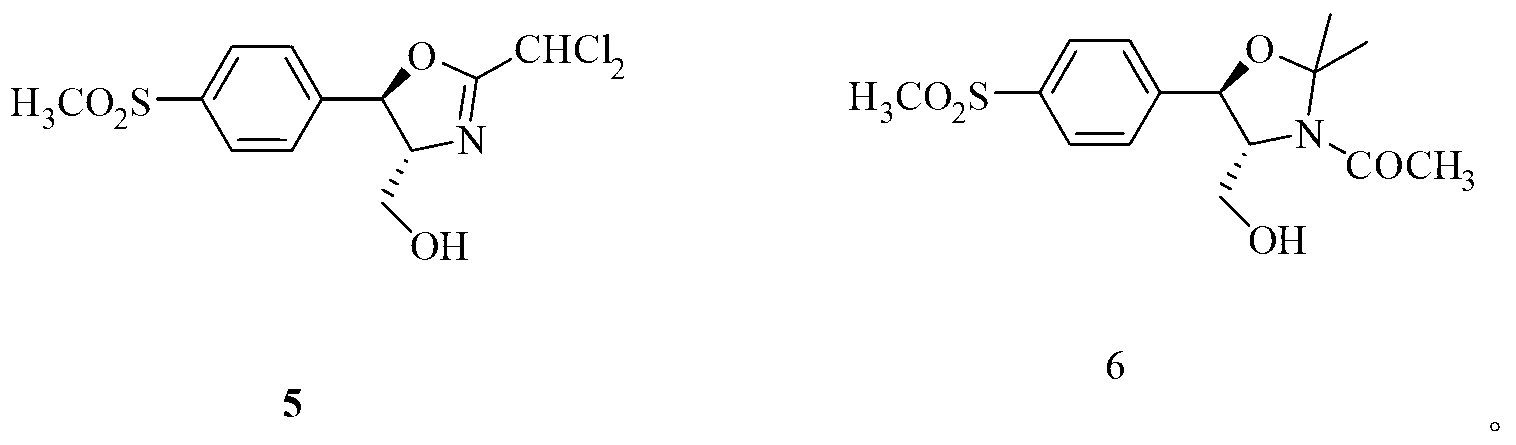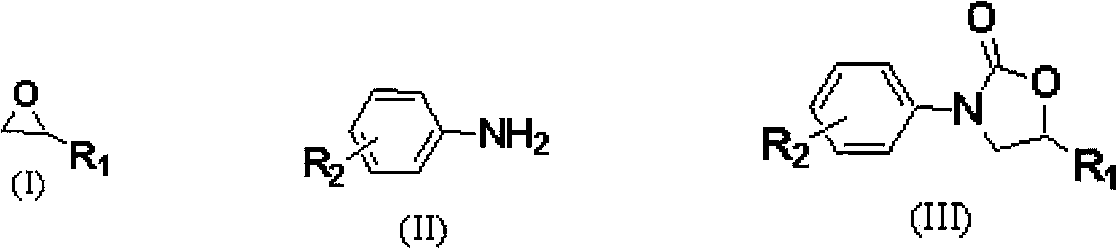Patents
Literature
157 results about "Oxazolidine derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
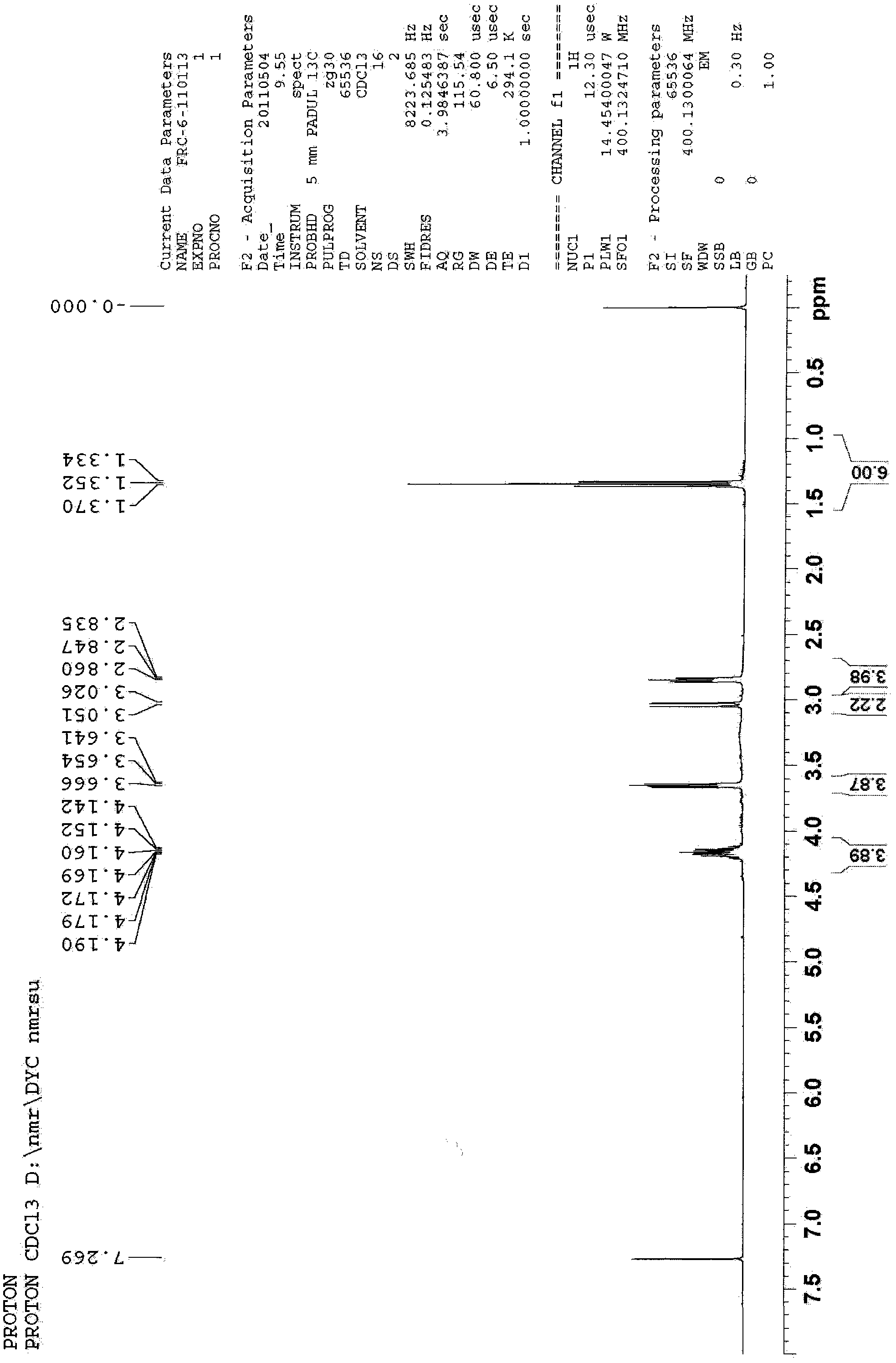
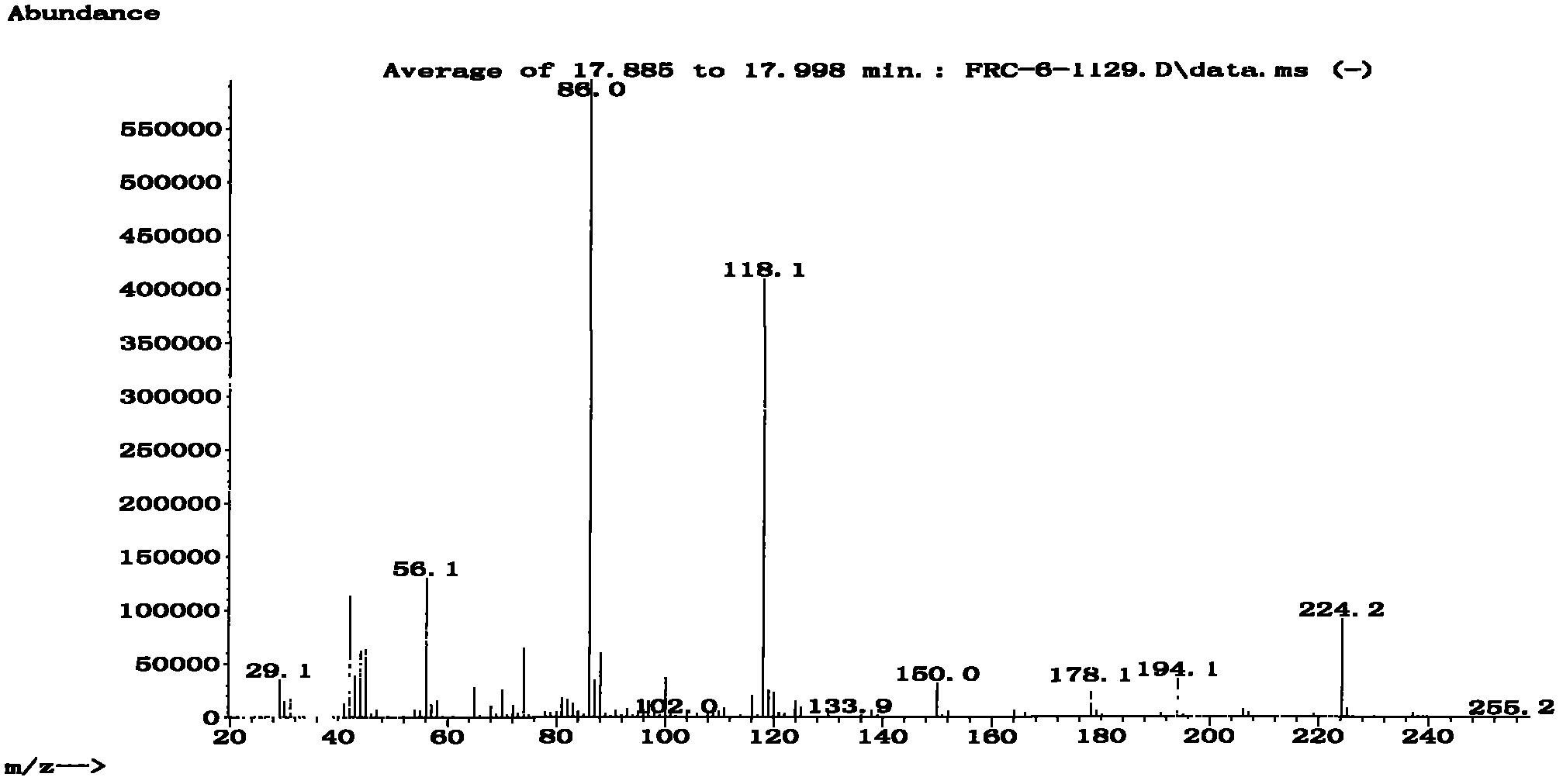
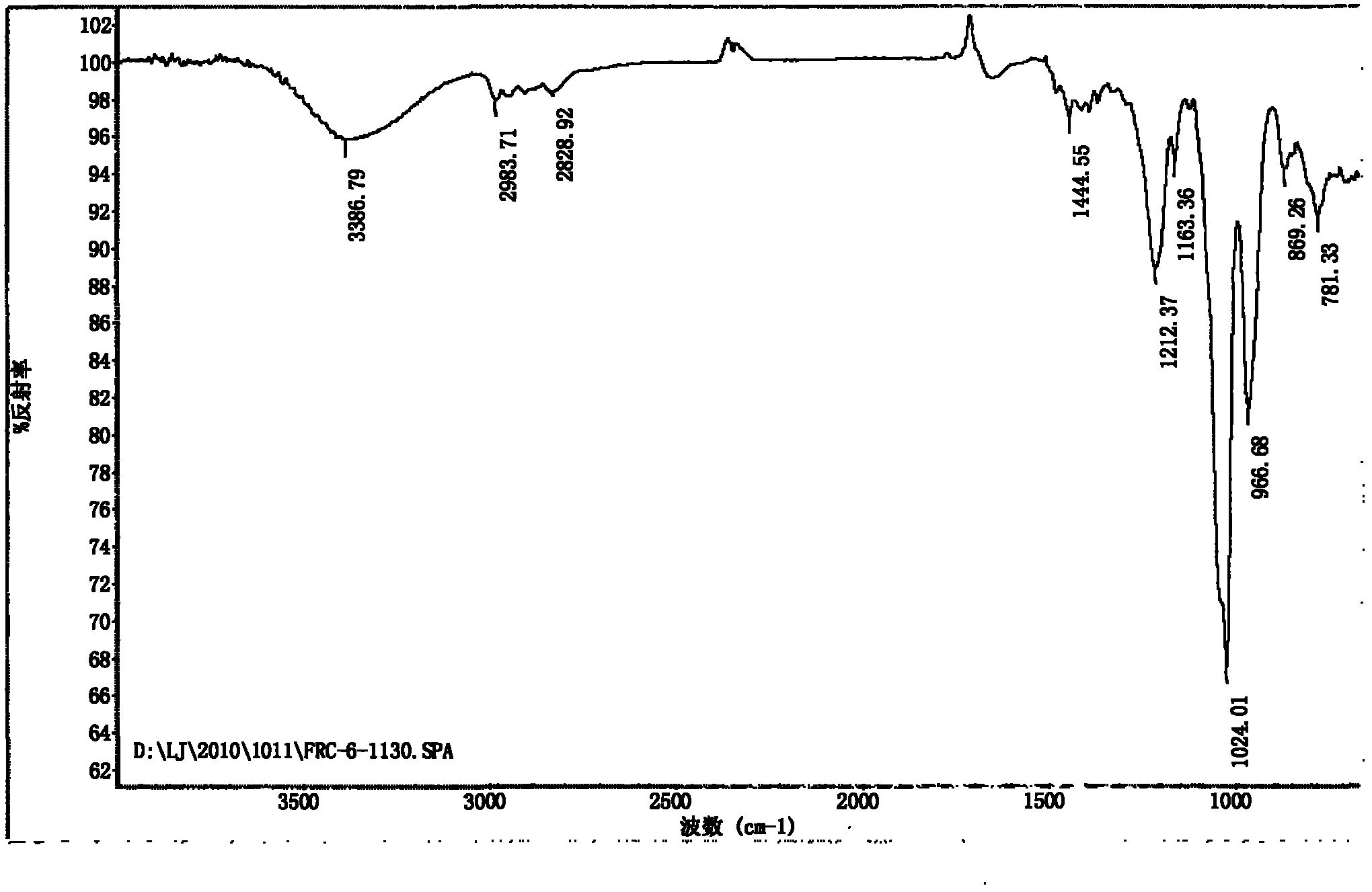
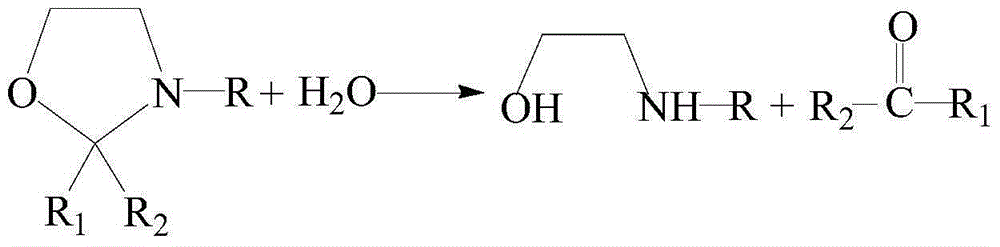
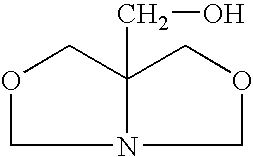
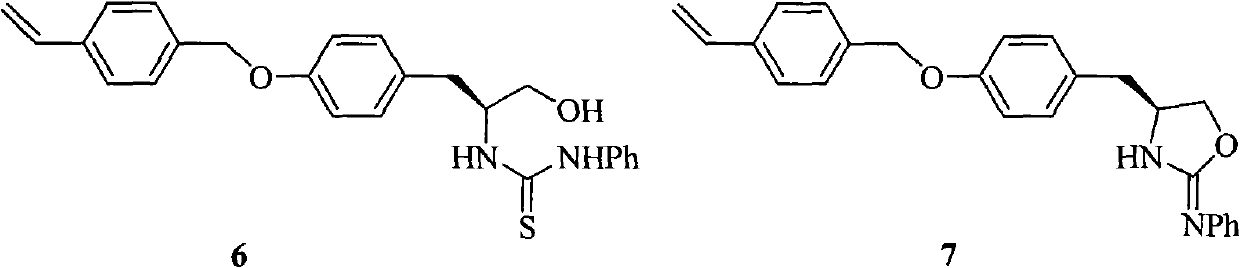
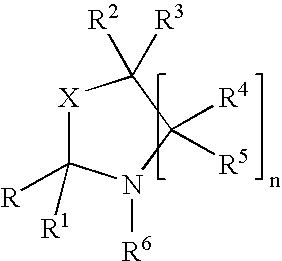
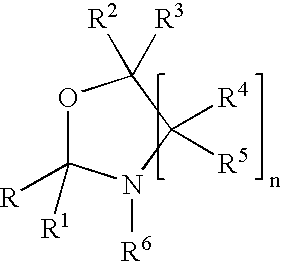
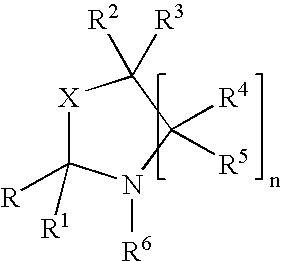
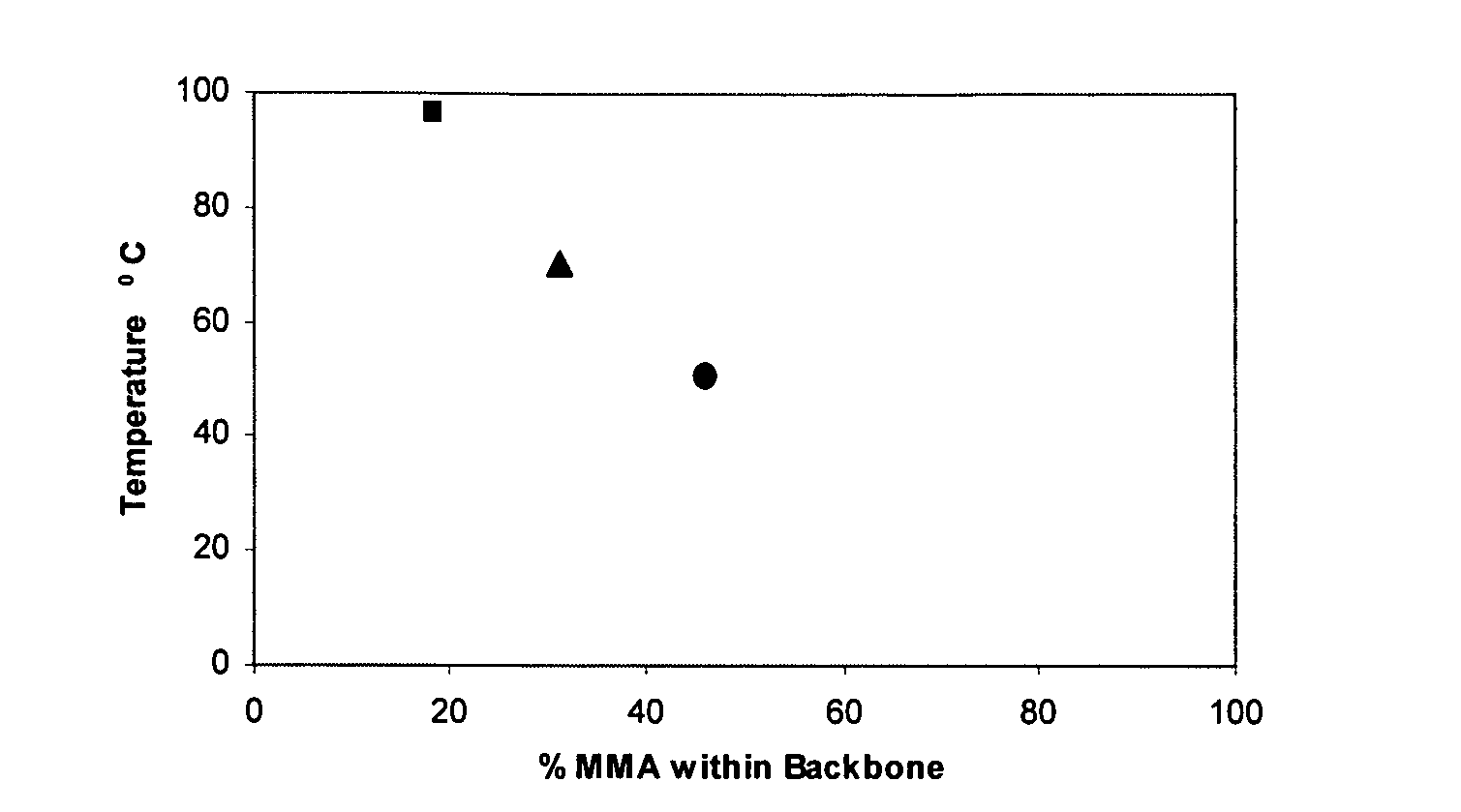
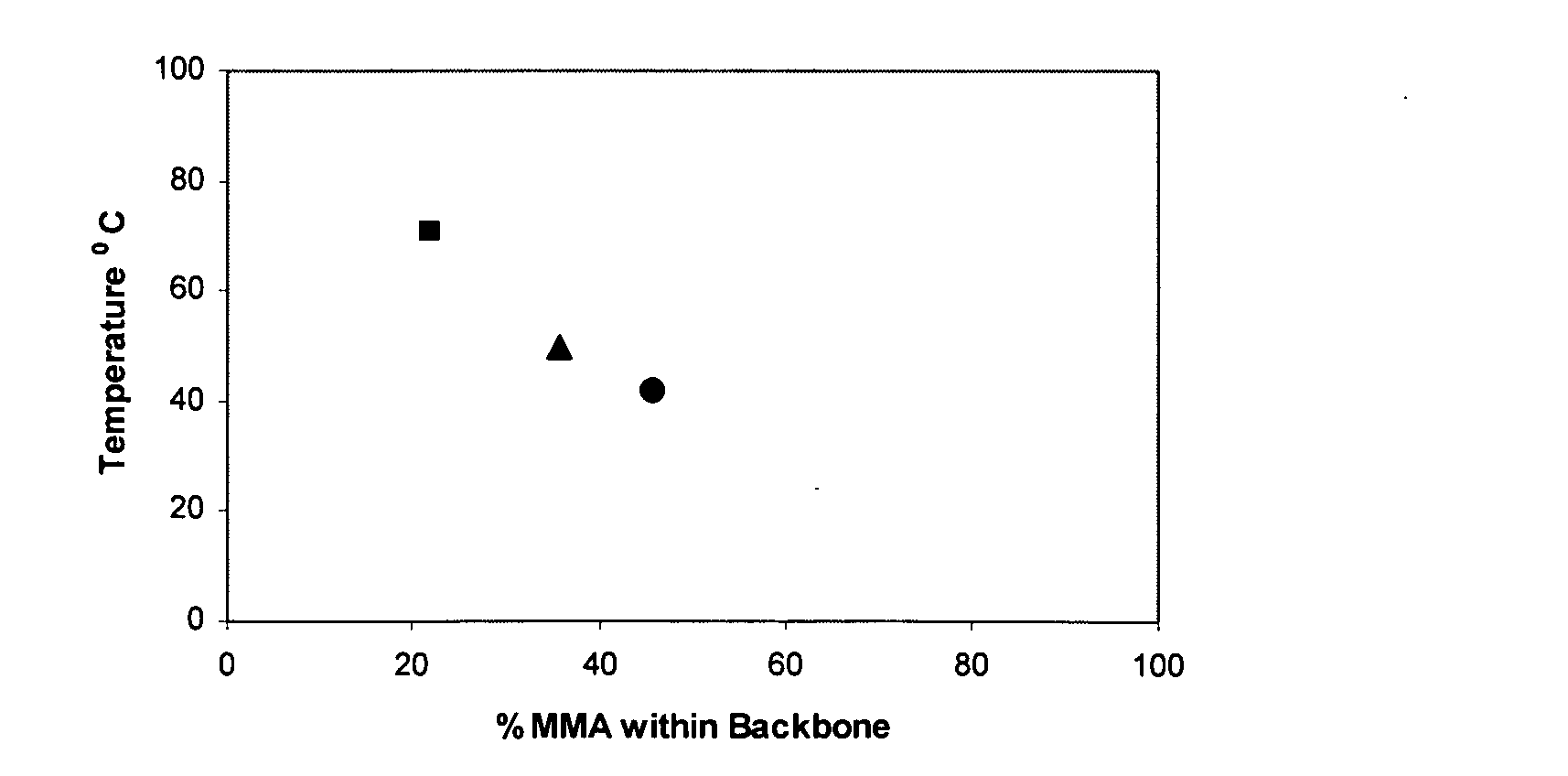
An oxazolidine is a five-membered ring compound consisting of three carbons, a nitrogen, and an oxygen. The oxygen and NH are the 1 and 3 positions, respectively. In oxazolidine derivatives, there is always a carbon between the oxygen and the nitrogen (or it would be an isoxazolidine).
Fragrance pro-accords and aldehyde and ketone fragrance libraries
InactiveUS7018978B2Extend your lifeEasy to prepareCosmetic preparationsOrganic chemistryFlavorOxazolidine E
