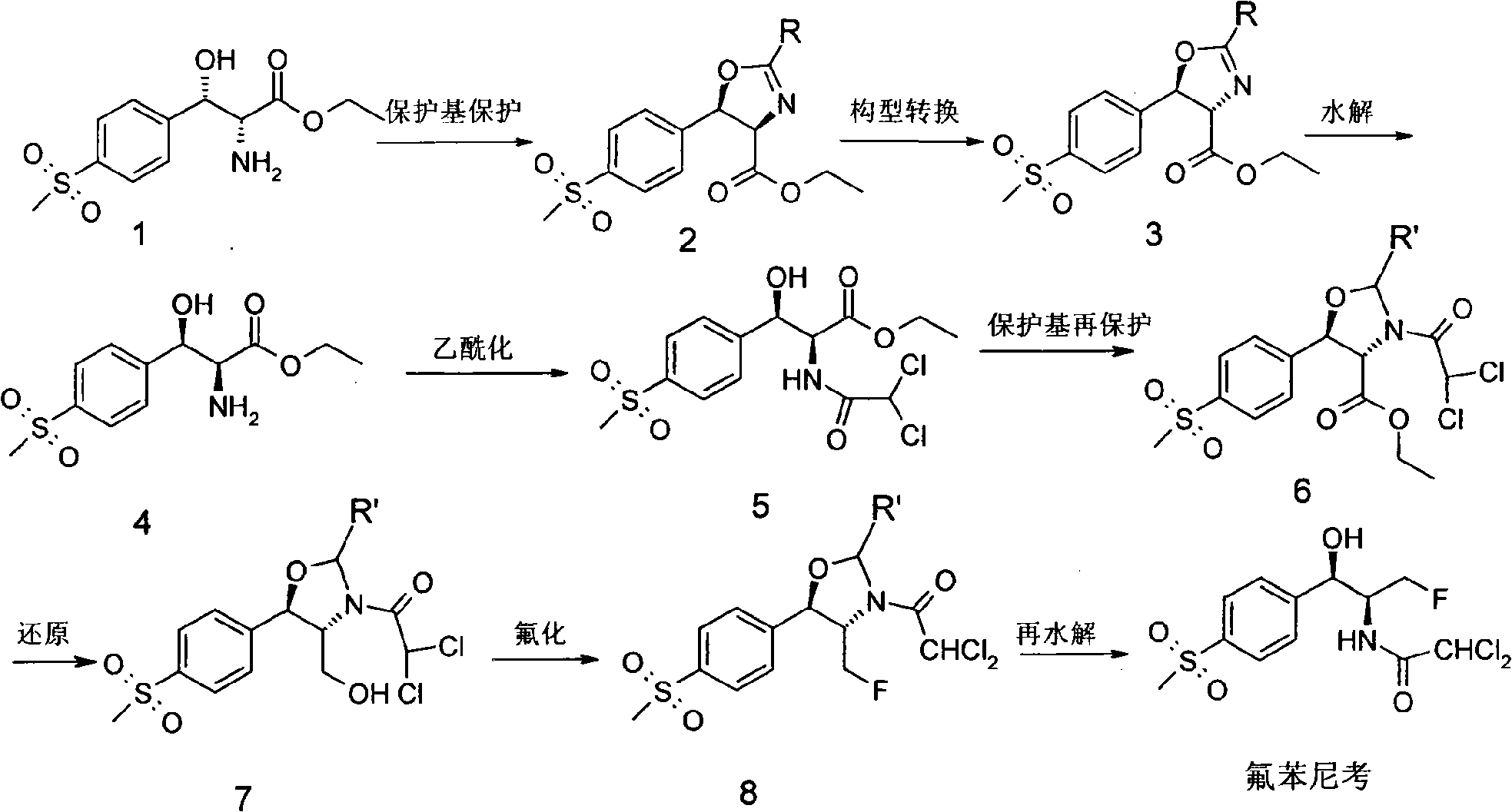
The invention discloses a synthesis method of synthesizing florfenicol. The synthesis method comprises the following steps: L-threo-(p-(methylsylfonyl) phenyl) serine ethyl ester which is used as raw material is processed by the protection by a protecting group, configurational transition, hydrolyzation, acetylation, re-protection, deoxidation, fluorination, and pre-hydrolyzation, so that the florfenicol is obtained, wherein, the protecting group R is one of benzoyl chloride, phthalic anhydride, cyanophenyl, and allyl compounds; the configurational transition is performed when sodium alcoholate or sodium methoxide exists. The raw material which is used in the method is a byproduct which is generated during the process of preparing thiamphenicol, is easy to get in the market, and is inexpensive; the technological operation is simple, the cost is low, the yield rate is high, and the synthesis method has industrialized value.
Owner:SHANGHAI RECORD PHARM CO LTD
Method for producing florfenicol soluble powder
InactiveCN101966340AImprove solubilityIncrease productivityAntibacterial agentsOrganic active ingredientsDiseaseSolubility
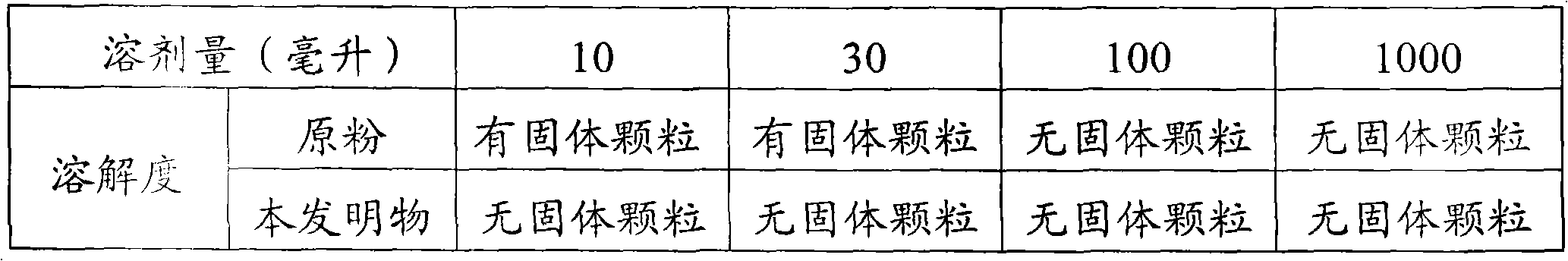
The invention relates to a method for producing florfenicol soluble powder. The method comprises the following technical steps of: 1, including florfenicol and 2-hydroxypropyl-beta-cyclodextrin; and 2, performing spray drying on included solution by a spray drying tower. By combining grinding inclusion and spray drying process, the production efficiency and the yield are high; the solubility of the prepared florfenicol-included powder is increased to be 263mg / ml (soluble) from original 13mg / ml (slight soluble) and enlarged by 20 times; and the product can be directly dissolved in water and diseases of animals can be treated by drinking the water.
Owner:WUXI ZHENGDA POULTRY
Cyclodextrin-included florfenicol quick-release water-soluble powder preparation and preparation method thereof
InactiveCN102160854AGood water solubilityImprove solubilityAntibacterial agentsPowder deliveryCyclodextrinDissolution
The invention relates to a cyclodextrin-included florfenicol quick-release water-soluble powder preparation and a preparation method thereof. The preparation is prepared by stirring florfenicol, cyclodextrin and water at a uniform speed and including by using an inclusion technology. The preparation can be directly dissolved in water to be taken by livestock, and has the characteristics of high medicine dissolution rate, high dissolubility, high bioavailability, good absorption and the like; and the medicine (florfenicol) is included in a carrier material, the bitterness of the florfenicol can be masked, and the intake or drinking of animals cannot be influenced by the bitterness of the medicine.
Owner:GUANGDONG YANGBLE BIOPHARMLS
Novel method for synthesizing thiamphenicol and florfenicol and its key intermediate product
InactiveCN1743308AEfficient constructionSolve the cumbersome operationOrganic chemistryOrganic compound preparationThiamphenicolChemical reaction
This invention relates to a synthetic method for thiamphenicol and florfenicol and their key intermediate. Through a series of chemical reaction-first, MCPBA oxidation or deshydroxyl fluorination in advance then MCPBA oxidation, then hydrolization for ring-opening, benzyl removal and finally acetylation, the raw material No. 7 compound is ultimately converted into thiamphenicol or florfenicol. In this method, the targeted compound's chiral center is efficiently configurated and the complicated and wasted resolution process is avoied.
