Method for preparing paclitaxel
A technology of paclitaxel and hydroxyl, applied in the field of preparing paclitaxel, can solve the problems of impossible to achieve, optimization, various steps not specifically disclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
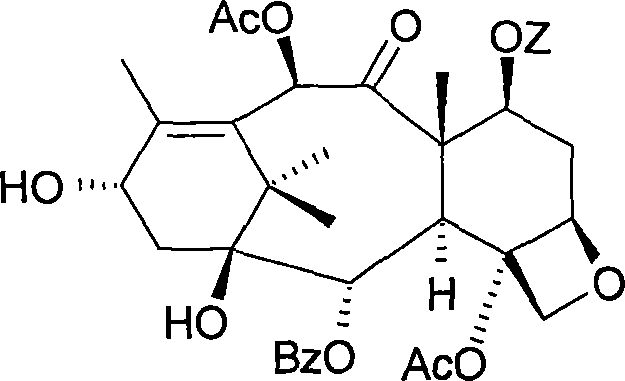
[0030] 7-o-triethylsilyl baccatin III
[0031]
[0032] 0.42 g (2.79 mmol) of triethylchlorosilane was added within 30 minutes to a stirred solution of 1 g (1.84 mmol) of 10-deacetylbaccatin in 2 ml of anhydrous pyridine at 5°C under an inert atmosphere. After reacting at 5°C for 24 hours, 0.37 g (3.62 mmol) of acetic anhydride was added within 30 minutes, and the reaction mixture was returned to room temperature. After 22 hours of reaction, 5 ml of a methanol / water (50 / 50) mixture were added within 30 minutes. The suspension was stirred for 30 minutes then filtered through a sintered glass.
[0033] After drying, 1.09 g of the above title compound was obtained in the form of a white solid (yield = 85%).
[0034] The resulting compound has the following characteristics:
[0035] 1 H NMR 400MHz (CDC1 3 ) (δppm): 8.11 (2H, d, J = 7.1Hz); 7.6 (III, t, J = 7.4Hz); 7.48 (2H, t, J = 7.7Hz); 6.46 (1H, s); 5.63 ( 1H, d, J = 7Hz); 4.96 (1H, d, J = 8.1Hz); 4.83 (1H, m); 4.49 ...
Embodiment 2
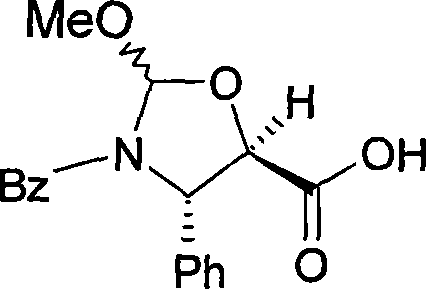
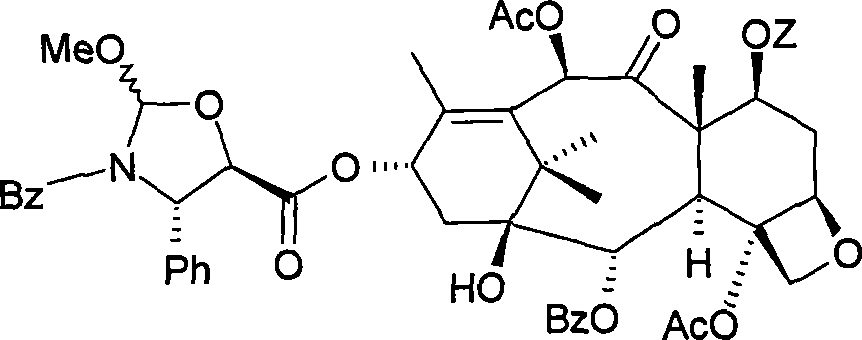
[0037] 13-o-[[(4S, 5R)-3-N-benzoyl-4-phenyloxazolidine-2RS-methoxy-5-yl]carbonyl Base]-7-o-triethylsilyl-baccatin III
[0038]
[0039] Under stirring in an inert atmosphere at room temperature, 1 g (1.43 mmol) of 7-o-triethylsilyl baccatin III was added to 0.58 g (1.77 mmol) of (4S,5R)-3-N-benzoyl -4-Phenyloxazolidine-2RS-methoxy-5-carboxylic acid in 6.5 ml of ethyl acetate. A solution of 0.73 g (3.54 mmol) of dicyclohexylcarbodiimide in 1 ml of ethyl acetate and 0.02 g (0.16 mmol) of 4-dimethylaminopyridine was added. After reacting for 1 hour, the insoluble matter was removed by filtration and the organic phase was concentrated under reduced pressure.
[0040] 1.36 g of the above title compound were obtained in the form of a pale yellow residue (yield = 95%).
[0041] The resulting compound has the following characteristics:
[0042] 1 H NMR 400MHz (CDCl 3 ) (δppm): 8.13 (2H, d, J = 7.3Hz); 7.75 (2H, d, J = 7.3Hz); 7.61 (1H, t, J = 7.4Hz); 7.50 (5H, m); 7.41 ( ...
Embodiment 3
[0044] paclitaxel
[0045]
[0046] Under an inert atmosphere, 2 mL (3 mmol) of 1.5 N aqueous HCl was added to 1 g (1 mmol) of 13-o-[[(4S,5R)-3-N-benzoyl -4-phenyloxazolidine-2RS-methoxy-5-yl]carbonyl]-7-o-triethylsilyl-baccatin III in a mixture of 8 ml ethyl acetate and 4 ml ethanol in solution. After 1 hour of reaction, 2 ml of water were added and the reaction mixture was stirred at 45° C. for another 3 hours. After adding 16 ml of ethyl acetate, the organic phase was washed with 11 ml of a saturated aqueous solution of sodium bicarbonate and then with 11 ml of a saturated aqueous solution of sodium chloride, and concentrated under reduced pressure.
[0047] The crude product was chromatographed on silica gel (10 μm) (eluent: cyclohexane / ethyl acetate, 5 / 5) and crystallized from a mixture of ethanol and water (25 / 75), whereby 0.65 g of Paclitaxel (Yield = 80%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com