The present invention relates to novel heterocyclic pro-fragrances, preferably oxazolidines, tertahydro-1,3-oxazines, thiazolidines, or tetrahydro-1,3-thiazines, more preferably oxazolidines, or tertahydro-1,3-oxazines, most preferably oxazolidines, which are capable of sustained release of fragrance raw material ketones and aldehydes and to fragrance delivery systems which comprise said pro-fragrances.
Owner:THE PROCTER & GAMBLE COMPANY
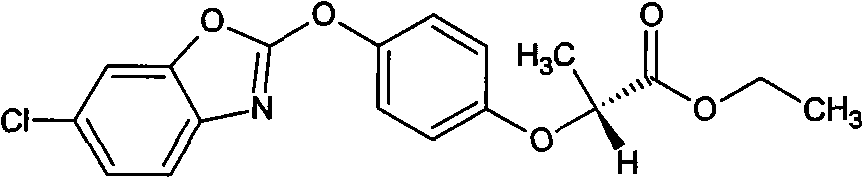
Process for preparing florfenicol
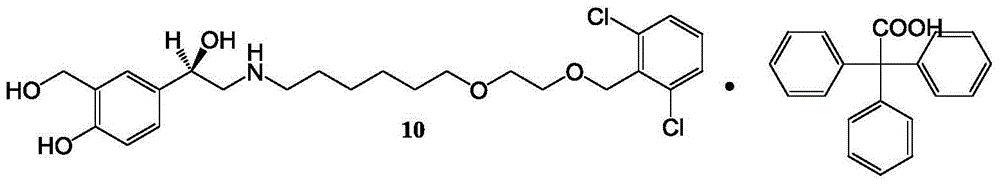
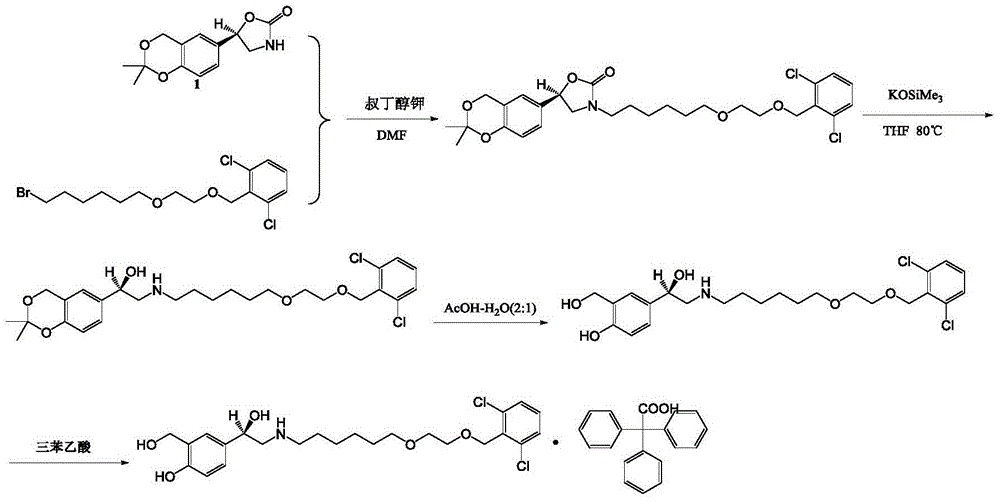
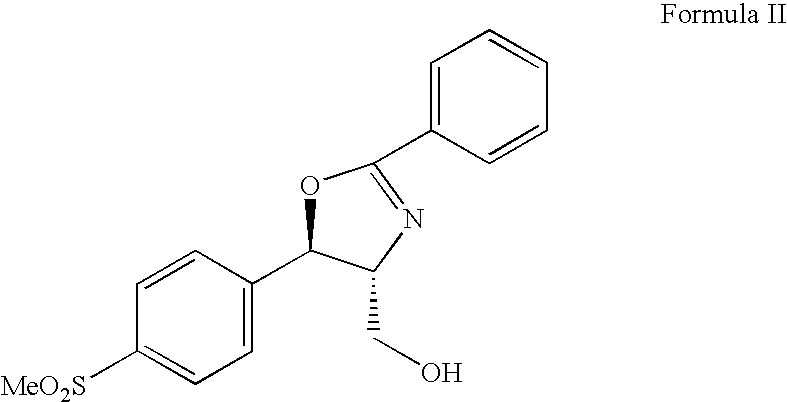
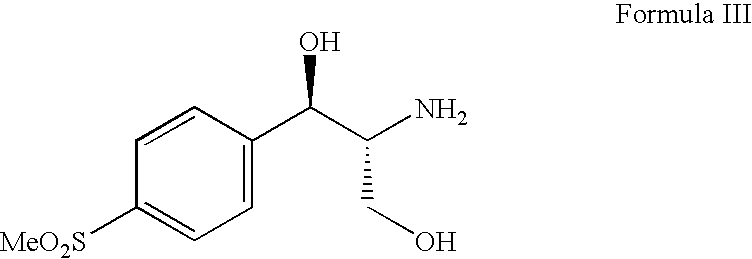
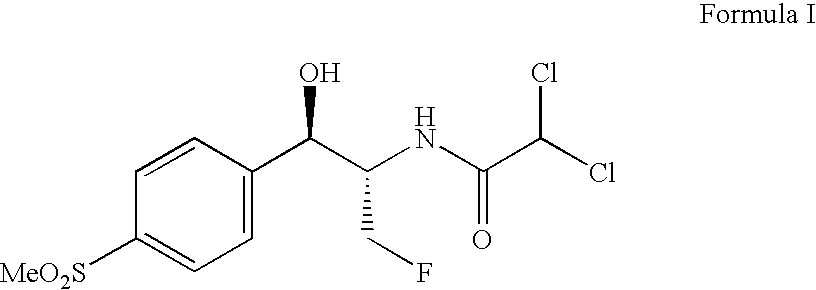
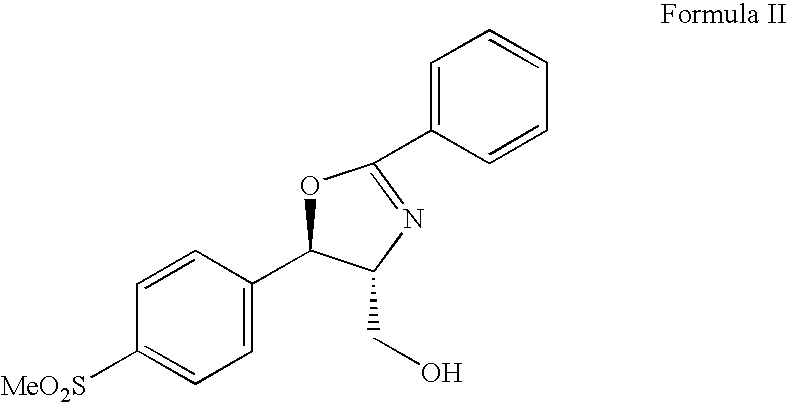
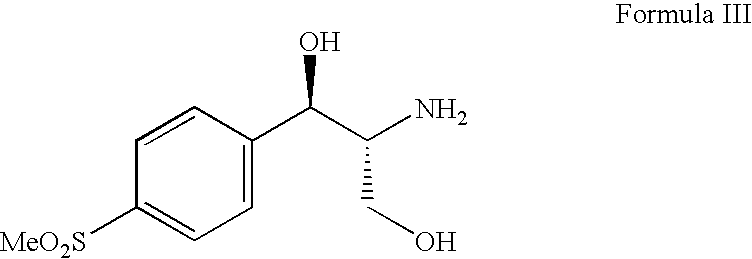
The present invention is directed to a new process of preparing highly pure Florfenicol. The invention is further directed to new oxazolidine derivatives useful in making Florfenicol and processes of making these derivatives. Examples of such intermediates include (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine; and (4S,5R)-3-acetyl-2,2-dimethyl-4-fluoromethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine.