Owner:SHAOXING MINSHENG PHARMA
Florfenicol composition with high bioavailability and preparation method thereof
ActiveCN105477642AImprove solubilityOvercome the disadvantage of being insoluble in waterPowder deliveryOrganic active ingredientsBioavailabilityProcess design
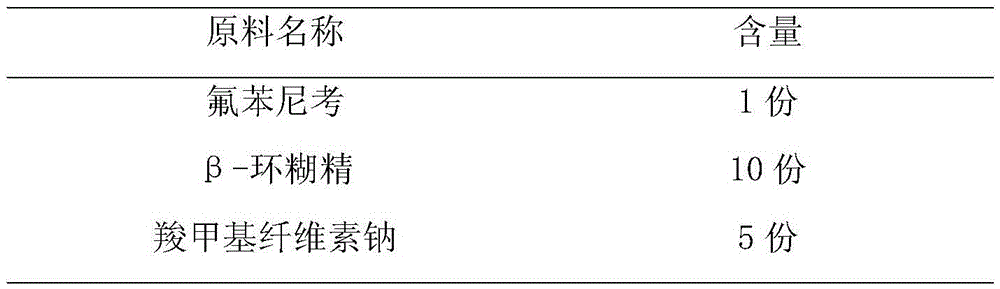
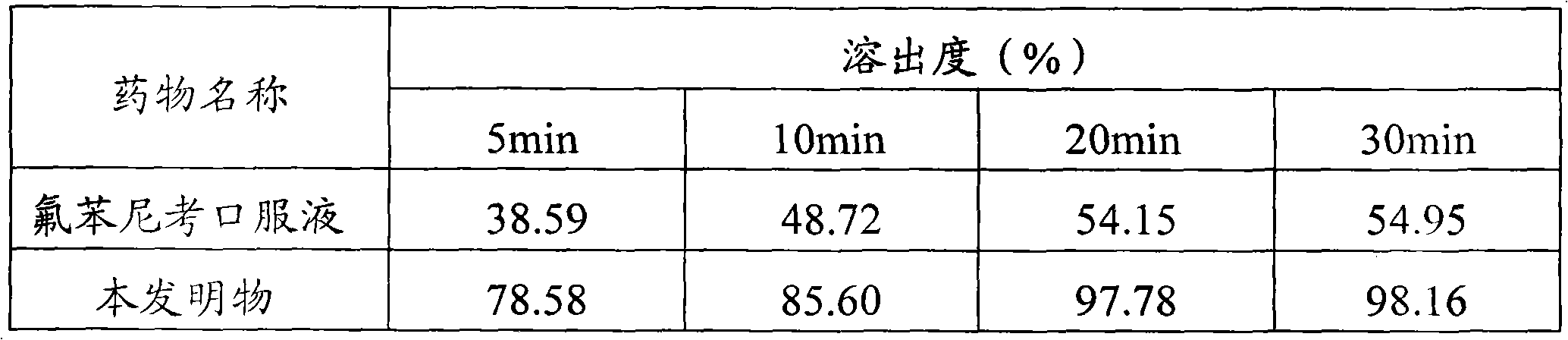
The invention discloses a florfenicol composition with high bioavailability. The florfenicol composition is prepared from, by weight, 1-5 parts of florfenicol, 2-50 parts of beta-cyclodextrin and 5-10 parts of absorption promoter. The florfenicol composition has the advantages that the florfenicol composition with high bioavailability is obtained by screening through a large amount of experiments, the absorption promoter is added on the basis of florfenicol cyclodextrin inclusion complex, the defect that florfenicol is difficult to dissolve in water is overcome while the oral bioavailability of the florfenicol can be increased greatly. A preparation method of the florfenicol composition is reasonable in process design, high in operability and capable of achieving industrial production.
Owner:CHINANIMAL NANJING VETERINARY DRUGS
Method for preparing solid florfenicol dispersion
InactiveCN102657614AImprove solubilitySimple preparation processAntibacterial agentsPowder deliveryPolyethylene glycolPoloxamer
The invention discloses a method for preparing solid florfenicol dispersion, and relates to the field of animal medicine. The solid florfenicol dispersion is uniformly and sequentially prepared from the following components in parts by weight: 15 to 25 parts of florfenicol (sieved with a sieve of 80 meshes), 15 to 25 parts of poloxamer, and 50 to 70 parts of polyethylene glycol-4000. The preparation method comprises the following steps of: (1) weighing 15 to 25 parts of florfenicol (sieved with the sieve of 80 meshes), 15 to 25 parts of poloxamer and 50 to 70 parts of polyethylene glycol-4000 by weight; (2) heating and melting the poloxamer and the polyethylene glycol-4000, stirring for 3 to 5 minutes till the mixture is uniform, adding the florfenicol, and stirring uniformly; and (3) quickly cooling the mixture obtained in the (2) to the temperature of 10 DEG C below zero, crushing, and thus obtaining the solid florfenicol dispersion. The preparation process is simple, and the dissolubility of the florfenicol is remarkably improved and is about 2 times that of a common florfenicol preparation (soluble powder or oral liquid).
Owner:QINGDAO LVMAN BIOLOGICAL ENG
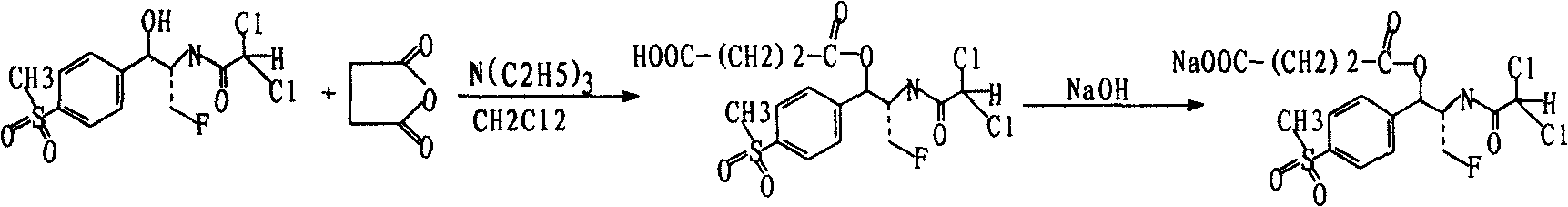
Preparation of florfenicol sodium succinate
InactiveCN101279941AImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsOrganic solventReaction temperature
Disclosed is a method to prepare florfenicol sodium succinate. Florfenicol reacts with succinic anhydride in organic solvent with triethylamine as activator under 10-15 DEG C to produce florfenicol ester succinate which then reacts with sodium hydroxide in organic solvent under 10-15 DEG C, producing florfenicol sodium succinate which is soluble in water. The mol ratio of the materials of florfenicol: triethylamine:succinic anhydride is 1:2-4:1.5-3. Florfenicol sodium succinate preparation can be made into injection, aqueous solution and lyophilized powder and can be used for disease prevention and treatment on animals.
Owner:阮明华
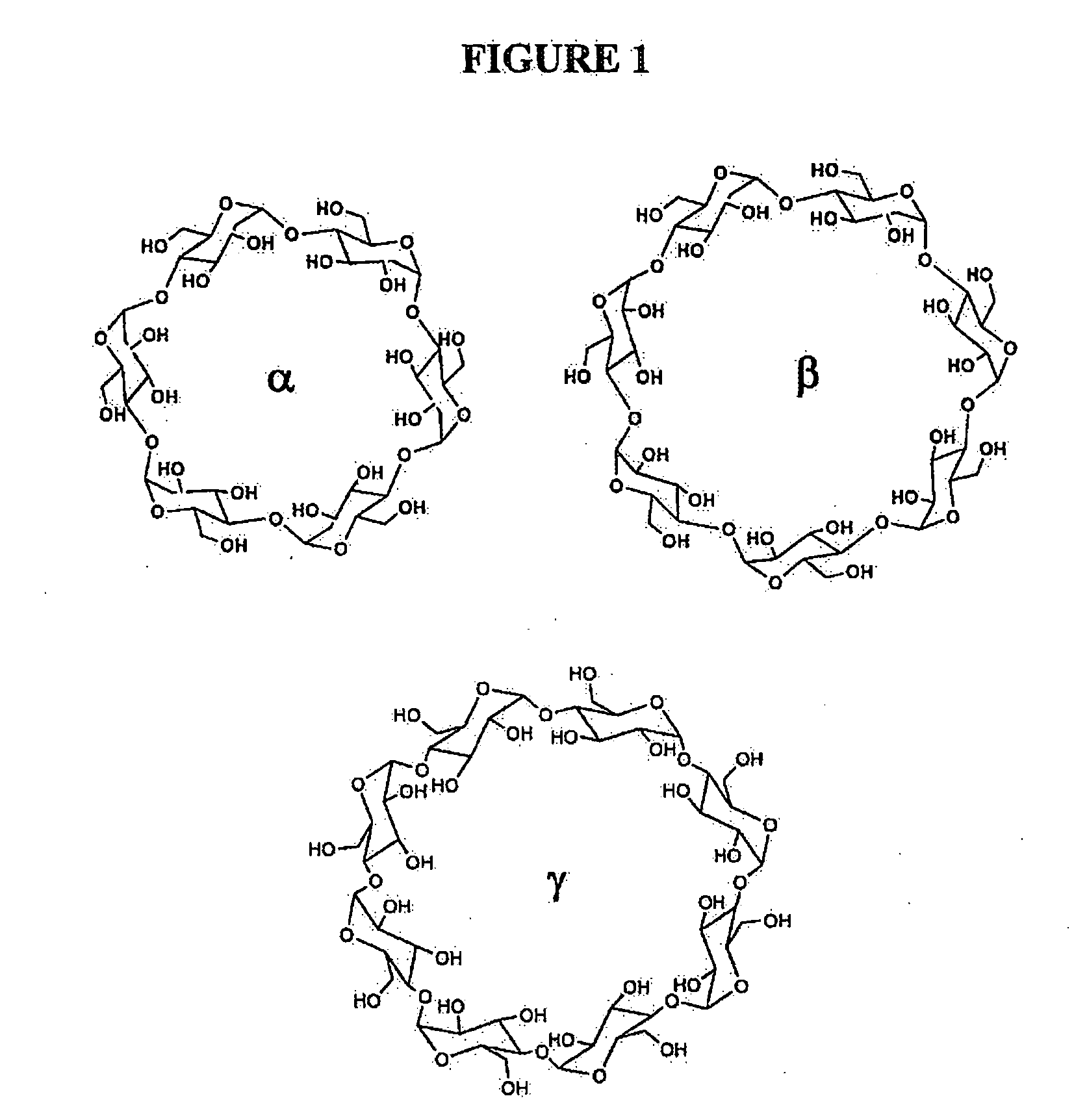
Compounds and methods for enhancing solubility of florfenicol and structurally-related antibiotics using cyclodextrins
InactiveUS20090062397A1Improve solubilityReduce the amount requiredAntibacterial agentsBiocidePolyethylene glycolCyclodextrin
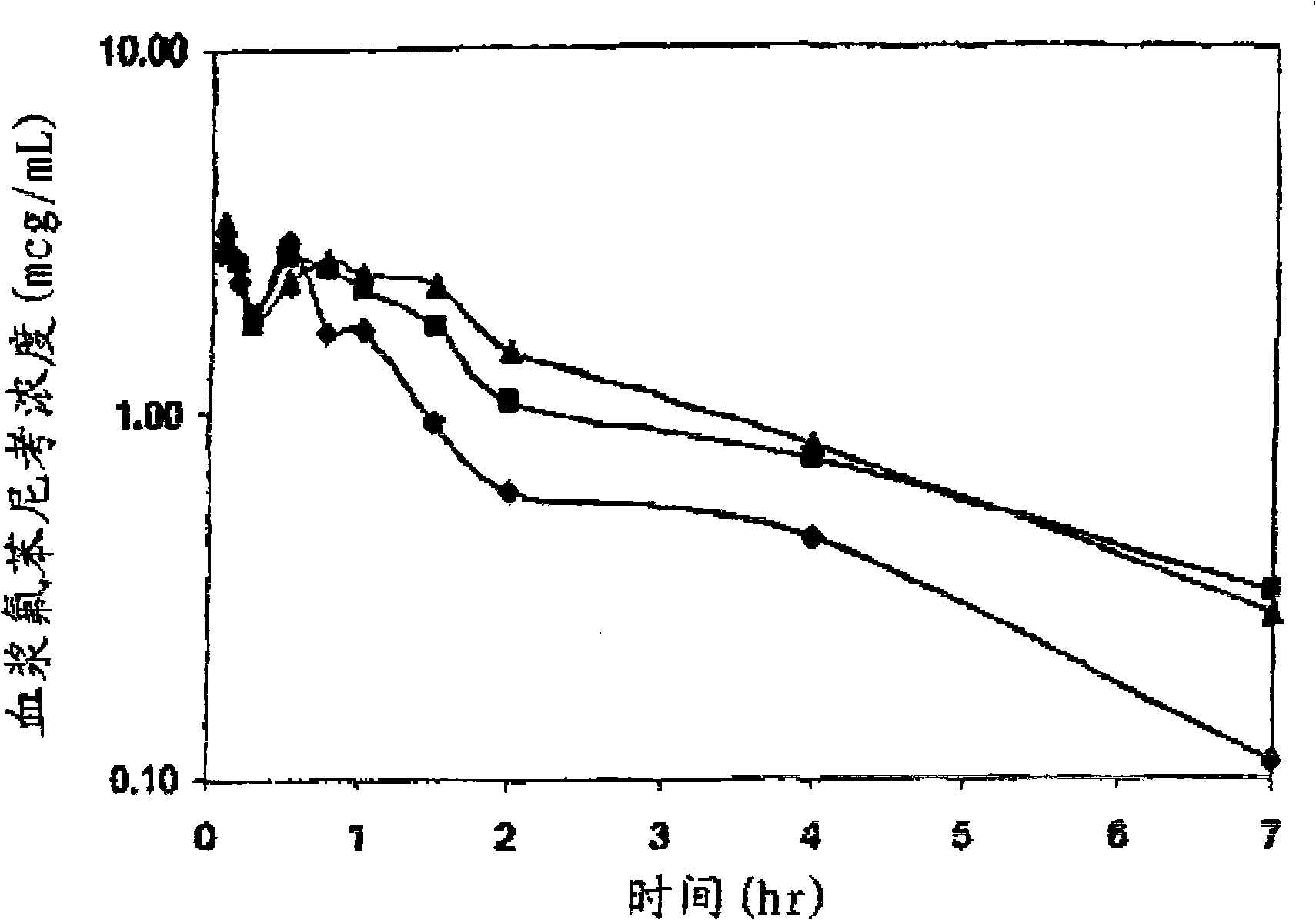