Owner:AUROBINDO PHARMA LTD
Composition and use
The present invention relates to a composition comprising: (i) an anti-microbial agent comprising a polymeric biguanide, alone or in combination with at least one other microbiologically active component selected from the group consisting of quaternary ammonium compounds, monoquaternary heterocyclic amine salts, urea derivatives, amino compounds, imidazole derivatives, nitrile compounds, tin compounds or complexes, isothiazolin-3-ones, thiazole derivatives, nitro compounds, iodine compounds, aldehyde release agents, thiones, triazine derivatives, oxazolidine and derivatives thereof, furan and derivatives thereof, carboxylic acids and the salts and esters thereof, phenol and derivatives thereof, sulphone derivatives, imides, thioamides, 2-mercapto-pyridine-N-oxide, azole fungicides, strobilurins, amides, carbamates, pyridine derivatives, compounds with active halogen groups, and organometallic compounds; and (ii) an amphoteric co-polymer of the Formula (1): wherein:[A] is of Formula (9), [B] is of Formula (10), [C] is of Formula (12), [D] is of Formula (13), and X is of Formula (11), wherein [A], [B], [C] and [D] may occur in any order; T is an optionally substituted substituent; L, G and Z each independently is an optionally substituted linking group; R1, R2 and R3 are each independently H, optionally substituted C1-20-alkyl or optionally substituted C3-20-cycloalkyl; R4 and R5 are each independently H or C1-4-alkyl; q is 15 to 1000; p is 3 to 50; J is an optionally substituted hydrocarbyl group; F is an acidic substituent; E is a basic substituent; m is 0 to 350; n is 1 to 75; v is 0 to 100; y is 1 to 100; b is 0, 1 or 2; s is 0 or 1; w is 1 to 4; and provided that at least one of R4 and R5 is H.
Owner:ARCH UK BIOCIDES LTD
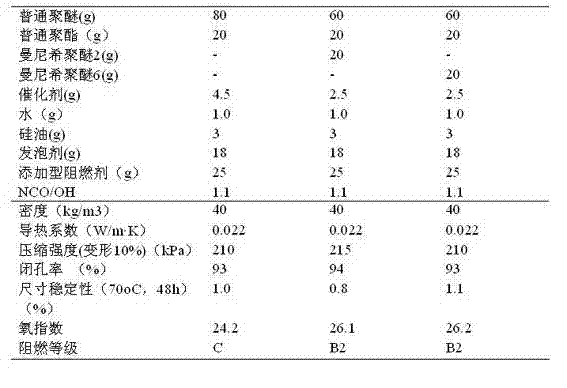
Preparation method and applications of reaction type Mannich polyether polyol with permanent flame retardant effect
The invention provides a preparation method of reaction type Mannich polyether polyol with permanent flame retardant effect. The preparation method of reaction type Mannich polyether polyol with permanent flame retardant effect comprises the following steps: reacting paraformaldehyde or formaldehyde solution with diethanol amine to obtain 3-ethoxyl-1,3-oxazolidine reaction liquid, and then carrying out reduced pressure distillation to obtain the product 3-ethoxyl-1,3-oxazolidine; reacting aromatic