The relatively low solubility of florfenicol (FEC) in water (1.3 mg / mL) limits its use in medicated drinking water systems for treatment of pulmonary disease of swine and poultry. Current formulations use a high volume organic solvent to reach the required FFC concentration of 13.5 mg / mL in an automated proportioner mixing tank system, with practical disadvantages for the users in the field. This invention relates to the effects of complex formation with natural and modified cyclodextrins on the aqueous solubility of FFC and antibiotics of related structure. Furthermore, this invention relates to the effects of polyethylene glycol (PEG-300) as a co-solvent in an FFC-cyclodextrin system to achieve the required FFC dose in the mixing tank system and to avoid high volumes of the organic solvent.
Owner:SCHERING PLOUGH ANIMAL HEALTH +2
Compound pharmaceutical composition for curing virus diseases of animals and preparation method thereof
ActiveCN101543589AReasonable compositionFormulation ScienceOrganic active ingredientsPowder deliveryTherapeutic effectForsythia
The invention provides a high-efficiency, rapid and safe compound pharmaceutical composition for curing virus diseases of animals and a preparation method thereof. The compound pharmaceutical composition comprises the following components of forsythia fruit, isatis root, bamboo leaf, scutellaria root, honeysuckle flower, isatis leaf, milkvetch root, longspur epimedium, glossy privet fruit, dried rehmannia root, red sage root, chuanxiong rhizome, dark plum, peppergrass, tendril-leaved fritillary bulb, tangerine peel, lilyturf root, liquorice and florfenicol. The pharmaceutical composition has the function of superactive antivirus, has strong antivirus and antibiosis actions, remarkable therapeutic effect, fast effect, complete treatment and no relapse, is not easy to generate drug resistance and can be effectively used for preventing and curing the virus diseases of animals. The compound pharmaceutical composition can be powder preparation formulation. The invention also provides feed containing the compound pharmaceutical composition.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Method for producing water soluble fluorophenylnico
InactiveCN1947699AImprove solubilityReduce manufacturing costAntibacterial agentsOrganic active ingredientsSolubilityWater soluble
A water-soluble florfenicol with high solubility (10000 PPM) and low cost is prepared from florfenicol powder and glucose powder through proportional mixing and stirring.
Owner:庄晓峰
Florfenicol soluble power and preparation method thereof
InactiveCN102697730AImprove solubilityIncrease contentAntibacterial agentsOrganic active ingredientsSolubilityPolyethylene glycol
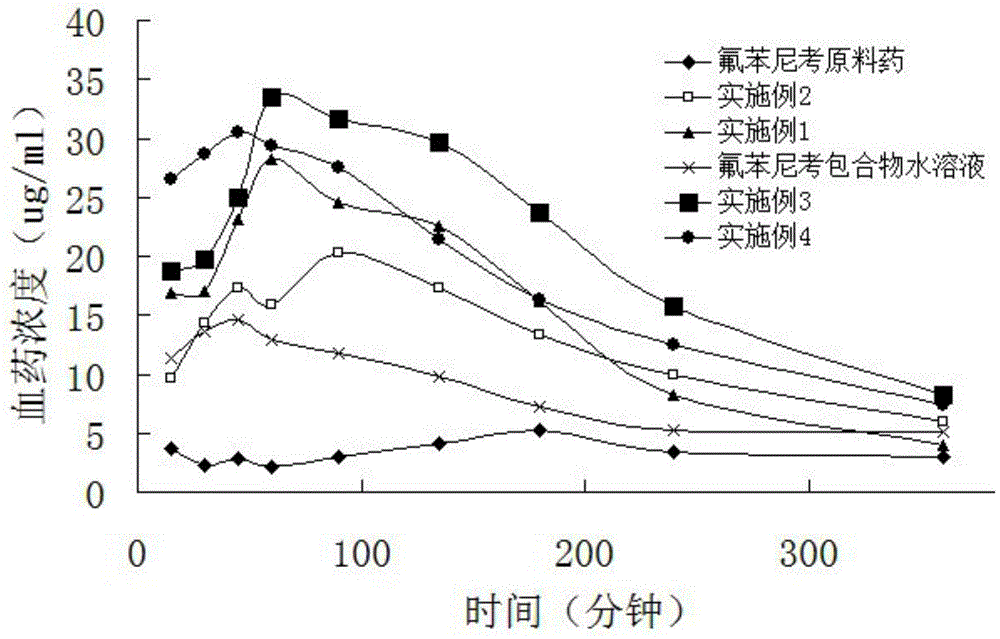
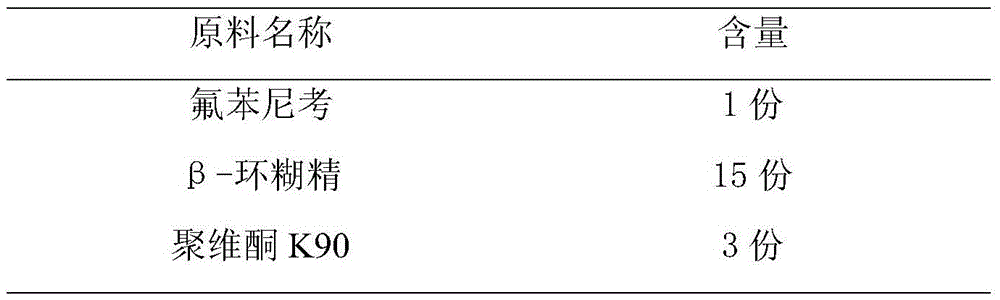
The invention discloses florfenicol soluble power. The florfenicol soluble power consists of by weight, florfenicol 65-75%, polyethylene glycol 6000 10-15%, polyvinyl pyrrolidone (PVP) 5-10% and carbomer 5-10%. By means of reasonable compounding of the florfenicol, the polyethylene glycol 6000, the PVP and the carbomer, the water solubility of the florfenicol is improved greatly, and the long-acting slow release effect is played. Due to the fact that the polyethylene glycol 6000, the PVP and the carbomer belong to high molecular polymers, the solution rate in a body is slow, the bioavailability of the florfenicol in the human body is improved fully, and the treatment effect of the florfenicol in an animal body is improved greatly (shown in figure). The florfenicol which is difficultly soluble in water is manufactured into the florfenicol soluble power capable of being soluble in water in clinical application, and the florfenicol soluble power is convenient and effective in the clinical application.