phenol compounds or / and melamine compounds with 3-ethoxyl-1,3-oxazolidine to obtain Mannich base as intermediate; and carrying out polymerization reaction between the intermediate Mannich base and epoxy compound to obtain crude product of reaction type Mannich polyether polyol with permanent flame retardant effect, then carrying out reduced pressure distillation to remove impurities with low boiling points, and thus obtaining finished product. The reaction type Mannich polyether polyol simultaneously has the high stability and thermal stability of rigid polyurethane foam and the flame resistance of phenolic foam, is applied to foaming rigid polyurethane foam, and has wide application prospect.
Owner:JIANGSU YOKE TECH
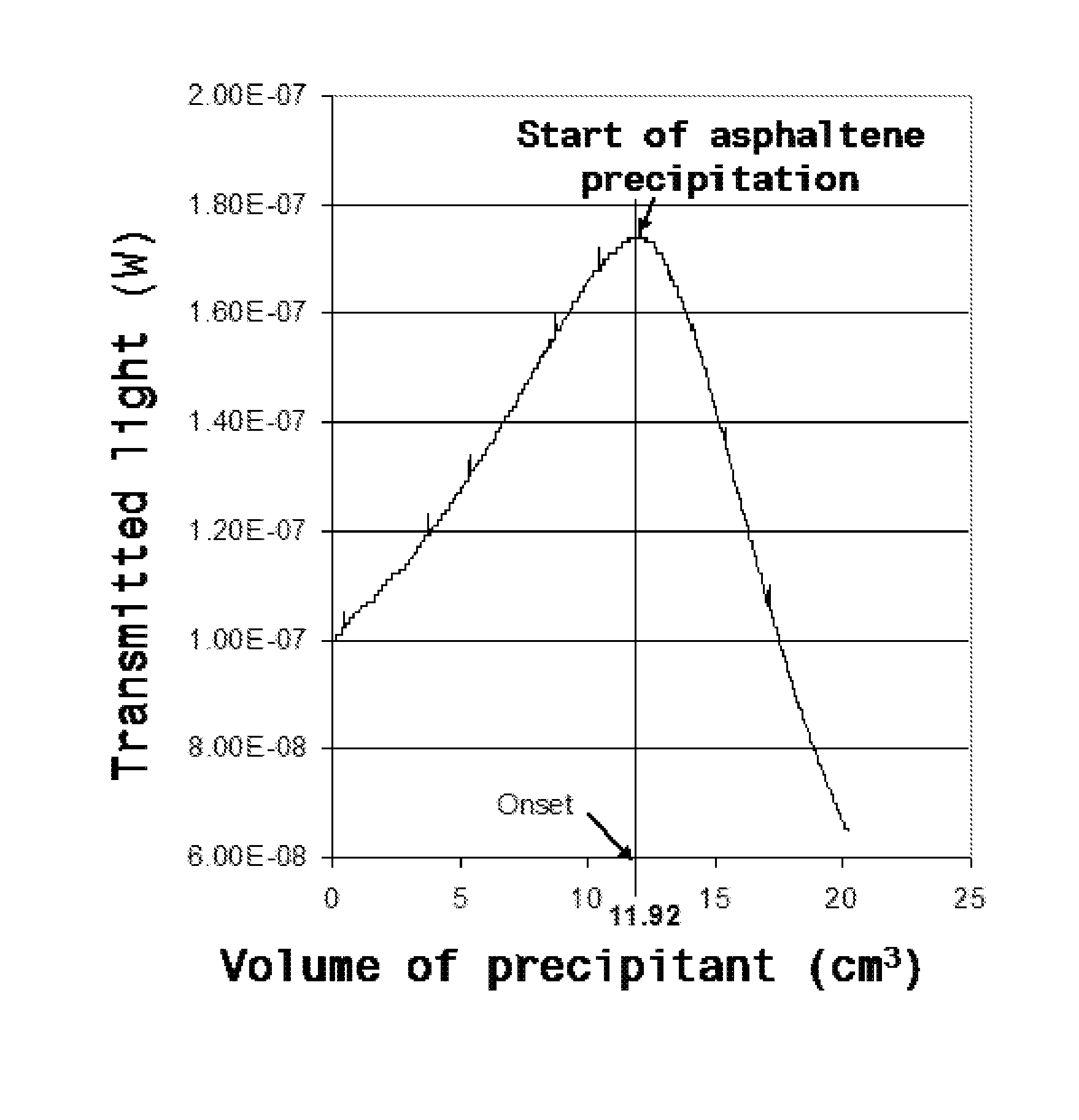
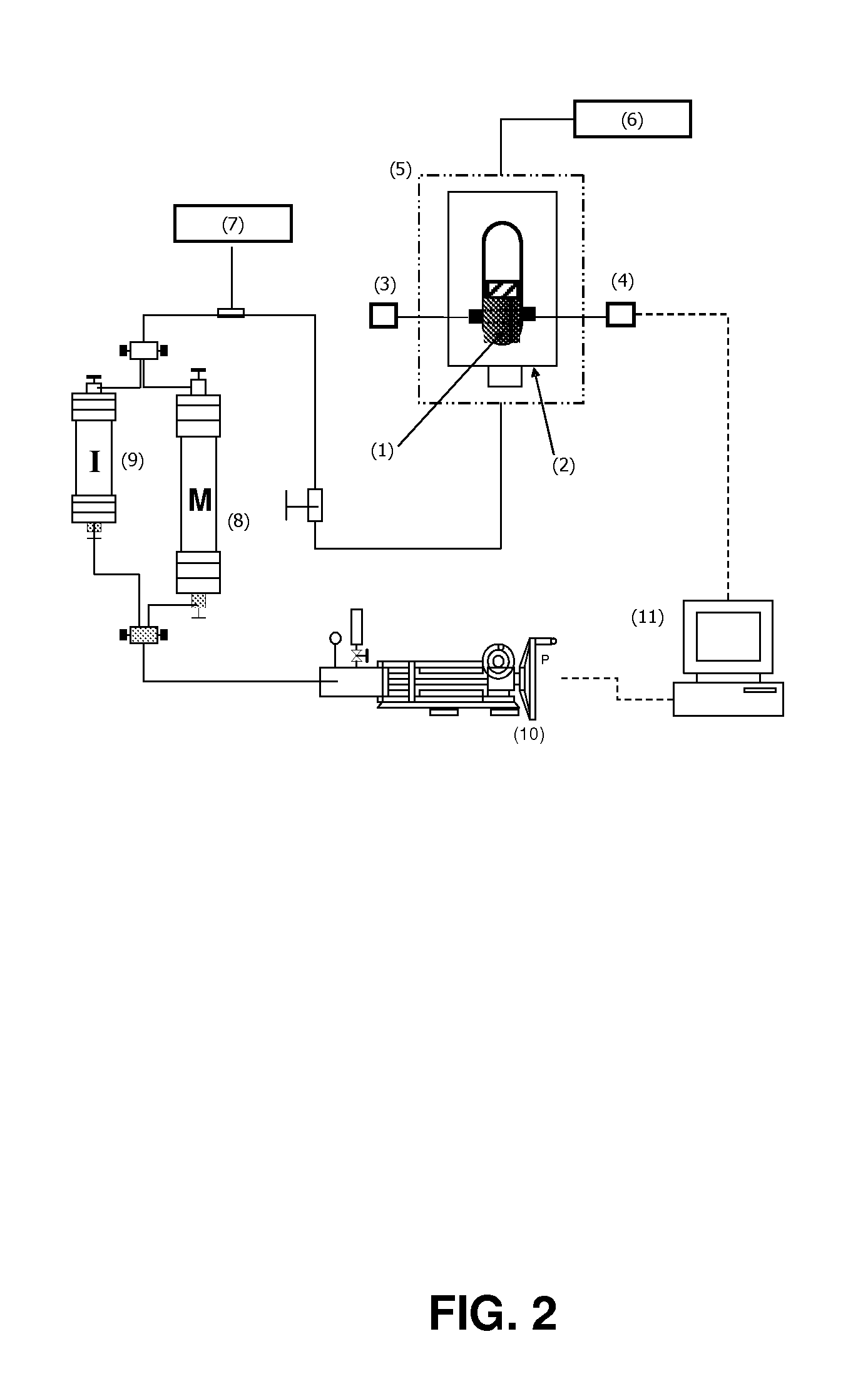
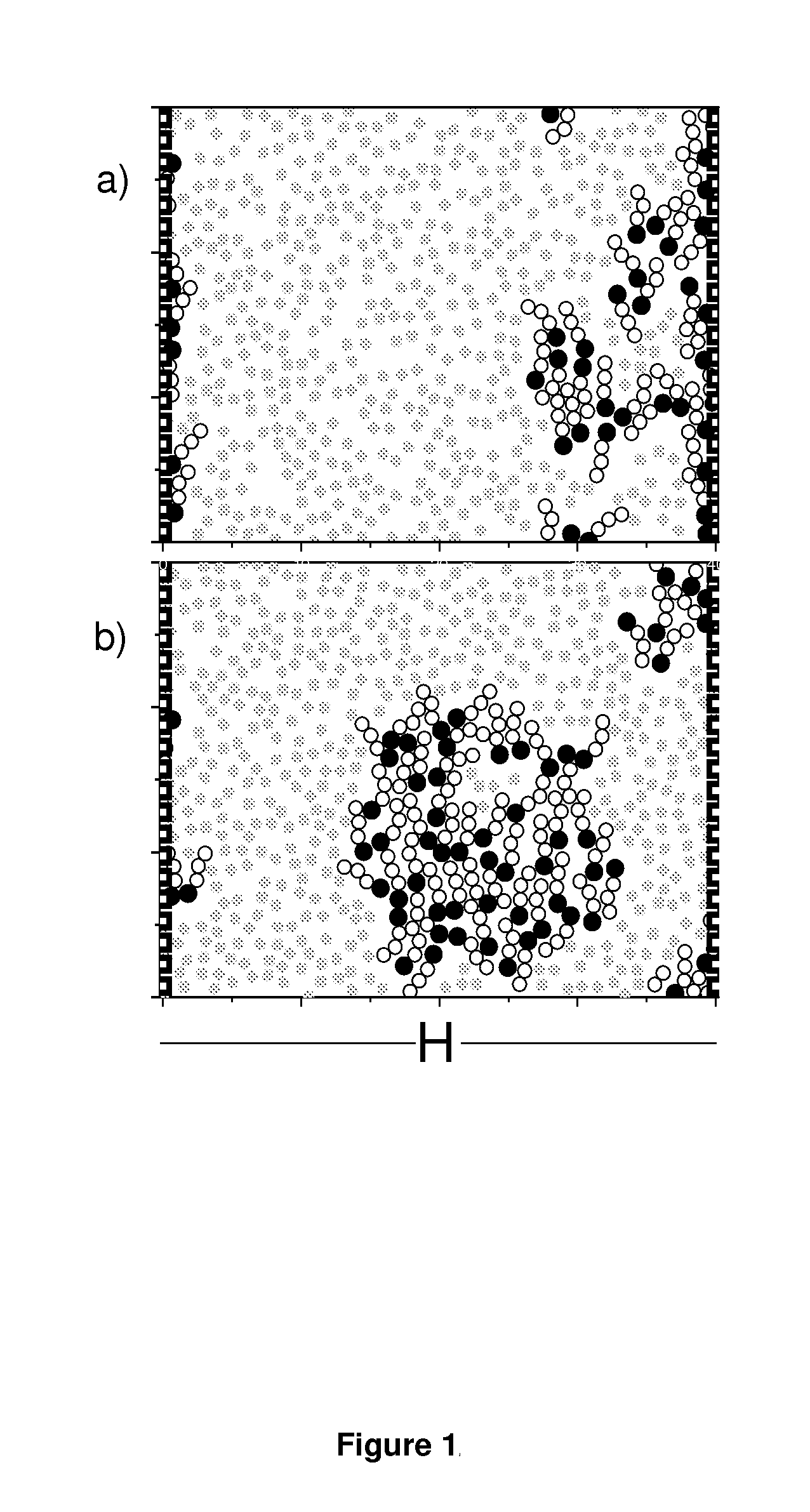
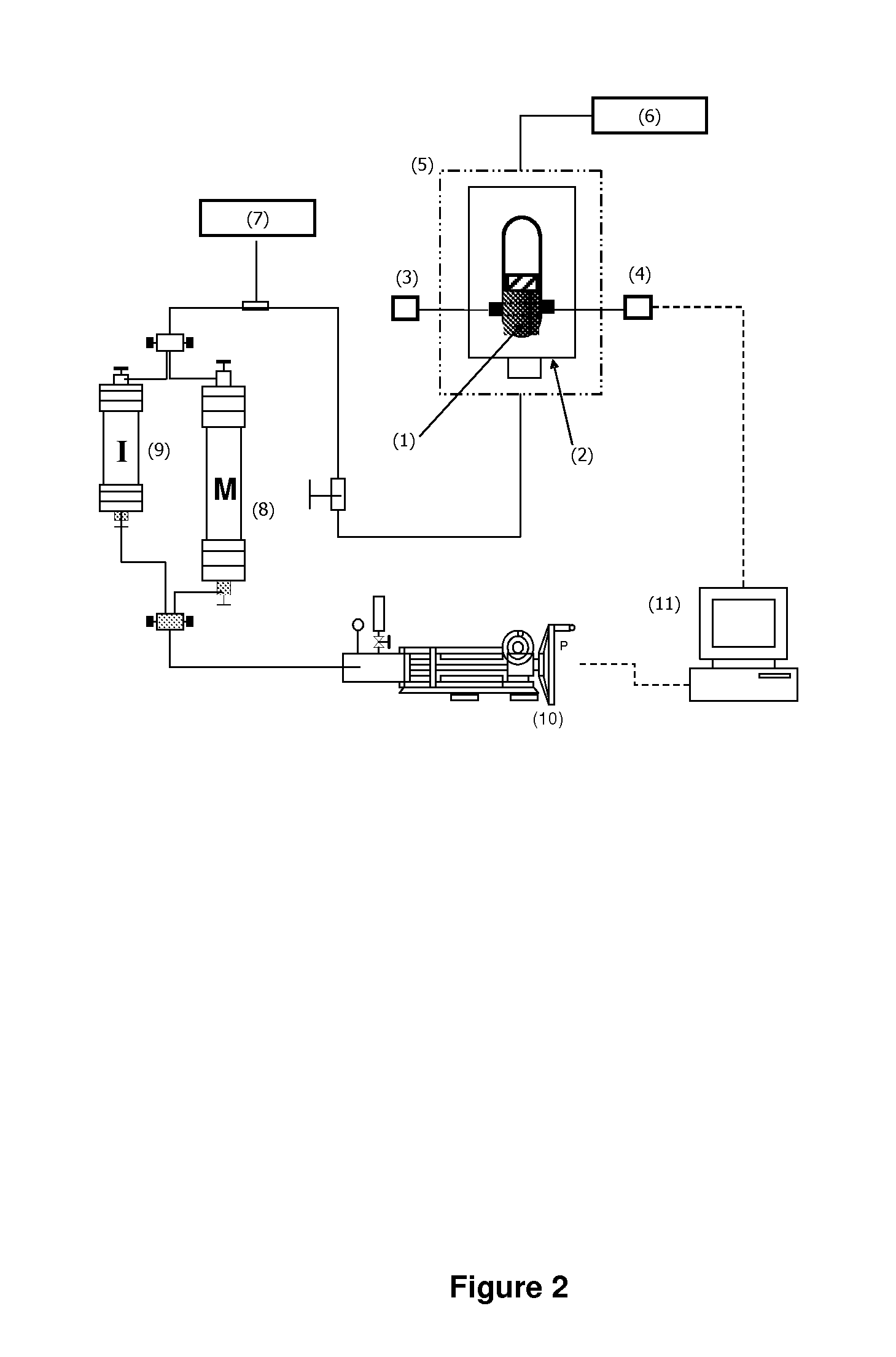
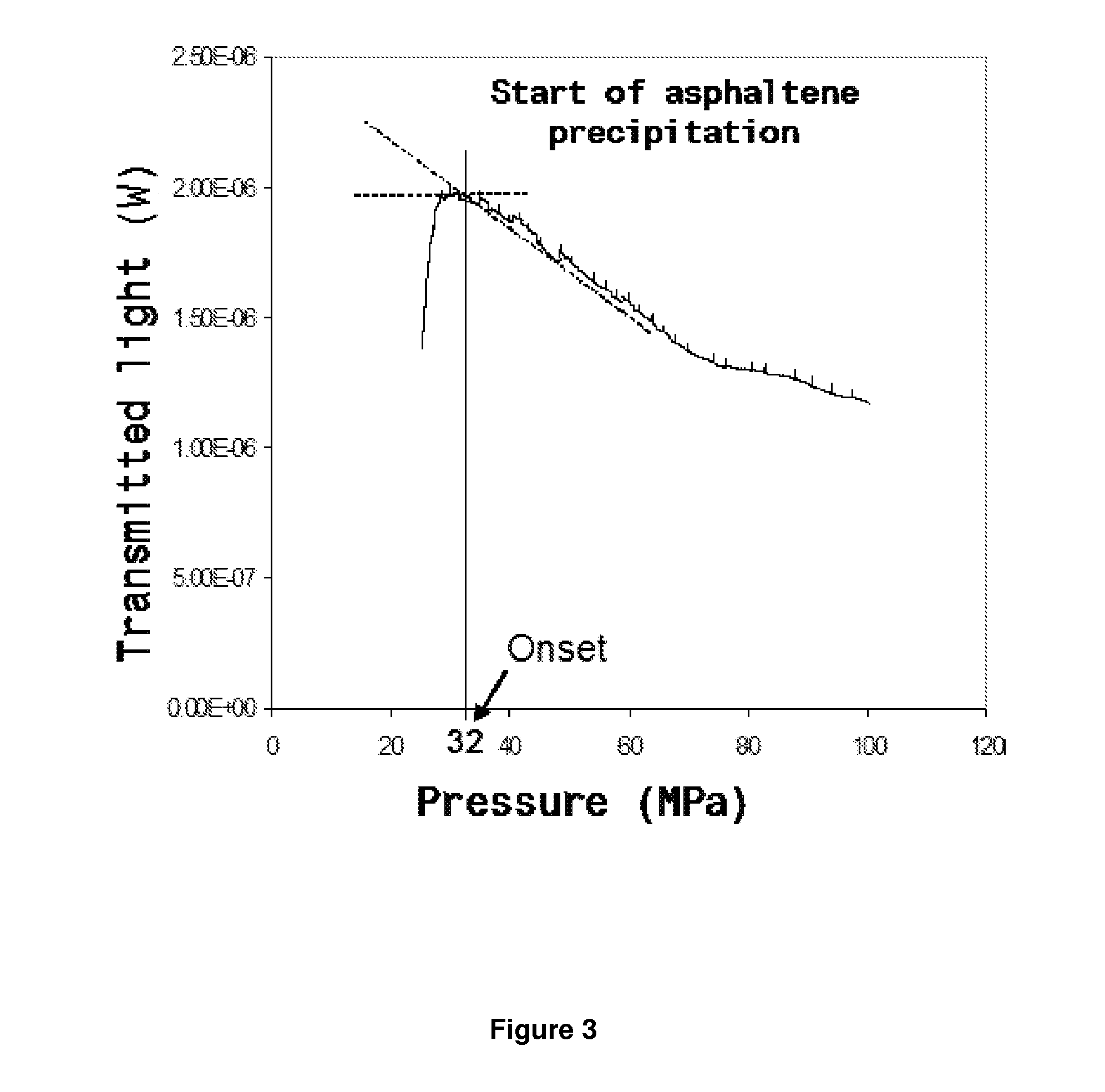
Formulations comprising an asphaltene-dispersing/inhibiting additive based on oxazolidines derived from polyalkyl or polyalkenyl N-hydroxyalkyl succinimides
The present invention relates to formulations of asphaltenes' inhibitor-dispersant additives based on oxazolidine derived from polyalkyl or polyalkenyl N-hydroxyalkyl succinimides. Said formulations can contain inert organic solvents, preferably including: toluene, mixtures of xylene, o-xylene, p-xylene, kerosene, turbo-fuel; or inert hydrocarbon solvents having boiling points within the range of gasoline and diesel; or inert hydrocarbon or organic solvents having a boiling point within a range from 75 to 300° C. The ratio in weight of inert organic solvents to additive that prevents and controls the precipitation and deposition of asphaltenes ranges from 1:9 to 9:1, preferably from 1:3 to 3:1.
Owner:INST MEXICANO DEL GASOLINEEO
Use of derivatives of methylene-bis-oxazolidine and compositions obtained thereby
InactiveUS6348483B1Improve solubilityEmission reductionBiocideDead animal preservationSolubilityOxazolidine
The present invention relates to stable microbicidal compositions which are characterized in that they comprise derivatives of methylenebisoxazolidine and 1H-benzimidazol-2-ylcarbamic acid. The compositions according to the invention can be employed in industrial products. The derivatives of methylenebisoxazolidine are used, in particular, for increasing the solubility of derivatives of 1H-benzimidazol-2-ylcarbamic acid in liquid preparations.