Owner:ZHENGZHOU HOUYI PHARMA
Water-Soluble Prodrugs of Florfenicol and its Analogs
The present invention discloses certain novel prodrugs of florfenicol and / or of florfenicol analogs, including prodrugs of salts pharmaceutically acceptable salts of florfenicol and its analogs, including nitrogen-containing esters of the secondary alcohol group of florfenicol and of its analogs, and pharmaceutically acceptable salts thereof, compositions containing them, and methods of administering them to subjects. In particular embodiments the prodrugs are sufficiently water-soluble to serve the functions needed of a water-soluble prodrug of florfenicol or of a water-soluble prodrug of a florfenicol analog. A certain subclass of the compounds also possesses the hydrolytic stability needed to maintain the prodrug in solution in the subject's system until appropriate conditions exist when the prodrug can hydrolyze, releasing florfenicol or the florfenicol analog in question.
Owner:SCHERING PLOUGH ANIMAL HEALTH +2
Compositions and method for treating infection in cattle and swine
ActiveUS20120077764A1Easy to manageAntibacterial agentsBiocideBovine respiratory diseaseThiamphenicol
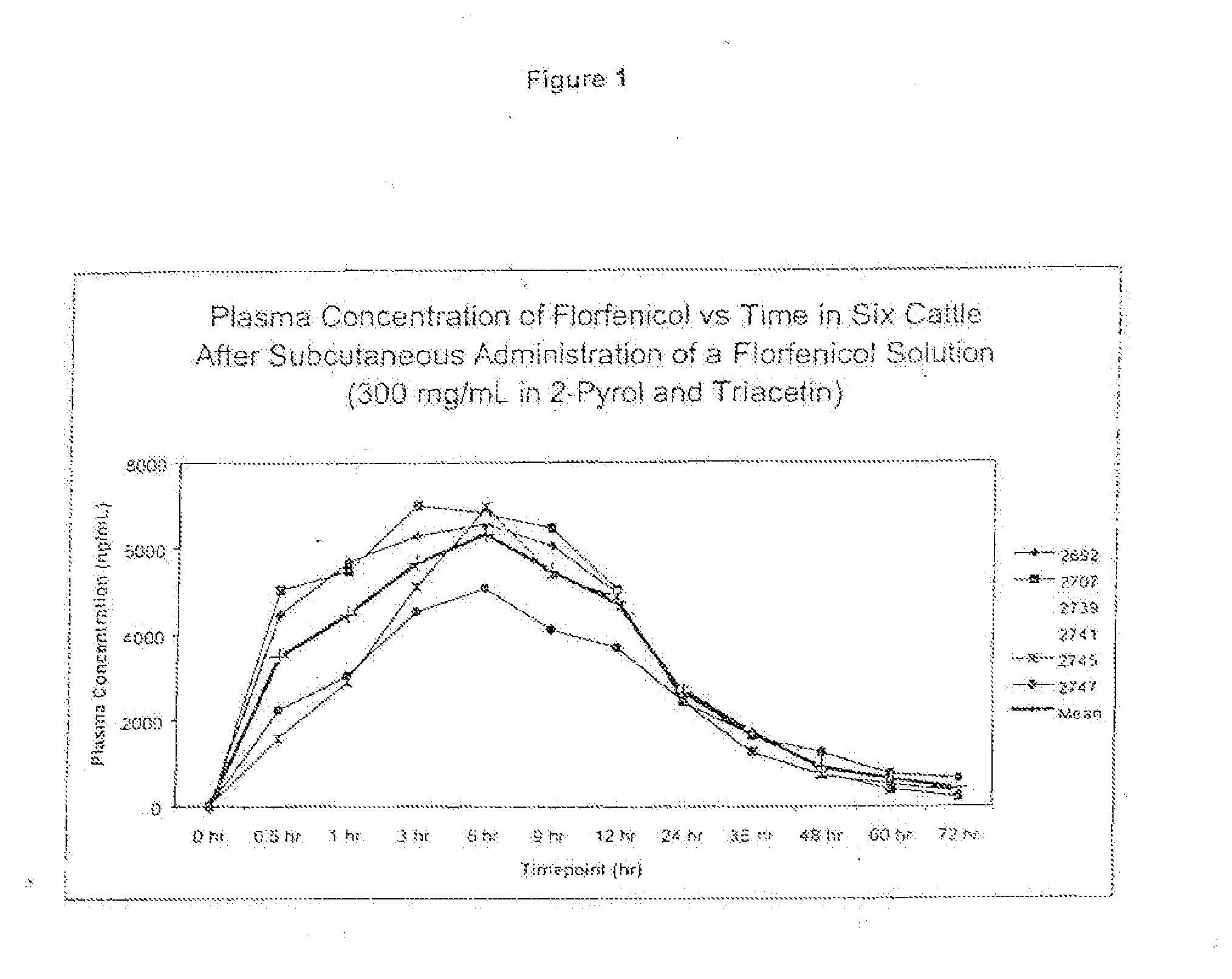
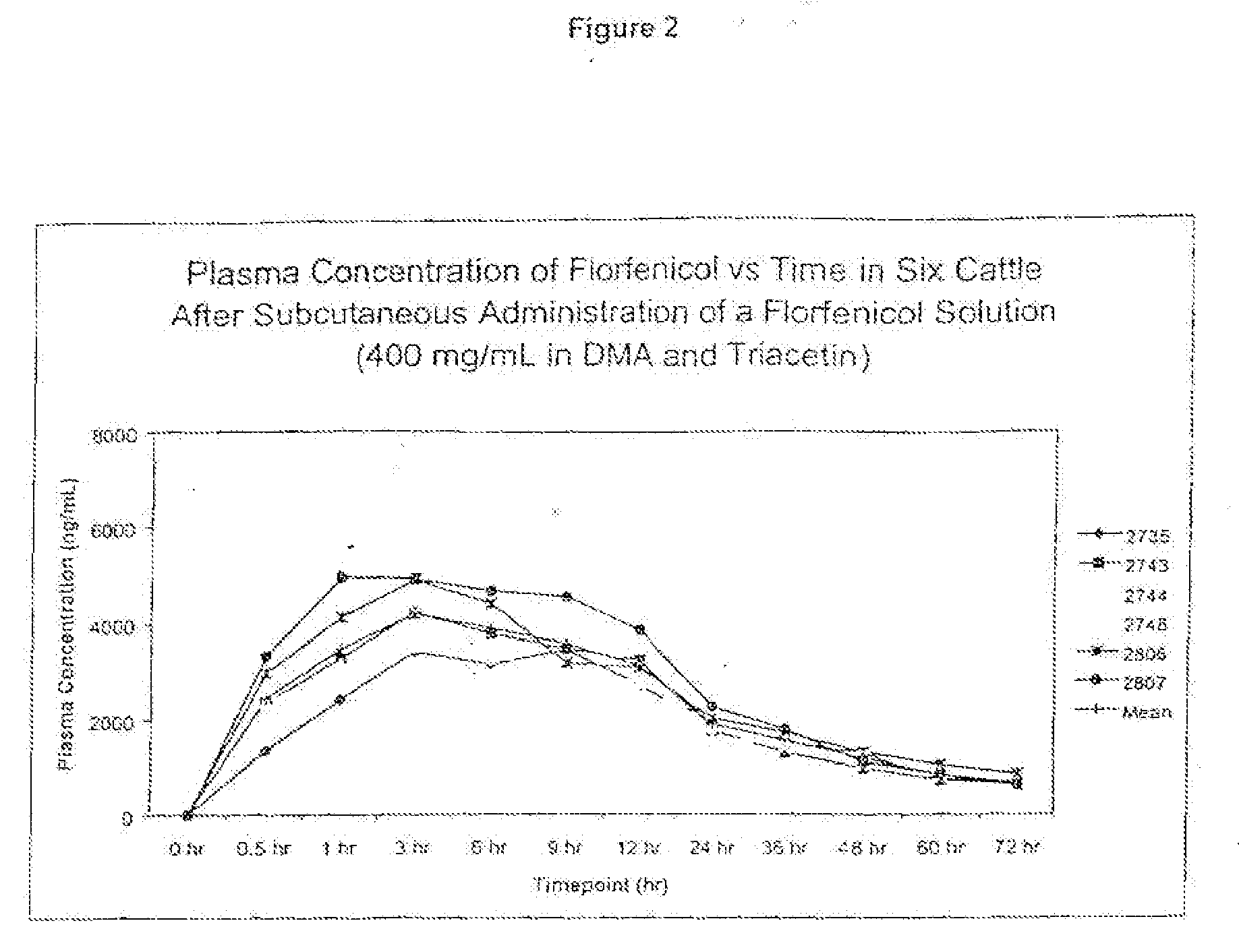
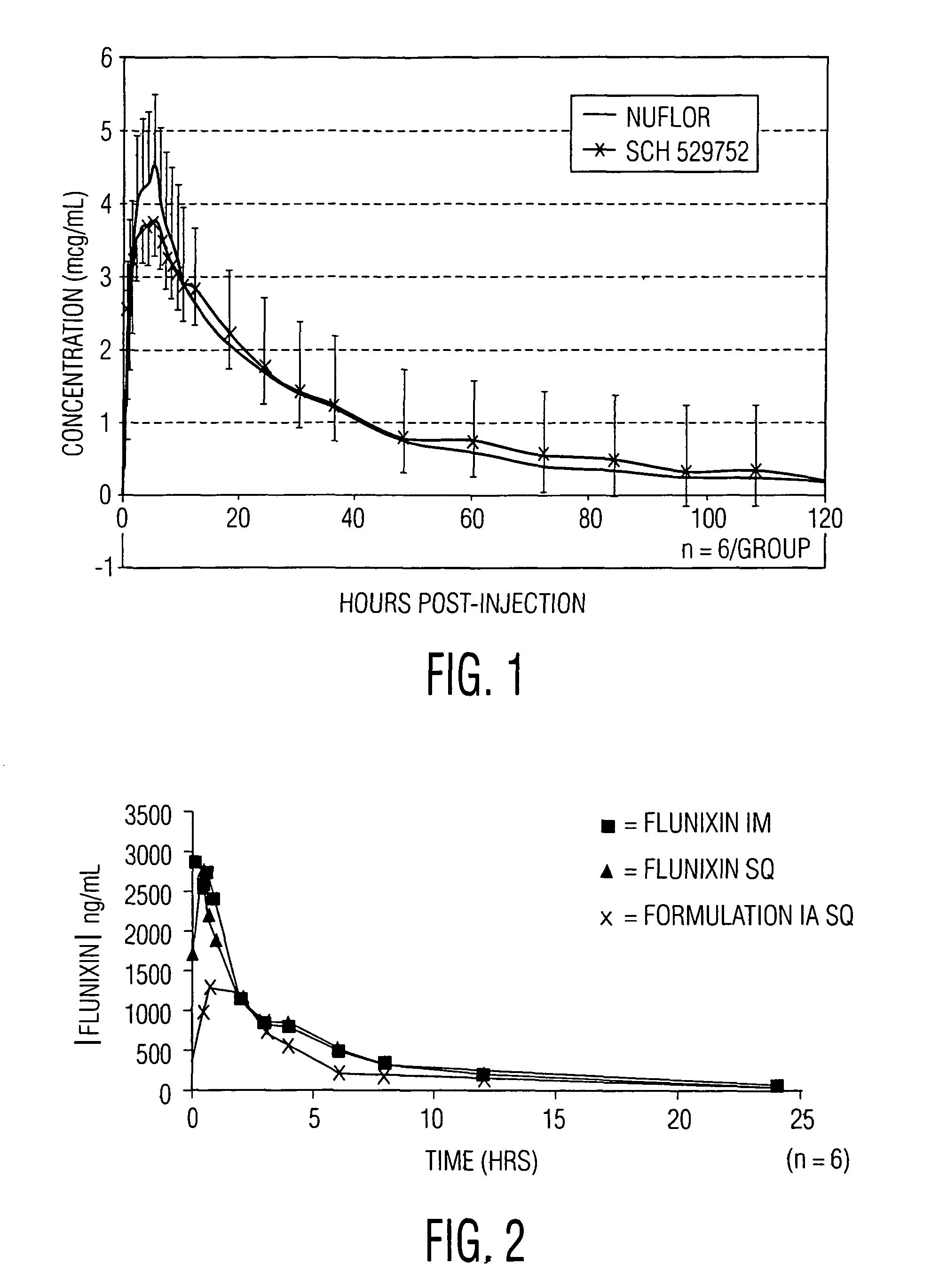
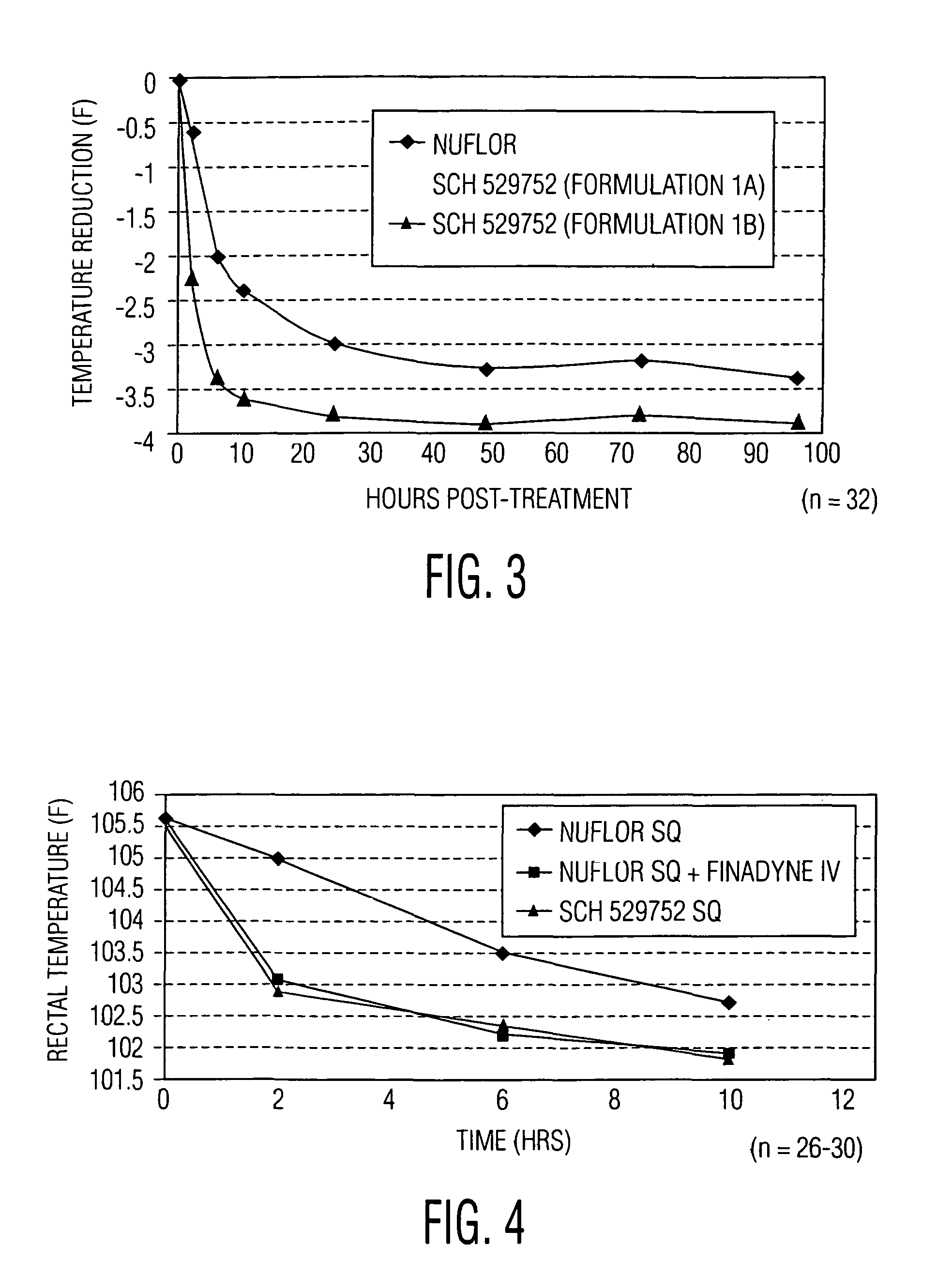
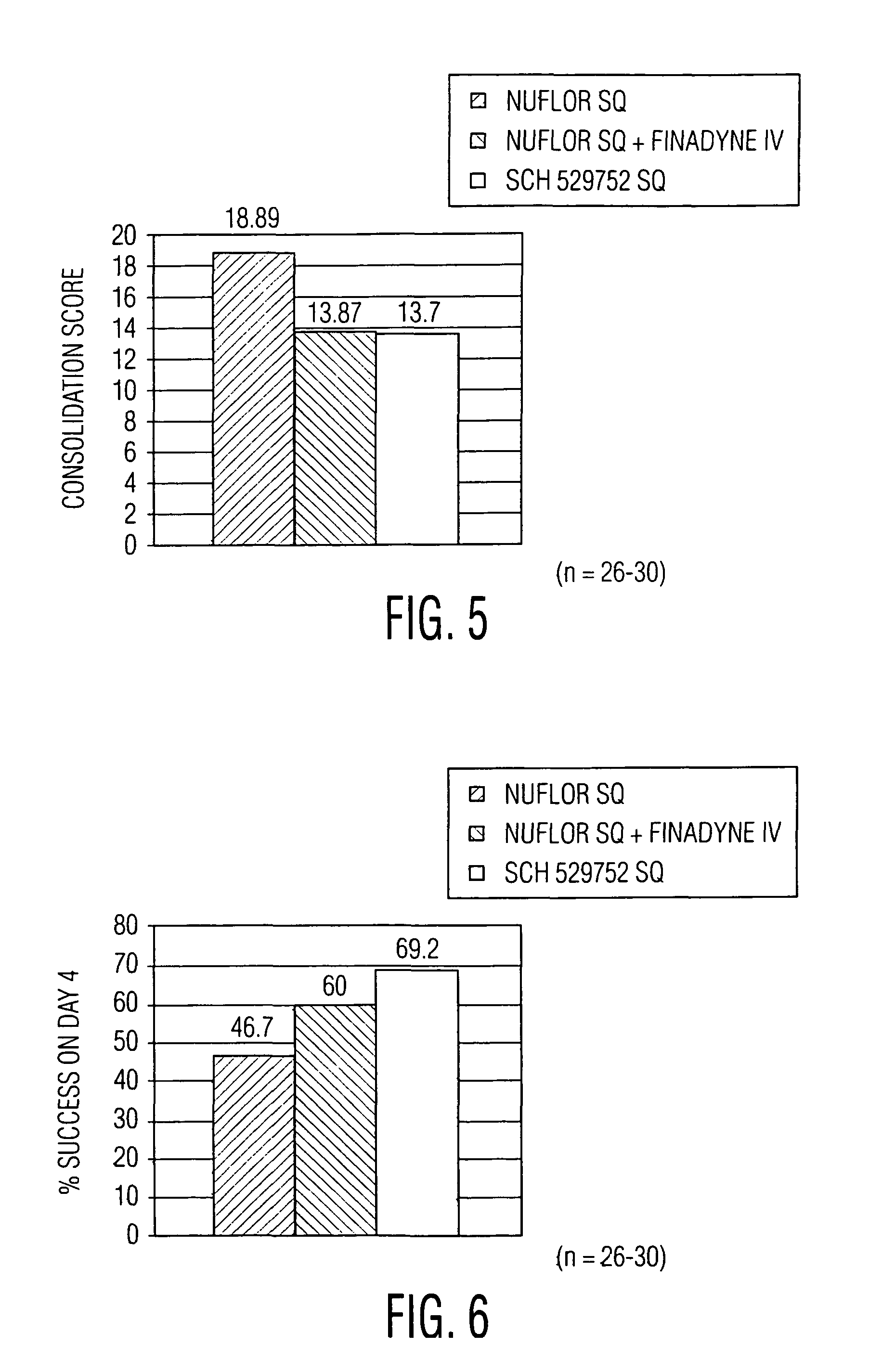
Novel formulations containing a fluorinated chloramphenicol or thiamphenicol derivative antibiotic such as florfenicol, and methods for using such formulations in the treatment and prevention of infectious diseases of bovines and swine, including bovine respiratory disease.