Owner:AIR LIQUIDE SANTE
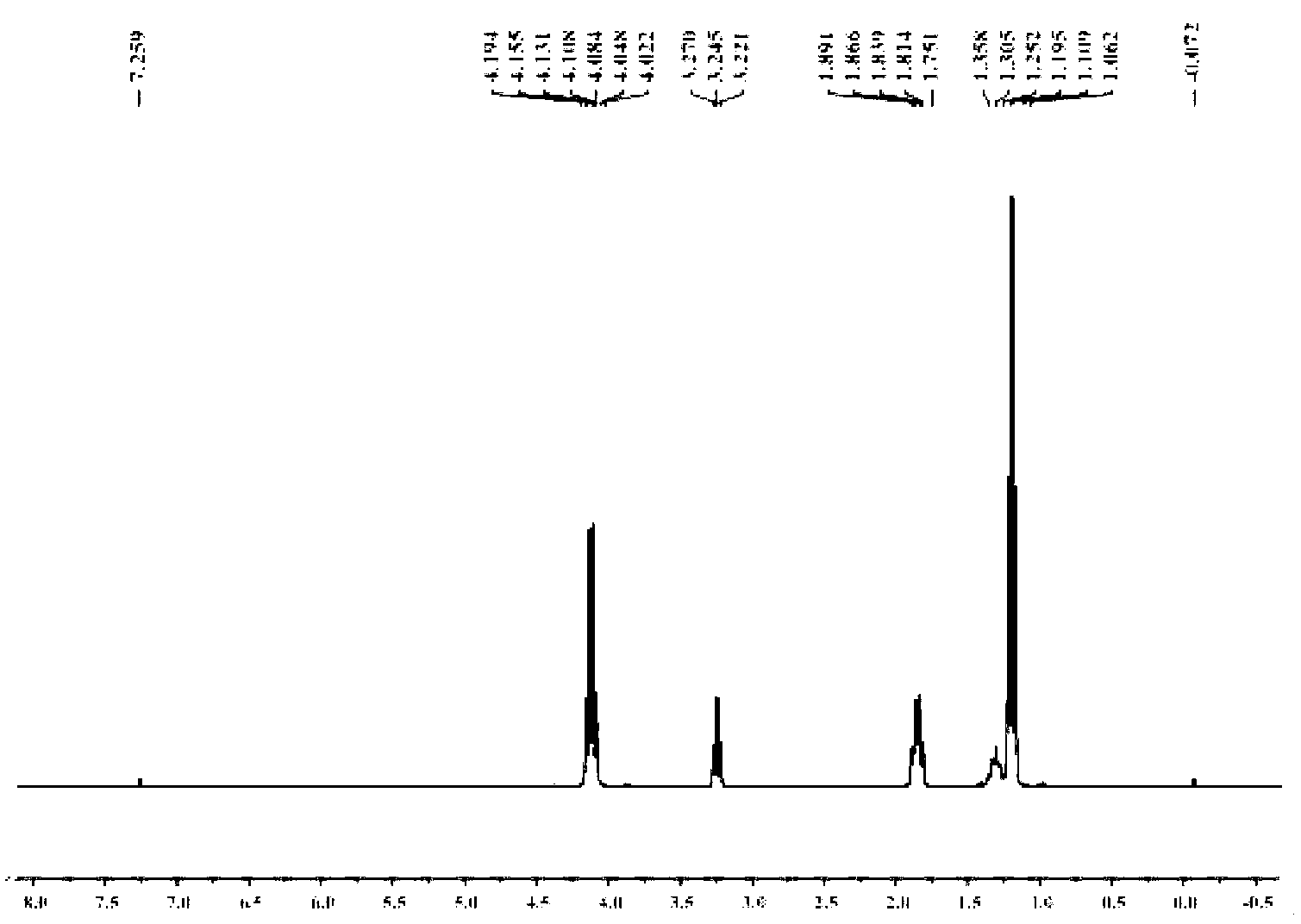
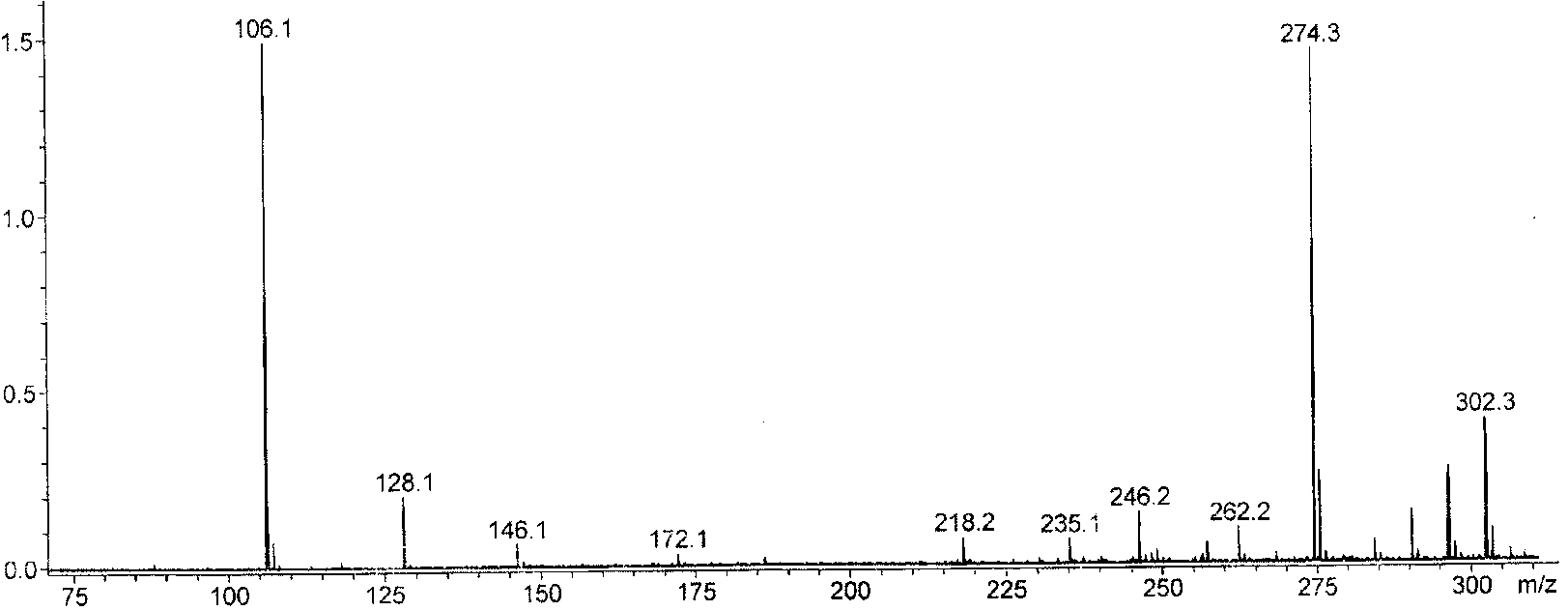
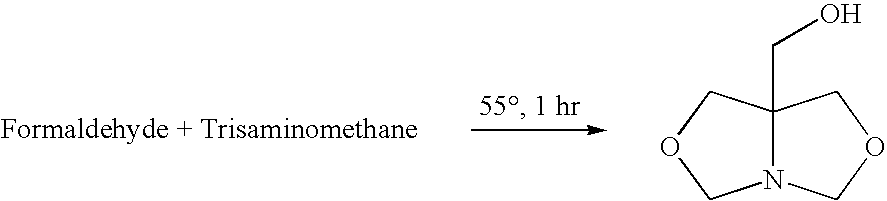
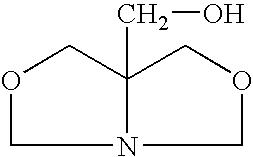
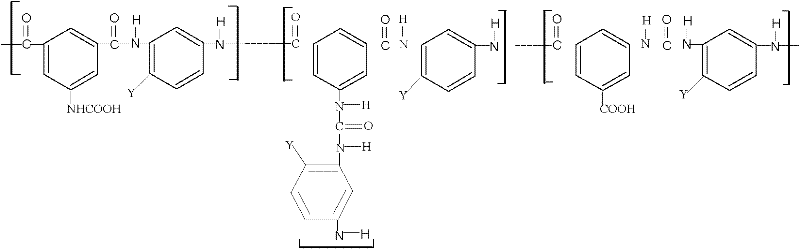
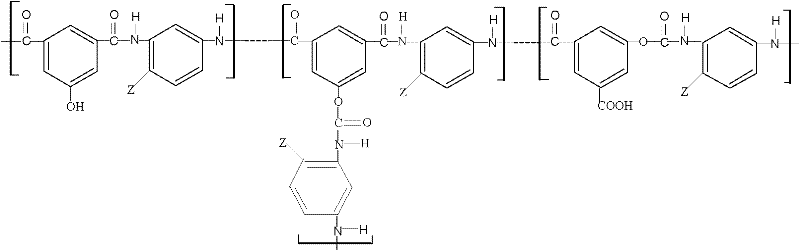
1-Aza-3,7-Dioxabicyclo[3,3,0]Octane Compounds and Monocyclic Oxazolidines as Pro-Fragrances
InactiveUS20090312231A1Low vapor pressureIncrease depositionCosmetic preparationsSilicon organic compoundsSilicic acidOxazolidine
Silicic acid esters to which fragrances are bound, preferably as 1-aza-3,7-dioxabicyclo[3,3,0]octane compounds or as monocyclic oxazolidines and that are suitable for adding fragrance to detergents and cleaning agents because they release the bound fragrances in hydrolysis.
Owner:HENKEL KGAA
A kind of preparation method of n,n-bis(2-hydroxyethyl)aminomethylphosphonic acid diethyl ester
ActiveCN102276645AReduce pollutionReduce manufacturing costGroup 5/15 element organic compoundsLiquid productDepolymerization
The invention provides a preparation method of diethyl N,N-bis(2- hydroxyethyl) aminomethylphosphonate, comprising the following steps: (1) carrying out depolymerization on paraformaldehyde; (2) carrying out ring formation on formaldehyde and diethanol amine to prepare 3-(2- hydroxyethyl)-1,3-oxazolidine; (3) reacting 3-(2- hydroxyethyl)-1,3-oxazolidine with diethyl phosphite in the presence of anhydrous Lewis acid catalysts; and (4) filtering and recovering the catalysts to obtain a yellow transparent liquid product. The overall yield of the whole process reaches more than 99 %, and the content of diethyl N,N-bis(2- hydroxyethyl) aminomethylphosphonate in the product is 94 %-96 %. The preparation method disclosed in the invention is simple and efficient, has the advantages of high product yield, high content of effective components in the product, reduction of temperature in dehydration process, shortening of time, and low energy consumption, reduces the water content of the final product to 0.1-0.2 %, and reduces the dosage of the catalysts by 20-50%. The preparation method is suitable for large-scale industrial production.
Owner:WANHUA CHEM NINGBO RONGWEI POLYURETHANE
Chrome free or less chrome main tanning agent for leather and preparation process thereof
InactiveCN1465718ALow priceRich varietyAntifouling/underwater paintsTanning treatmentReduced doseTannin
The leather tanning agent having no chrome or having little chrome is made up by using amine material containing carboxymethyl group and aliphatic aldehyde compound through the processes of N-carboxymethylation reaction and ring-closing reaction. In addition to oxazolidine naphthenicbase its molecular structure also contains several functional groups of aldehyde group, carboxyl group, hydroxyl group and quaternary amino-group. Said main tanning agent can be combined with vegetable tannin extract, also can be combined with chrome for tanning leather, and can raise wet and thermal stabilizing of the finished leather and reduce dose of chrome by 30-40%.
Owner:SICHUAN UNIV
Novel oxazolidine, preparation method, application, and mono-component polyurethane waterproof paint
InactiveCN105061347AImprove stabilityImprove film qualityOrganic chemistryPolyurea/polyurethane coatingsOxazolidinePrepolymer
The invention discloses novel oxazolidine, a preparation method, application, and mono-component polyurethane waterproof paint. The novel oxazolidine is named as 4-mehtyl-3-(2-phenylcarbinol)-1,3-oxazolidine. The novel oxazolidine is used as a latent curing agent of the mono-component polyurethane waterproof paint. During the production process of the waterproof paint, the novel oxazolidine is added into the raw materials, one naked hydroxy group of the novel oxazolidine will react with the polyurethane prepolymer formed by the raw materials, thus the novel oxazolidine is grafted to the polyurethane prepolymer, and the novel oxazolidine (latent curing agent) can be more evenly dispersed in the waterproof paint. If the waterproof paint is stored for a long time, the grafted novel oxazolidine will be dissolved out continuously, the stability of the waterproof paint is improved, the latent curing effect is improved, and avoided is the phenomenon that the latent curing agent is precipitated from the mono-component polyurethane waterproof paint or the latent curing agent aggregates in the waterproof paint. After the waterproof paint is used, the quality of the film formed by the waterproof paint is improved.
Owner:BEIJING ORIENTAL YUHONG WATERPROOF TECH CO LTD
Spraying polyurea elastic coating and preparing method thereof
ActiveCN105255333ASolve the problem of blisteringGuaranteed storage stabilityPolyurea/polyurethane coatingsPolyolOxazolidine
The invention relates to a coating, in particular to a spraying polyurea elastic coating. The spraying polyurea elastic coating is composed of a component A and a component B. The component A is prepared from, by mass, 55% to 65% of polyisocyanate and 35% to 45% of polyether polyol. The component B is prepared from, by mass, 45% to 55% of amine-terminated polyether, 34% to 45% of amine chain extenders, 5% to 10% of polyfunctionality oxazolidine, 0.5% to 1% of defoaming agents and 0.5% to 1% of dispersing agents. According to the spraying polyurea elastic coating, as the polyfunctionality oxazolidine is added into the dewatered amine-terminated polyether, hydroxyl terminated polyether and the amine chain extenders and can be firstly reacted with a small amount of moisture generated in the use process, the transport process and the use process to generate amino-and-hydroxyl-containing open-loop compounds, then the compounds are reacted with isocyanate groups of polyurea curing agents to be cured, carbon dioxide gas is not generated in the whole curing process, and the defect that a paint film is bubbly is well overcome.
Owner:FOSHAN KESHUN BUILDING MATERIAL CO LTD
Preparation of chemical compounds
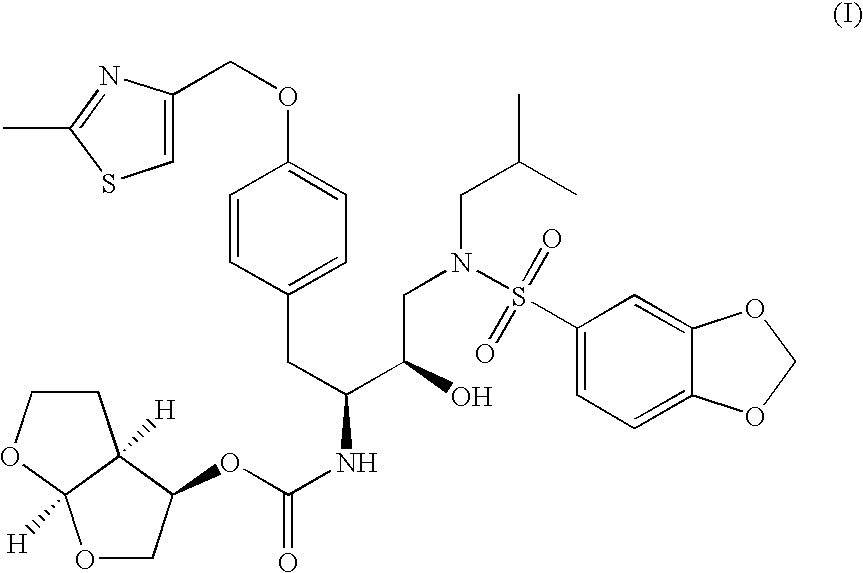
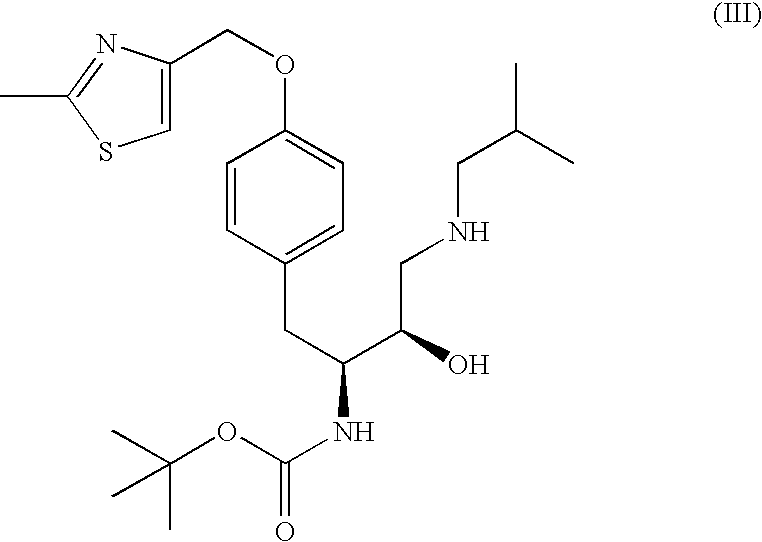
The present invention is directed to processes for the preparation of N-(3R, 3aS, 6aR)-hexahydrofuro[2,3-b]furan-3-yl-oxycarbonyl-, (4S,5R)-4-[4-(2-methylthiazolo-4-methyloxy)-benzyl]-5-i-butyl-[(3,4-methylenedioxyphenyl)sulfonyl]-aminomethyl-2,2-dimethyl-oxazolidine.