Owner:INTERVET INC
Compound florfenicol injection for animal
InactiveCN102008490AHigh degree of complexationNot perishableAntibacterial agentsTetracycline active ingredientsOrganic solventAntioxidant
The invention discloses compound florfenicol injection for an animal. Every 1,000ml of the compound florfenicol injection contains 50 to 300g of florfenicol, 50 to 300g of doxycycline hydrochloride, 150 to 800g of organic solvent, 5 to 90g of complexing agent and 0.2 to 1g of antioxidant. The compound florfenicol injection has the advantages that: (1) the preparation is high in complexation degree and stable in quality, and the doxycycline hydrochloride injection is instable in property; (2) the injection is low in dosage and convenient to use, and the injection twice can be finished at one time; (3) the injection greatly saves the labor cost for veterinary medicine under an intensive feeding condition; and (4) the production of the preparation is controllable in terms of technical conditions, the injection is suitable for industrial production, various raw and auxiliary materials are needed to be accurately weighed in the production process, only the reaction temperature and the reaction time are needed to be controlled without any special technical requirement, and the production technology is easy to master and control.
Owner:JIANGXI NUCLEAR IND TIANDIHE PHARMA
Compound fluorophenylnico injection, prepn. method and use thereof
InactiveCN1861084AImprove survival rateIncrease weightAntibacterial agentsTetracycline active ingredientsMedicineTrimethoprim
A compound florfenicol injection for treating the pneumonia and bacterial lung infection of pig is prepared from florfenicol, doxycycline, dimethyl formamide, magnesium dichloride, polyvinyl pyrrolidone, trimethoprim and propanediol. Its preparing process is also disclosed.
Owner:TIANJIN SHENGJI GRP CO LTD
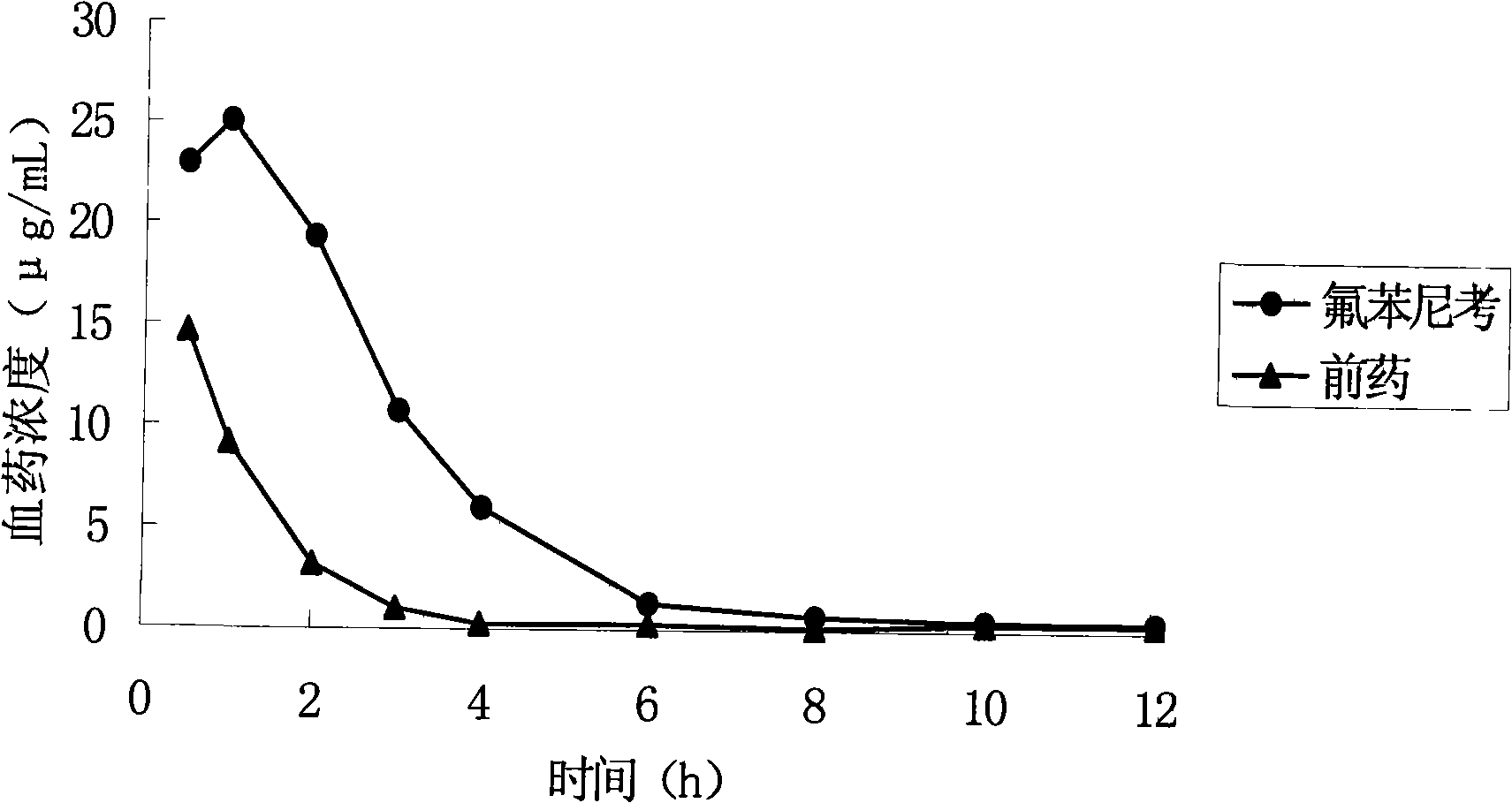
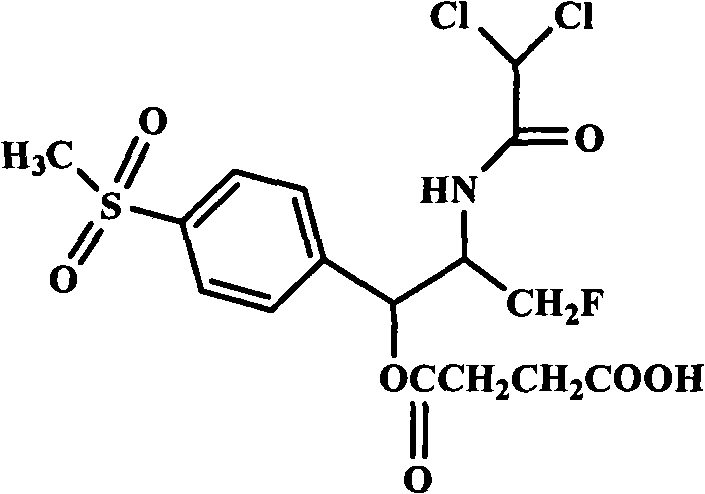
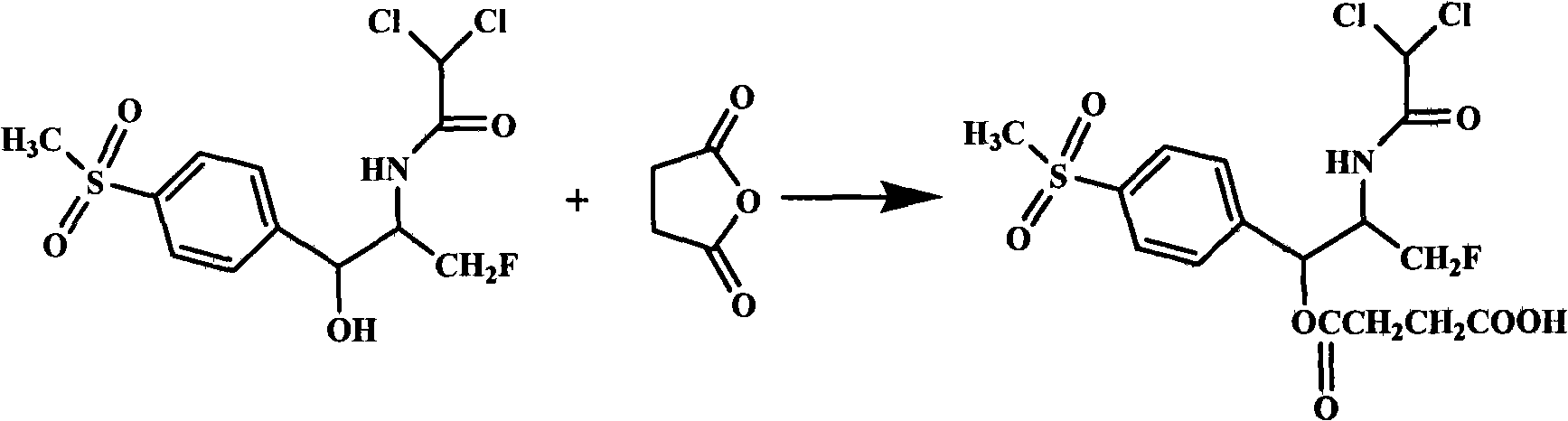
High-water-solubility florfenicol prodrug quickly released in vivo
InactiveCN101289416AThe synthesis process is simpleMedication convenienceOrganic chemistryOrganic compound preparationSolubilityArginine
The invention relates to a florenicol succinic acid monoester and a preparation method thereof, and the florenicol succinic acid monoester can be obtained by the esterification of the florenicol and succinic anhydride. Simultaneously, the invention also relates to acceptable salts and preparation methods thereof of the florenicol succinic acid monoester pharmacy, such as sodium salt, potassium salt, calcium salt and magnesium salt etc., and salts that are formed with basic amino acids and comprises lysine and arginine salts. By testing, the water-solubility of the corresponding salt is more than 500 mg / ml. The water-soluble derivatives disclosed by the invention quickly release the florenicol after being hydrolyzed in bodies and the derivatives can be used as high water-soluble prodrugs of the florenicol.
Owner:SOUTHWEST UNIV
Suspension emulsion of florfenicol, preparation method and application thereof
InactiveCN101444484ASimple manufacturing methodReduce manufacturing costAntibacterial agentsOrganic active ingredientsOil phaseBiocompatibility
The invention discloses a suspension emulsion of florfenicol, a preparation method and application thereof. The suspension emulsion is prepared by 10-30% (W / V) of florfenicol raw medicine, 20-40% of (W / V) of oil phase, 5-20% (W / V) of emulsifier and the balance of water; furthermore, additive can be added when necessary. The suspension emulsion of florfenicol prepared by the method has good stability, good biocompatibility, and obvious slow-release effect, can be applied to the preparation of slow-release injection of florfenicol, fills up the national technical blank, inaugurates a novel technical field for the application of florfenicol. The preparation method of the suspension emulsion is simple and feasible and has low manufacture cost.
Owner:SOUTH CHINA AGRI UNIV
Chloramphenicol universal monoclonal antibody hybridoma cell strain and application thereof
ActiveCN104263701AHigh affinityHigh detection sensitivityMicroorganism based processesTissue cultureBALB/c1,3-Propanediol
Owner:JIANGNAN UNIV
Method for detecting thiamphenicol, florfenicol and residues of metabolite florfenicol amine in egg simultaneously
The invention discloses a method for detecting thiamphenicol, florfenicol and residues of metabolite florfenicol amine in an egg simultaneously. The method comprises the following steps: extracting, purifying and concentrating egg samples; and detecting the obtained product through a fluorescent detector at a position with 225 nm excitation waves and 285 nm transmission waves by taking solution of acetonitrile-monosodium orthophosphate as a mobile phase and by using 5 micrometer C18 column of 250 mm X 4.6 mm and at a flow speed of between 1.0 and 1.2 mL / min, wherein the solution of acetonitrile-monosodium orthophosphate has a concentration of 0.01 mol / L and contains 0.005 mol / lauryl sodium sulfate and 0,1 percent triethylamine; and the volume ratio of the acetonitrile to the solution of NaH2PO4 is 30-37: 70-65. The method for detecting the thiamphenicol, the florfenicol and the residues of metabolite florfenicol amine in the egg simultaneously has the advantages of low cost and high sensitivity.
Owner:YANGZHOU UNIV
Compound florfenicol injection and preparation method and application thereof
ActiveCN102188422AReasonable compositionSignificant effect on infectionAntibacterial agentsHydroxy compound active ingredientsBovine respiratory diseaseFLUNIXIN MEGLUMINE
The invention discloses compound florfenicol injection and a preparation method and application thereof. The compound florfenicol injection comprises main medicaments, injection solvent and local anodyne; and the main medicaments are florfenicol and flunixin meglumine. The compound florfenicol injection is safe and convenient to use, controllable in quality, stable in preparation process, suitable for industrialized production and low in cost; and the compound florfenicol enhances the prevention and treatment effects of porcine or bovine respiratory diseases, improves the survival rate of pigs or cattle, and is suitable for popularization and application.
Owner:GUANGDONG WENS DAHUANONG BIOTECH
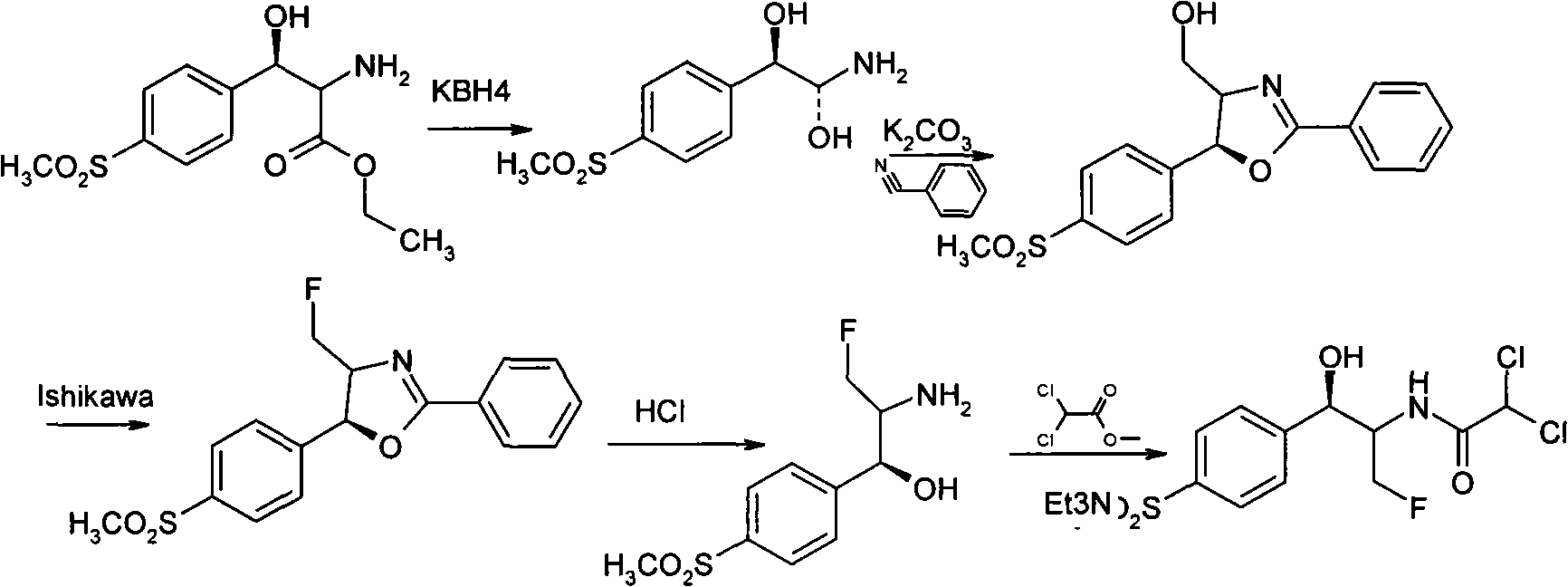
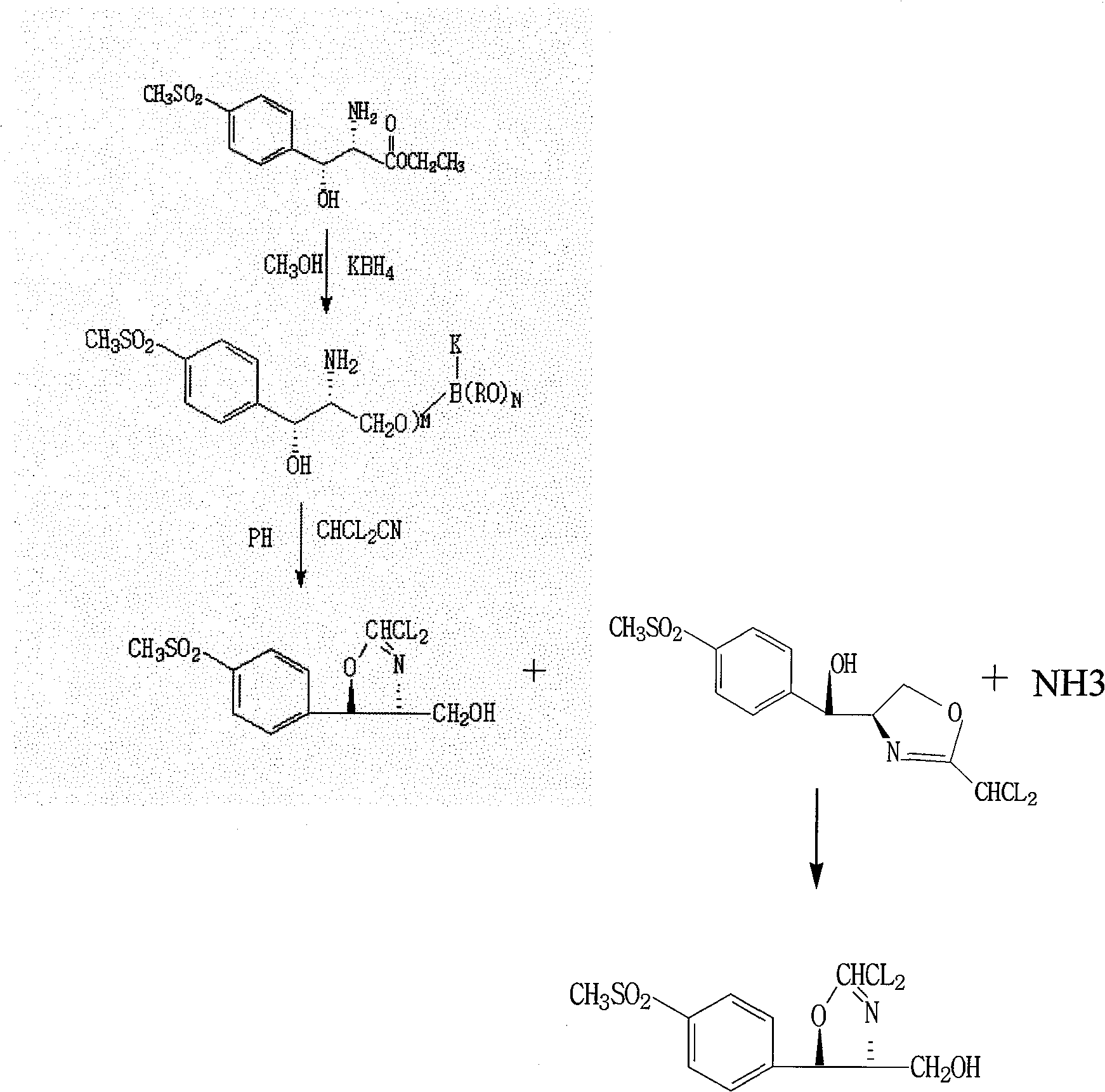
Preparation method of D-threo-2-(dichloromethyl)-4, 5-dihydro-5-(p-(methylsulfonyl) phenyl)-4-oxazole methanol
ActiveCN101550110AFew stepsEasy to operateOrganic chemistryPotassium borohydrideReaction temperature