Owner:MARTIN MICHAEL TOLAR +2
Non-crystallizing bis-oxazolidines
Owner:SIKA TECH AG
Asphaltene-dispersing/inhibiting additive based on oxazolidines derived from polyalkyl or polyalkenyl N-hydroxyalkyl succinimides
The present invention relates to formulations of asphaltenes' inhibitor-dispersant additives based on oxazolidine derived from polyalkyl or polyalkenyl N-hydroxyalkyl succinimides. Said formulations can contain inert organic solvents, preferably including: toluene, mixtures of xylene, o-xylene, p-xylene, kerosene, turbo-fuel; or inert hydrocarbon solvents having boiling points within the range of gasoline and diesel; or inert hydrocarbon or organic solvents having a boiling point within a range from 75 to 300° C. The ratio in weight of inert organic solvents to additive that prevents and controls the precipitation and deposition of asphaltenes ranges from 1:9 to 9:1, preferably from 1:3 to 3:1.
Owner:INST MEXICANO DEL GASOLINEEO
Star-like oxazolidine latent curing agent and preparation method as well as use thereof
ActiveCN103289038AReduce the presence of air bubblesHigh tensile strengthOrganic chemistryTanning treatmentFiltrationFractionation
The invention discloses a star-like oxazolidine latent curing agent and a preparation method as well as an application of the latent curing agent. The latent curing agent is characterized by being prepared by the following steps of: adding 0.01-0.1 part of sodium ethoxide, 5-60 parts of tetraethyl 1,1,5,5-pentane-terminated tetraformate and 15-120 parts of 2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidine into a reaction kettle, uniformly mixing the above raw materials, under the protection of nitrogen, increasing the temperature to 105-115 DEG C, carrying out reflux reaction for 2.5-3.5h, after the fractionation of ethanol is finished, increasing the temperature of a reaction solution to 115-125 DEG C to fractionate residual 2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidine, when the amount of 2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidine is not changed any more, stopping heating, dropwise adding 10-50 parts of butanone solution of p-toluenesulfonic acid at the concentration of 20-40% while stirring, carrying out reaction for 25-35min, neutralizing the reaction solution using acid until the pH (Potential of Hydrogen) is 7-7.5, adding 0.05-0.1 part of calcium oxide into the neutralized reaction solution, continuing the reaction for 0.5-1.5h, carrying out hot filtration at 75 DEG C to obtain 6-80 parts of thick semisolid tetra-2,2-dimethyl-N-hydroxyethyl-1,3-oxazolidinyl 1,1,5,5-pentane-terminated tetraformate.
Owner:ZHEJIANG GREAT CHEM SCI & TECH
Antimicrobial oxazolidine/iodopropynyl-butyl carbamate composition containing less than 0.1wt% free formaldehyde
This invention relates to a broad spectrum antimicrobial composition, effective against gram negative and gram positive bacteria and fungi, which comprises a synergistic composition comprising iodopropynyl butyl carbamate and a bicyclic hydroxymethyl oxazolidine containing less than 0.1% of free formaldehyde.
Owner:TROY TECH II
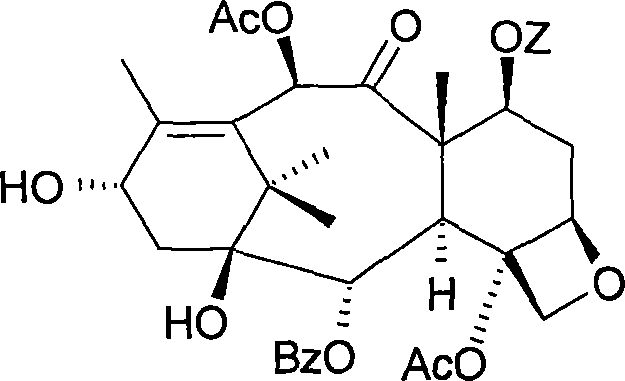
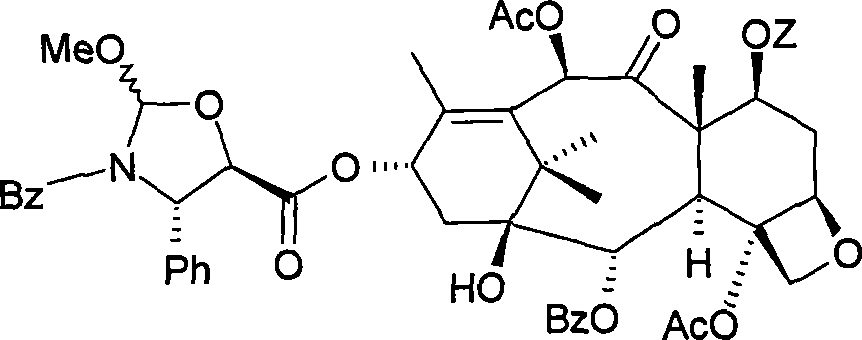
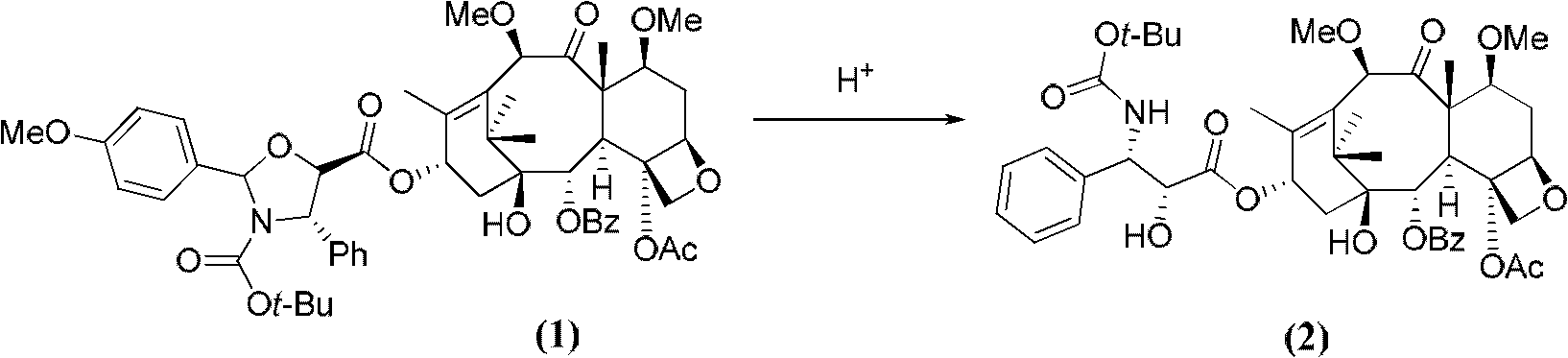
Method for preparing paclitaxel
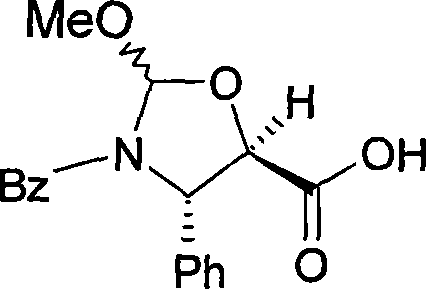
The invention concerns a method for preparing paclitaxel characterized in that it consists in starting with 10-deacetylbaccatine in accordance with a ''one-pot'' reaction including the following three steps: a) protecting the hydroxy radical in position 7 of 10-deacetylbaccatine with a silylated radical, then b) acetylating the hydroxy radical in position 10, c) optionally crystallizing the resulting baccatine III derivative, followed by condensation of (4S,5R)-3-N-benzoyl-2RS-methoxy-4-phenyl-l,3- oxazolidine-5-carboxylic acid, by esterifying in position 13 the acetylated 10-baccatine III derivative previously obtained, then opening the oxazolidine of the cyclic side chain and simultaneously releasing the hydroxy radical in position 7.
Owner:SOC DETUD & DE RES & DEV & INGIE PHARMA SERIPHARM
Coating graphite protective lubricant, and preparation method and application method thereof
ActiveCN103820198AAvoid high temperature oxidationStrong adhesionLubricant compositionDiacetone alcoholCarvacryl acetate
The invention discloses a coating graphite protective lubricant, and a preparation method and application method thereof. The coating graphite protective lubricant comprises the following ingredients by mass: 1.5-2 parts of monoethanolamine, 2.2-3 parts of sodium silicate, 1.8-2 parts of gelatins, 4.7-5 parts of sodium borate, 0.4-0.5 part of oxazolidine, 1.3-1.5 parts of sodium methylene bis-naphthalene sulfonate, 4.2-5 parts of ethylene-vinyl acetate copolymer, 0.8-1 part of crylic acid-2-ethylhexyl, 2.2-3 parts of diacetone alcohol, 14-15 parts of graphite and 60-62 parts of distilled water or deionized water. According to the invention, the coating graphite protective lubricant doesn't fall off, can't be oxidized, and has a good lubricating property and rejection capability.
Owner:QINGDAO YUANDA GRAPHITE
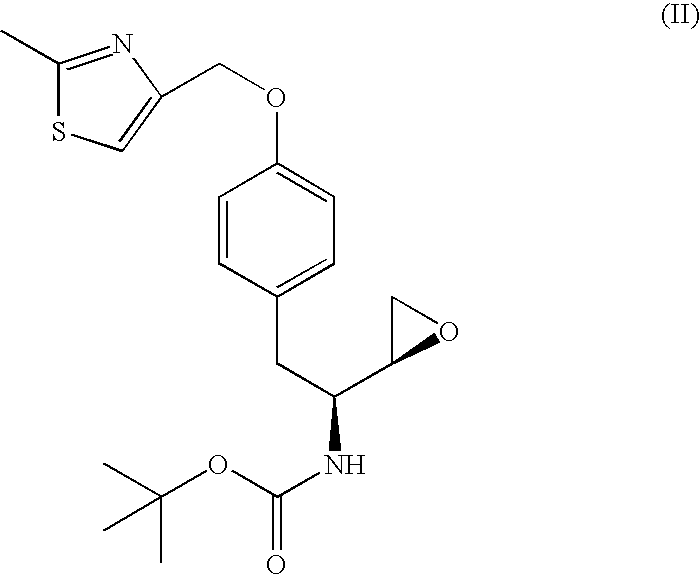
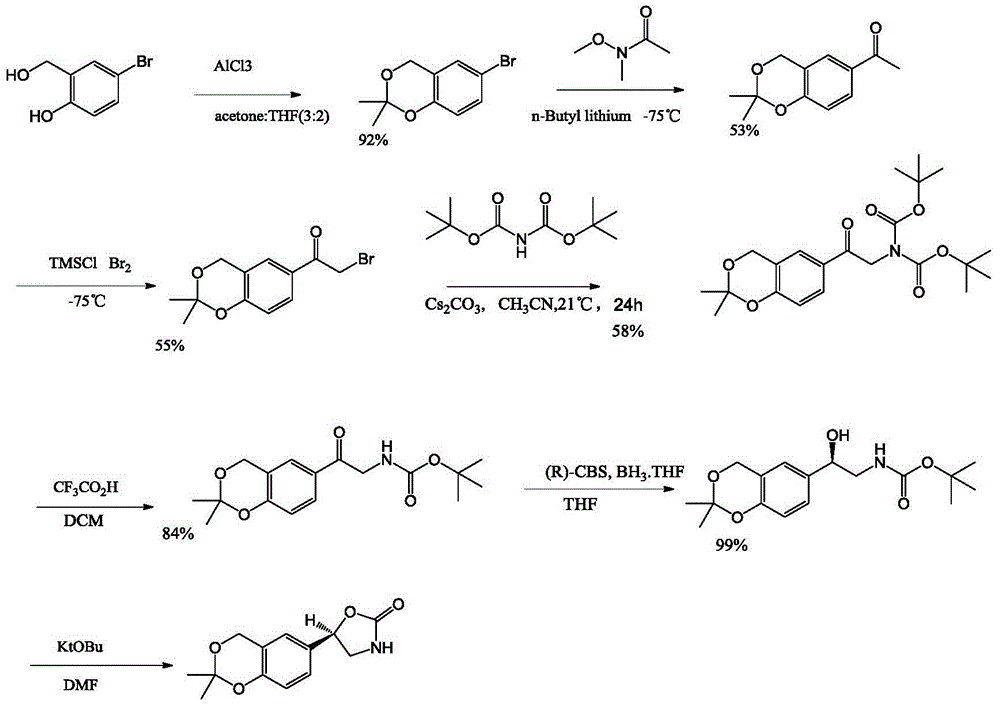
Vilanterol intermediate, preparation method and application thereof
ActiveCN105646285AHigh yieldReduce yieldCarbamic acid derivatives preparationOrganic compound preparationBenzeneCarboxylic acid
The invention provides a new Vilanterol intermediate compound 2. According to the invention, primary amino is introduced into a compound 5 through Delepine reaction, then di-tert-butyl dicarbonate is employed for amino protection, and the strategy greatly improves the yield and atom utilization rate. On the other hand, cheap and easily available urotropin is adopted in the invention to lower the cost, thus being easy for industrial large-scale production. Compared with the prior art, the yield of the method involved in the invention is increased to 65% by three-step reaction, also use of expensive di-tert-butyl iminodicarboxylate and cesium carbonate is avoided, the cost is reduced, the operation is simple, and the conditions are mild. Therefore, the method is suitable for industrial preparation of Vilanterol and its key intermediate (5R)-5-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-1, 3 oxazolidine-2-one. (formula of compound 2).
Owner:SHANGHAI INST OF PHARMA IND +1
Process for preparing florfenicol
The present invention is directed to a new process of preparing highly pure Florfenicol. The invention is further directed to new oxazolidine derivatives useful in making Florfenicol and processes of making these derivatives. Examples of such intermediates include (4R,5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine; and (4S,5R)-3-acetyl-2,2-dimethyl-4-fluoromethyl-5-[4-(methylsulfonyl)phenyl]-1,3-oxazolidine.
Owner:AUROBINDO PHARMA LTD
Composition and method for curing a resorcinol resin
InactiveUS6541576B1Good adhesionEmission reductionWood layered productsThin material handlingOxazolidinePHENOL/RESORCINOL
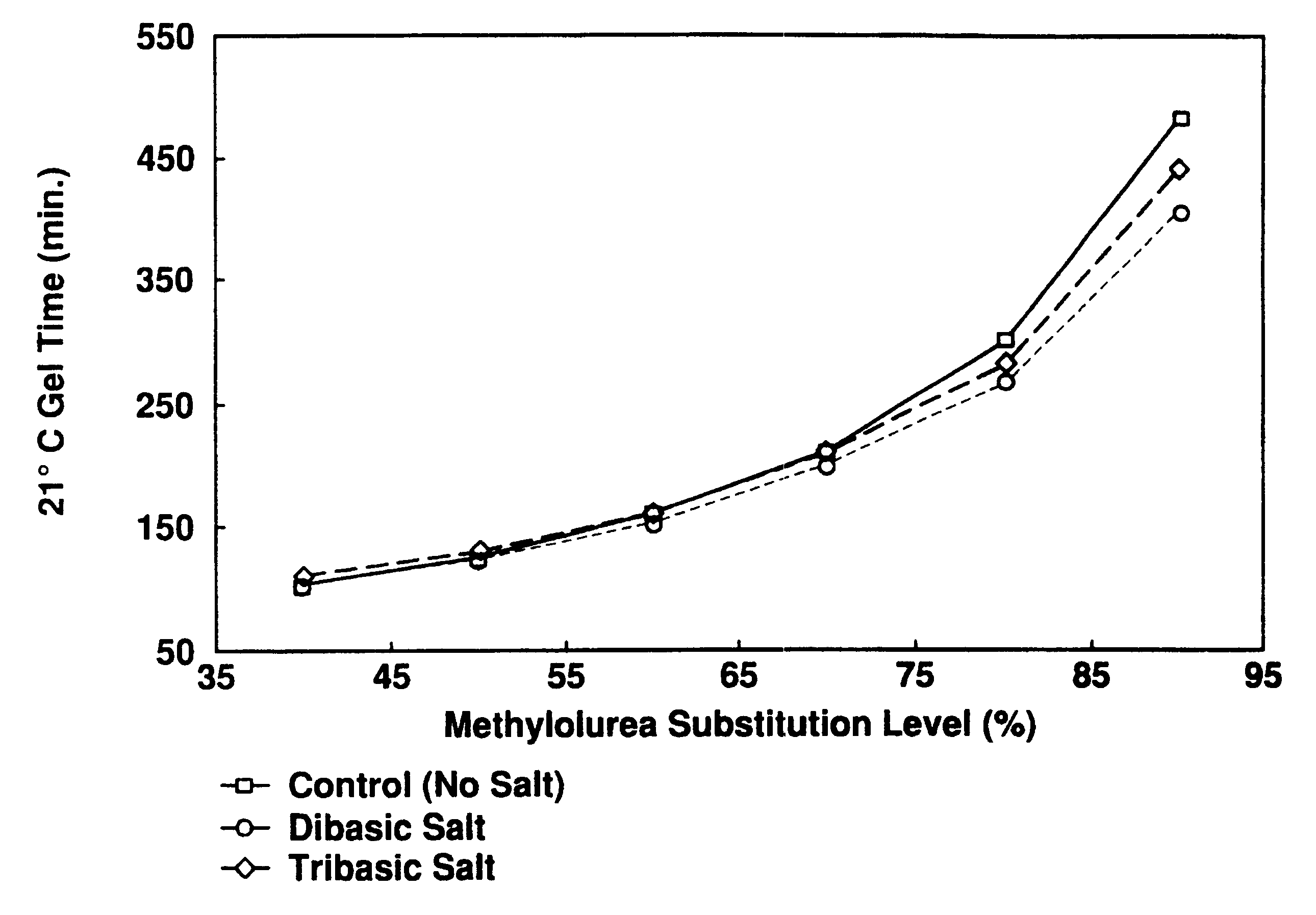
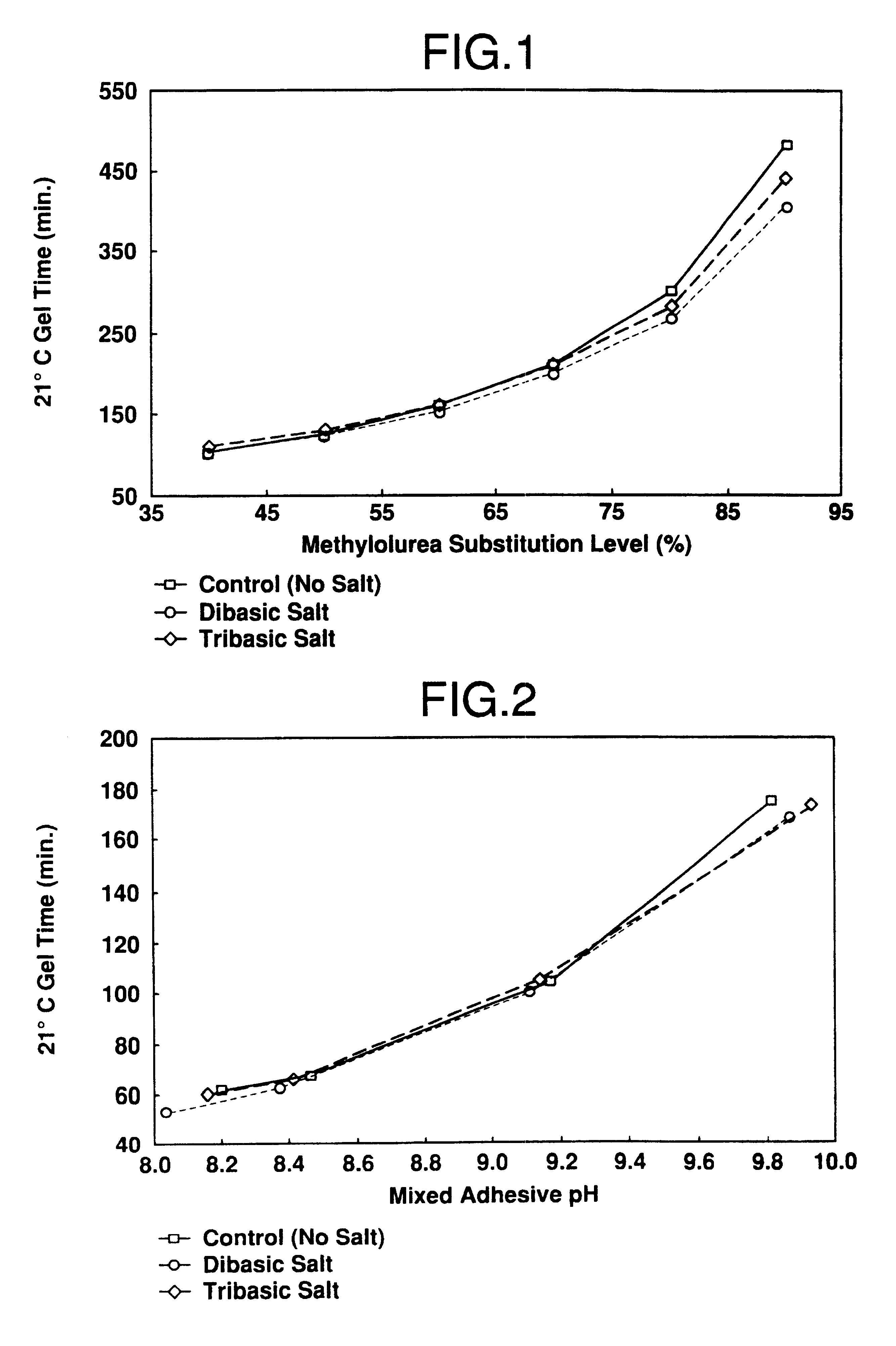
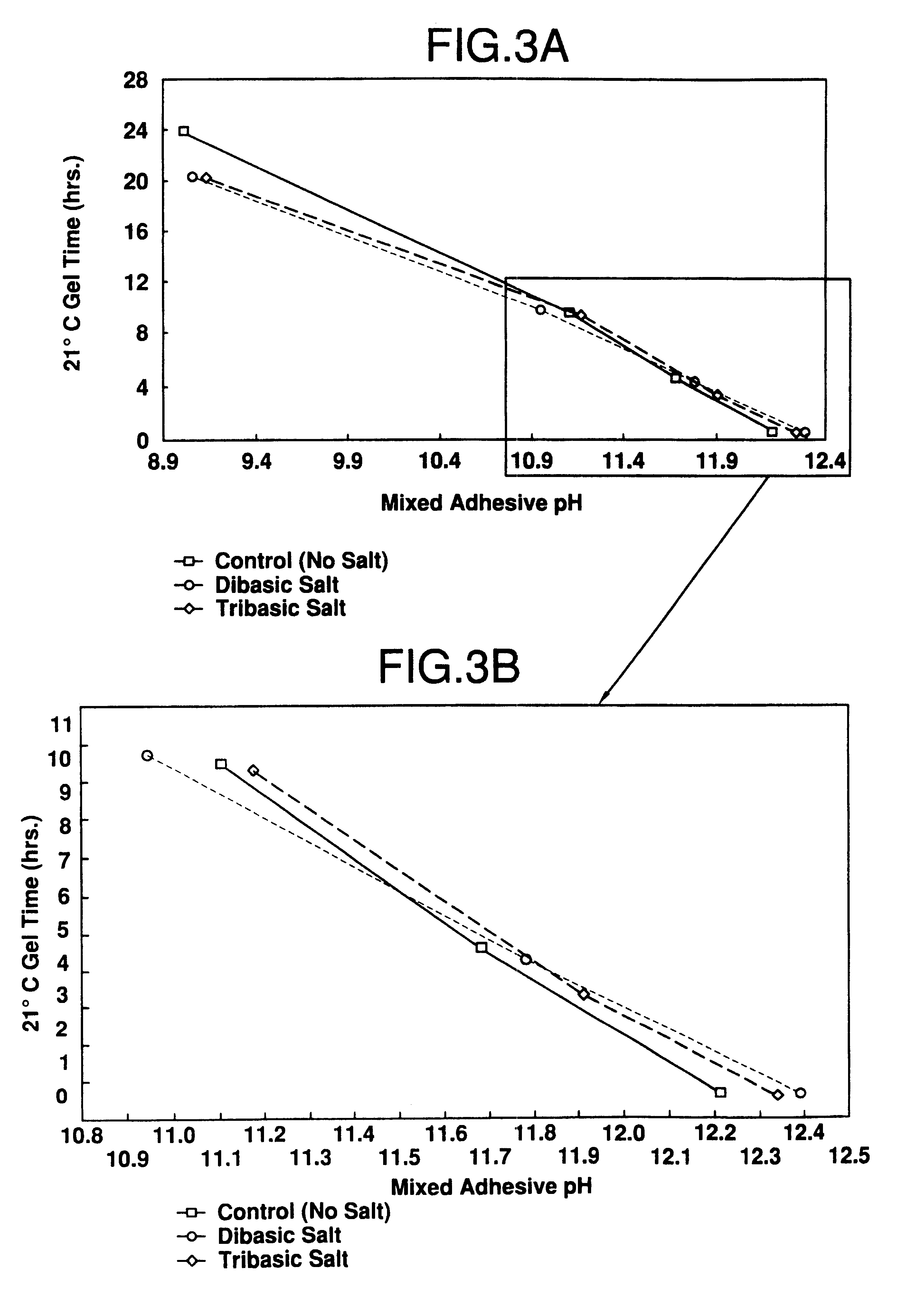
A catalyst curing system or hardener for resorcinolic resins, such as resorcinol-formaldehyde or phenol-resorcinol-formaldehyde resins including methylolurea. The methylolurea may be used in combination with oxazolidine in ratios ranging from 5:95 to 95:, by molar basis. The hardener crosslinks the resins which permits reduction or "fuming" of formaldehyde as well as control of gel times of the resulting adhesive. The hardener of the present invention if of particular utility in adhesives for use in the forest products industry.
Owner:HEXION INC
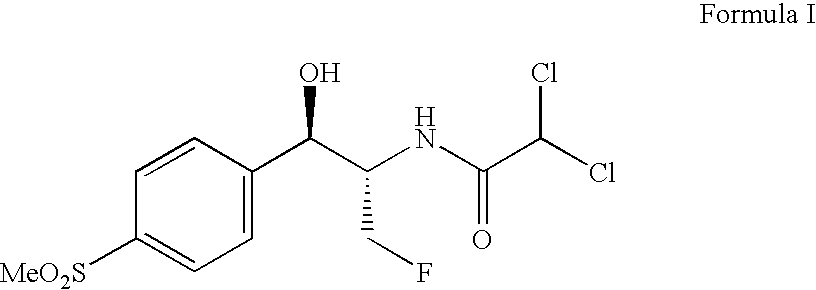
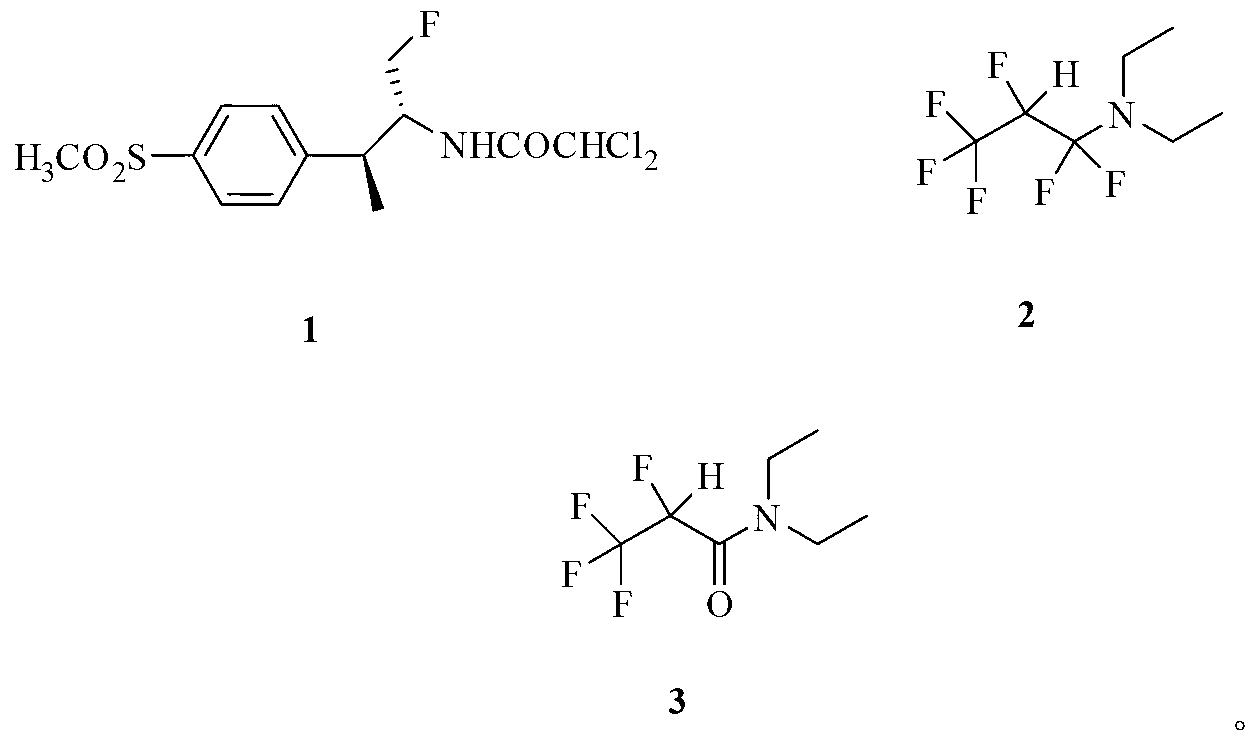
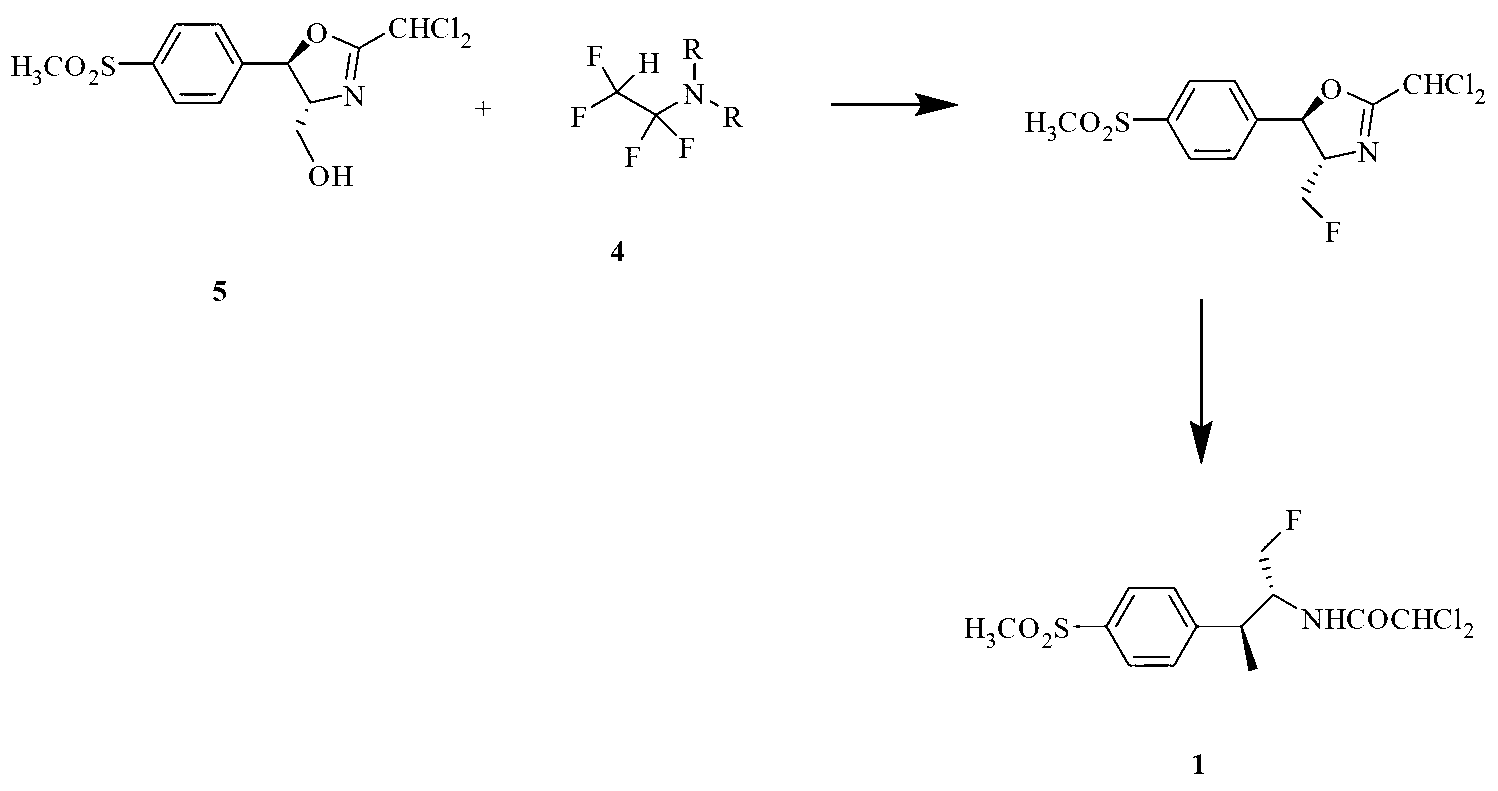
Application of fluorinating agent in florfenicol preparation technology
InactiveCN103254103AEasy to makeLow priceOrganic chemistryOrganic compound preparationHydrogenStructural formula
The invention relates to an application of a fluorinating agent in a florfenicol preparation technology. Particularly, a fluoridation reaction is performed between a fluorinating agent and a florfenicol intermediate (4R, 5R)-2-(dichloromethyl) 4,5-dihydro-5-(4-methylsulfone phenyl)-4-oxazol methanol (5) or (4R, 5R)-3-acetyl-2,2-dimethyl-4-hydroxymethyl-5-(4-methylsulfone phenyl) oxazolidine (6), and then florfenicol is obtained by hydrolysis, wherein the fluorinating agent has the structural formula shown in the specification. The fluorinating agent is simple to prepare and is cheap; with small molecular weight, a small amount of the fluorinating agent is used; and a byproduct can be conveniently and comprehensively utilized.