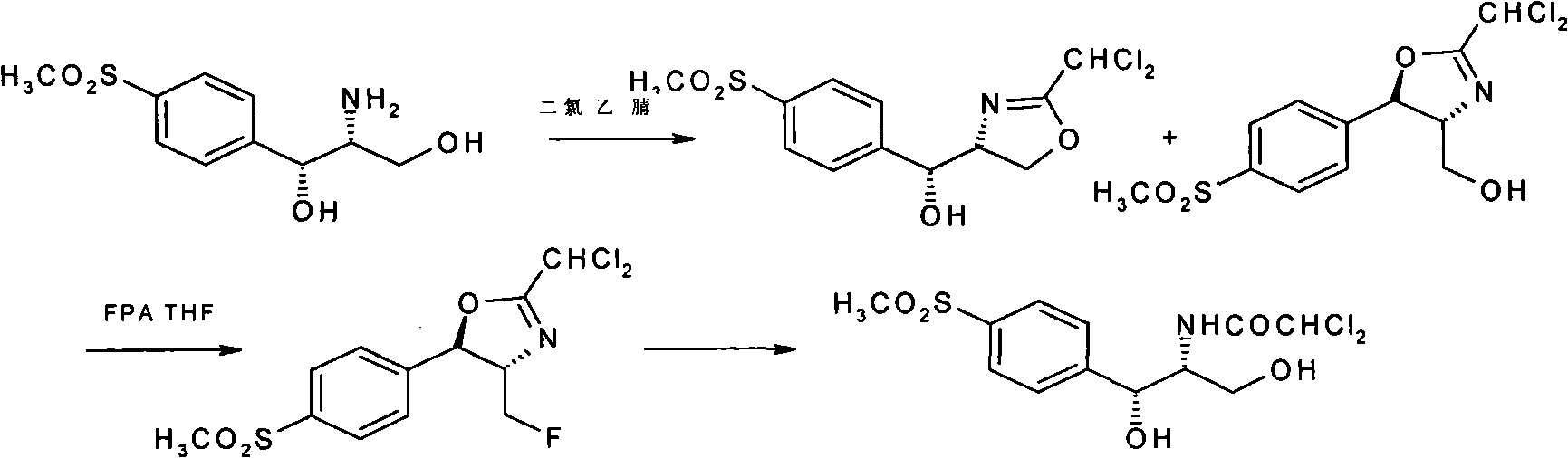
The invention relates to a preparation method of florfenicol intermediate, in particular to a preparation method of D-threo-2-(dichloromethyl)-4, 5-dihydro-5-(p-(methylsulfonyl) phenyl)-4-oxazole methanol, which comprises the detailed steps as follows: 100 portions of D-p-methylsulfino phenyl ethyl serinate (I) are dissolved in 500 to 700 portions of methanol; then 24 to 27 portions of potassium borohydride are added; the reaction temperature is between 30 and 50 DEG C and the reaction time is between 4 and 8 hours; 80 to 150 portions of methanol are recycled and 230 to 300 portions of glycerol are added; then acid is used for neutralizing the reaction solution to adjust the pH of the solution to be between 7 and 10; and 42 to 45 portions of dochloroacetonitril are added to generate the D-threo-2-(dichloromethyl)-4, 5-dihydro-5-(p-(methylsulfonyl) phenyl)-4-oxazole methanol (II). The preparation method has the characteristics of few technique steps, simple operation and low cost.
Owner:JIANGSU HANSYN PHARMA
In-situ gel formulation for florfenicol injection and preparation method thereof
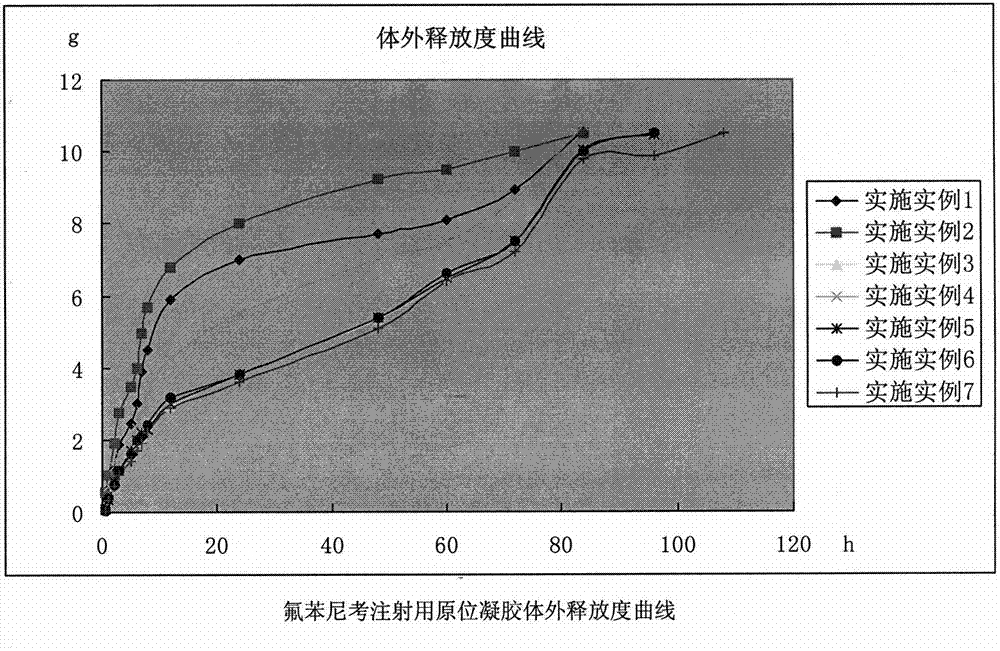
The invention belongs to the field of pharmaceutical formulations specially used for animals, and relates to an in-situ gel formulation for florfenicol injection and a preparation method thereof. The in-situ gel formulation is prepared from antibacterial drugs, an in-situ gel substratum and other indispensable auxiliary materials, wherein the antibacterial drugs are specially used for animals; the in-situ gel substratum is accredited in the field. The preparation method is illustrated.The in-situ gel formulation disclosed by the invention is freely flowing liquid at room temperature, forms the semisolid gel after intramuscular injection or hypodermic injection administration, realizes only one-time administration within one treatment course, has the advantages of easiness for preparation, stability in property, convenience for administration, long administration effect lasting time, definite treatment effect, high animal adaptability, no toxic or side effect or adverse reaction, and the like, achieves the broad-spectrum antibacterial effect and is suitable for preventing and treating various sensitive bacteria infection of the animals, such as pigs, sheep, dogs, cats, cattle, and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Compositions and method for treating infection in cattle and swine
Owner:INTERVET INC
Soluble florfenicol powder and preparation method thereof
ActiveCN104922073AImprove bioavailabilityEasy to useAntibacterial agentsOrganic active ingredientsSolubilityIrritation
The invention belongs to the fields of animal medicine and manufacturing thereof, and in particular relates to soluble florfenicol powder and a preparation method thereof. The invention provides soluble florfenicol powder which is prepared from the following components in percentage by weight: 1 to 10% of florfenicol, 1 to 15% of cosolvent, 1 to 5% of phagostimulant and the balance of filler. The soluble florfenicol powder disclosed by the invention is better in solubility in water and can realize drug therapy in a drinking water manner when sick animals do not eat feed; original unpleasant odor and irritation of florfenicol are covered; feed intake of medicine by the sick animals can be ensured effectively; a good treatment effect within a short period is realized, and treatment cost is reduced, so that the soluble florfenicol powder is popular among a majority of farmers.
Owner:GUANGDONG GALLOPER VETERINARY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com