Owner:NANTONG JINLI OIL & FAT IND CO LTD
Compound herbicide for paddy fields
The invention relates to an agricultural chemical compound herbicide for once preventing and killing weeds in paddy fields. The agricultural chemical compound herbicide is prepared by compounding fenoxaprop-p-ethyl and bensulfuron methyl, adding a compound safener of mefenpyr-diethyl and 3-benzenesulfonyl-oxazolidine-2-ketone and relative additives, not only expands a weed spectrum and once prevents and kills the weeds in the paddy fields, but also solves the hazard problem of the fenoxaprop-p-ethyl applied to the paddy fields and has wider prospects of market promotion and application.
Owner:HANGZHOU UDRAGON CHEMICAL CO LTD
Process for preparing paclitaxel
InactiveUS6130336ASpeed up the processEasy to disassembleOrganic chemistryAntineoplastic agentsOxazolidine EBenzoyl chloride
PCT No. PCT / KR97 / 00157 Sec. 371 Date Feb. 23, 1999 Sec. 102(e) Date Feb. 23, 1999 PCT Filed Aug. 25, 1997 PCT Pub. No. WO98 / 08832 PCT Pub. Date Mar. 5, 1998The present invention elates to a process for preparing paclitaxel represented by formula (1) characterized in that: (a) an oxazolidine derivative represented by formula (2) or its salt in which X represents halogen, is coupled with a 7-trihaloacetyl-baccatin III represented by formula (3) in which R1 represents trihaloacetyl, in a solvent in the presence of a condensing agent to produce an oxazolidine substituent-containing taxane represented by formula (4) in which X and R1 are each as previously defined; (b) the oxazolidine ring is opened in a solvent in the presence of an acid, and the product thus obtained is reacted with benzoyl chloride in the presence of a base to produce a protected paclitaxel wherein the hydroxy group at 7-position is protected with trihaloacetyl group represented by formula (5) in which R1 is as previously defined; (c) then the protecting group at 7-position is removed by ammonia or a salt of ammonia with a weak acid in a solvent.
Owner:HANMI PHARMA
Method for removing protective groups and preparing dimethoxy taxane compound
InactiveCN102424672AWon't happenEasy to operateOrganic chemistryBulk chemical productionEpoxyTaxadiene
The invention relates to a method for removing protective groups and preparing a dimethoxy taxane compound. The invention belongs to the technical field of medicines used for treating prostate cancer. According to the invention, an acid is dissolved in methanol; silica gel with a specification of 100-800 meshes is added to the solution, and the solution is sufficiently stirred under room temperature; the solution is dried by rotary evaporation, such that acid-activated silica gel is obtained; the acid-activated silica gel is dissolved in an organic solvent; a compound 3-Boc-2-p-methoxy phenyl-4-phenyl-(2R,4S,5R)-1,3-oxazolidine-5-carboxylate 4alpha-acetoxy-2alpha-benzoyloxy-5beta,20-epoxy-1beta-hydroxyl-9-oxygen-7beta,10beta-dimethoxy-11-taxadiene-13alpha ester is added to the solution; the mixture is continuously stirred, and is subject to a reaction; an obtained reaction liquid is processed through pump filtration; an obtained filtrate is dried by condensation, and is processed through silica gel column chromatography, such that the product dimethoxy taxane compound is obtained. According to the invention, the protective groups are removed in an acidic environment, such that the dimethoxy taxane compound is prepared. The method is advantaged in simple operation, suitability for industrialized productions, no by-product during the reaction process, improved reaction yield, and improved product purity.
Owner:JIANGSU HONGDOUSHAN BIOLOGICAL TECH
Linear polystyrene-supported (4S)-oxazolidine-2-benzimine as well as preparation method and application thereof
InactiveCN101914174ARealize recyclingHigh optical purityOrganic compound preparationCarboxylic acid amides preparationPolystyreneOxazolidine
The invention relates to linear polystyrene-supported (4S)-oxazolidine-2-benzimine which has the following structural formula, wherein m:n=1:1-4, and Mw=8300-14000. In the invention, (4S)-oxazolidine-2-benzimine is supported on linear polystyrene to prepare the linear polystyrene-supported (4S)-oxazolidine-2-benzimine as a chiral auxiliary reagent to induce asymmetric reaction into homogeneous reaction, and the reaction is rapid and convenient for on-line detection. The chiral auxiliary reagent not only reserves the high yield and the high stereoselectivity of the asymmetric reaction induced by the oxazolidine-2-benzimine as the chiral auxiliary reagent to obtain a chiral compound with high optical purity, bus also realize the recycling of the (4S)-oxazolidine-2-benzimine. The synthesized chiral compound with high optical purity is used as the precursor, the intermediate and the final product of medicines, agricultural chemicals, spices and functional materials.
Owner:HUBEI UNIV
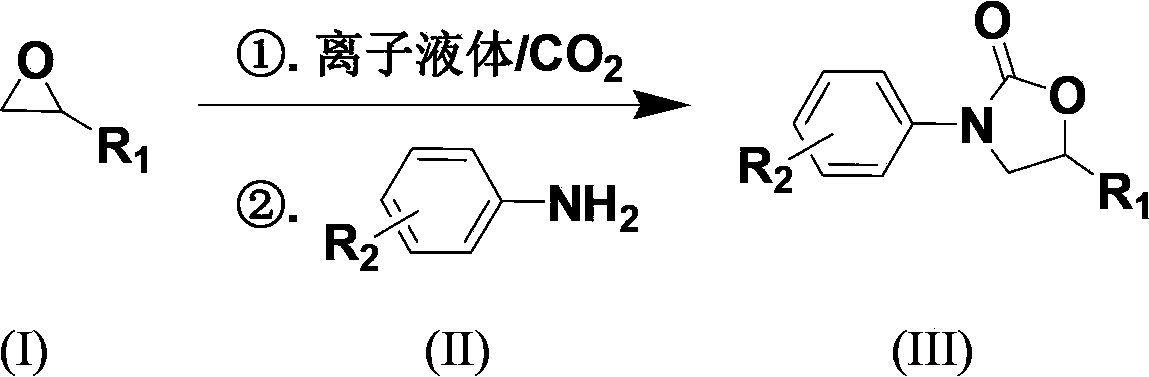
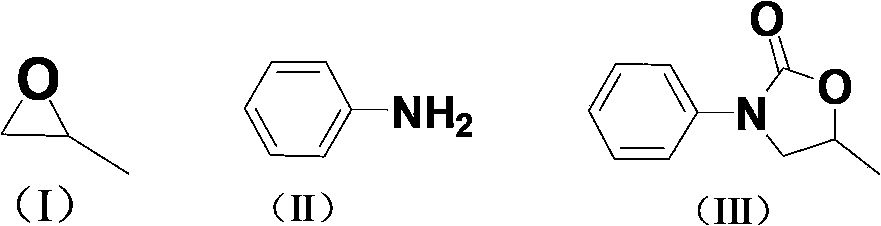
Carbon dioxide one-pot method for directly preparing oxazolidine-2-one compounds
InactiveCN103570640ARaw materials are cheapFew reaction stepsOrganic chemistryOrganic solventAniline
The invention discloses a carbon dioxide one-pot method for directly preparing oxazolidine-2-one compounds. The method comprises the following steps: adding a catalytic amount of ionic liquid into epoxy compounds, carrying out reactions under a primary pressure of CO2, then adding aniline or aniline derivatives, and making the reactions going on so as to prepare the oxazolidine-2-one compounds. The carbon dioxide one-pot method has the advantages of no need to separate intermediate products, little using amount of catalyst, safe reaction operation, mild reaction conditions, no addition of any organic solvent in the reaction process, and environment-friendliness.
Owner:EAST CHINA NORMAL UNIV
Method for preparing amphiprotic tanning agents in aryl-sulfone type
A preparation method for bisexual tanning agent takes 4,4' dihydroxy sulfobenzide, phenol sulfuric acid and methanol as the raw material to prepare a methanol condensate, takes diethanolamine and methanol as the raw material to prepare 3-ethoxyl oxazolidine, then to condensate the methanol condensate and 3-ethoxyl oxazolidine to prepare a bisexual tanning agent.
Owner:SHAANXI UNIV OF SCI & TECH
Biocidal composition of 2,6-dimethyl-m-dioxane-4-ol acetate and methods of use
Provided are compositions comprising 2,6-dimethyl-m-dioxane-4-ol acetate and a biocidal compound selected from the groups consisting of: a biocidal oxazolidine; 1-(3- chloroallyl)-3,5,7-triaza-1-azoniaadamantane; and tris(hydroxymethyl)-nitromethane. The compositions are useful for controlling microorganisms in aqueous systems.
Owner:NUTRITION & BIOSCIENCES USA 1 LLC
Antibacterial oxidation-resistant composite reverse osmosis membrane
ActiveCN102512982AImprove antibacterial propertiesImprove oxidation resistanceSemi-permeable membranesPolyamideReverse osmosis
The invention relates to a high-molecular composite membrane, in particular to an antibacterial oxidation-resistant composite reverse osmosis membrane for water treatment. The antibacterial oxidation-resistant composite reverse osmosis membrane comprises a non-woven fabric layer, a porous supporting layer and a polyamide polymer desalination layer on the porous supporting layer, and is characterized by being prepared by the following steps of: (1) soaking the polyamide polymer desalination layer in a formaldehyde solution, taking out the polyamide polymer desalination layer and washing with purified water; (2) soaking the polyamide polymer desalination layer in a solution containing hydroxymethyl hydantoin or hydroxymethyl ethyleneurea or hydroxymethyl oxazolidine, and then drying; and (3) soaking the treated polyamide polymer desalination layer in a solution containing active chlorine, taking out and then washing with the purified water to obtain the antibacterial oxidation-resistant composite reverse osmosis membrane. The antibacterial oxidation-resistant composite reverse osmosis membrane disclosed by the invention further has the characteristics of easy preparation, high desalination rate and large water flux, and also has wide application.
Owner:徐州世恒环保科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![1-Aza-3,7-Dioxabicyclo[3,3,0]Octane Compounds and Monocyclic Oxazolidines as Pro-Fragrances 1-Aza-3,7-Dioxabicyclo[3,3,0]Octane Compounds and Monocyclic Oxazolidines as Pro-Fragrances](https://images-eureka.patsnap.com/patent_img/e1e34178-1a59-4433-a0f2-2566c4401c25/US20090312231A1-20091217-C00001.png)
![1-Aza-3,7-Dioxabicyclo[3,3,0]Octane Compounds and Monocyclic Oxazolidines as Pro-Fragrances 1-Aza-3,7-Dioxabicyclo[3,3,0]Octane Compounds and Monocyclic Oxazolidines as Pro-Fragrances](https://images-eureka.patsnap.com/patent_img/e1e34178-1a59-4433-a0f2-2566c4401c25/US20090312231A1-20091217-C00002.png)
![1-Aza-3,7-Dioxabicyclo[3,3,0]Octane Compounds and Monocyclic Oxazolidines as Pro-Fragrances 1-Aza-3,7-Dioxabicyclo[3,3,0]Octane Compounds and Monocyclic Oxazolidines as Pro-Fragrances](https://images-eureka.patsnap.com/patent_img/e1e34178-1a59-4433-a0f2-2566c4401c25/US20090312231A1-20091217-C00003.png)