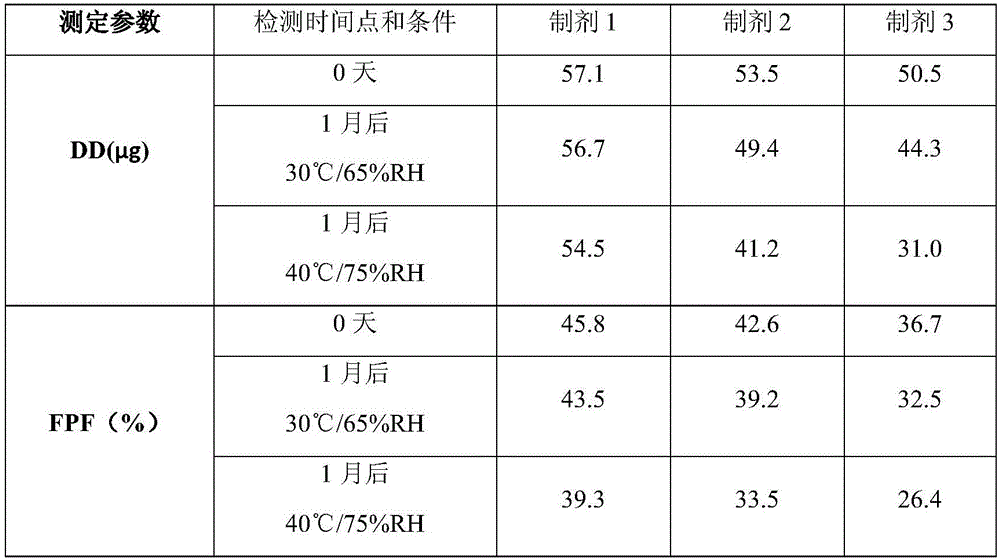
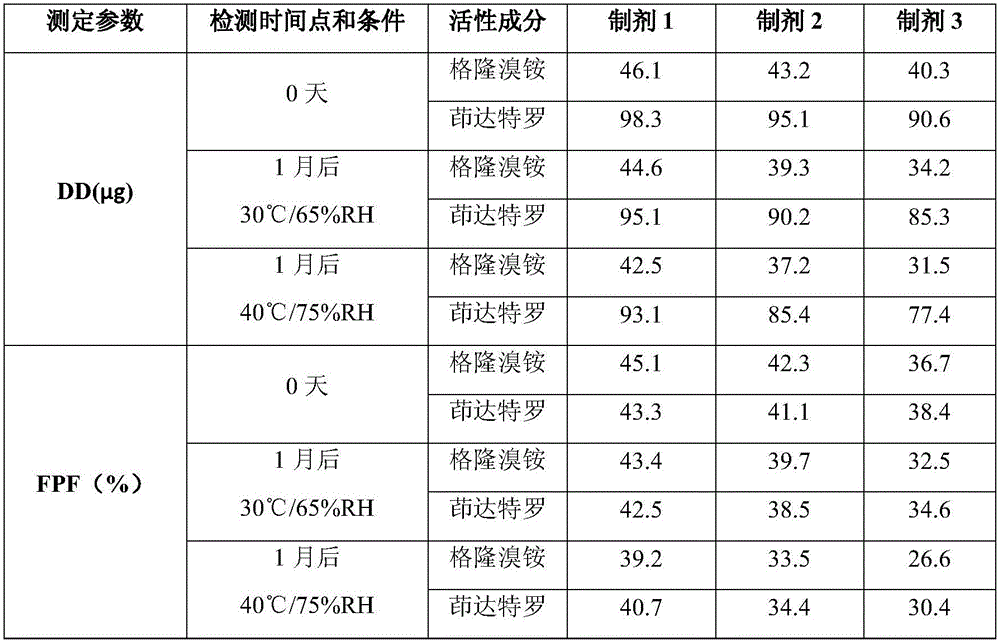
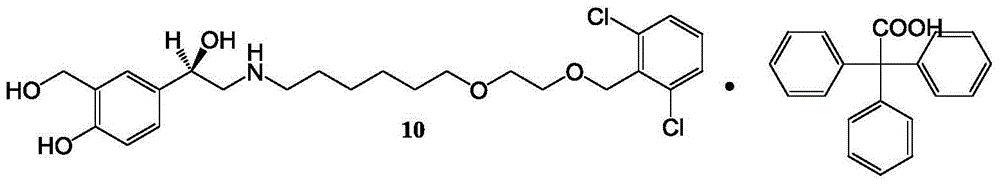
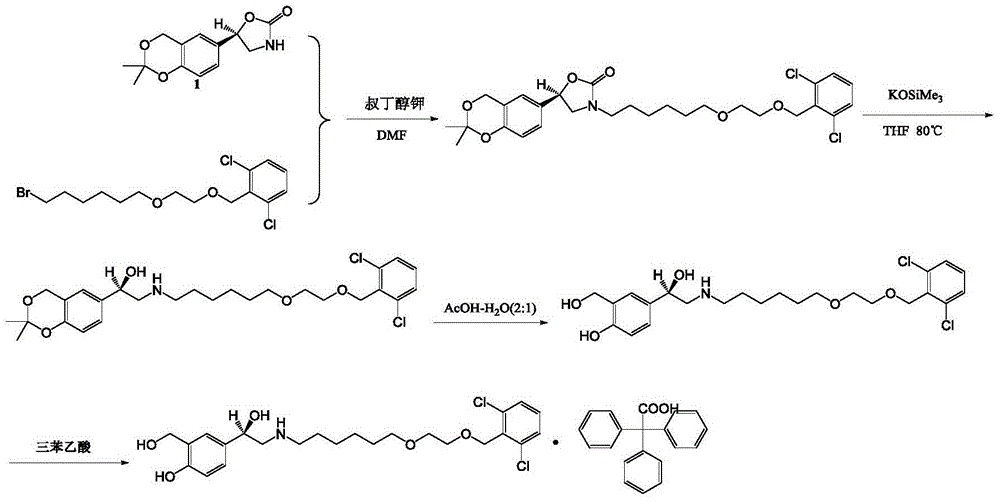
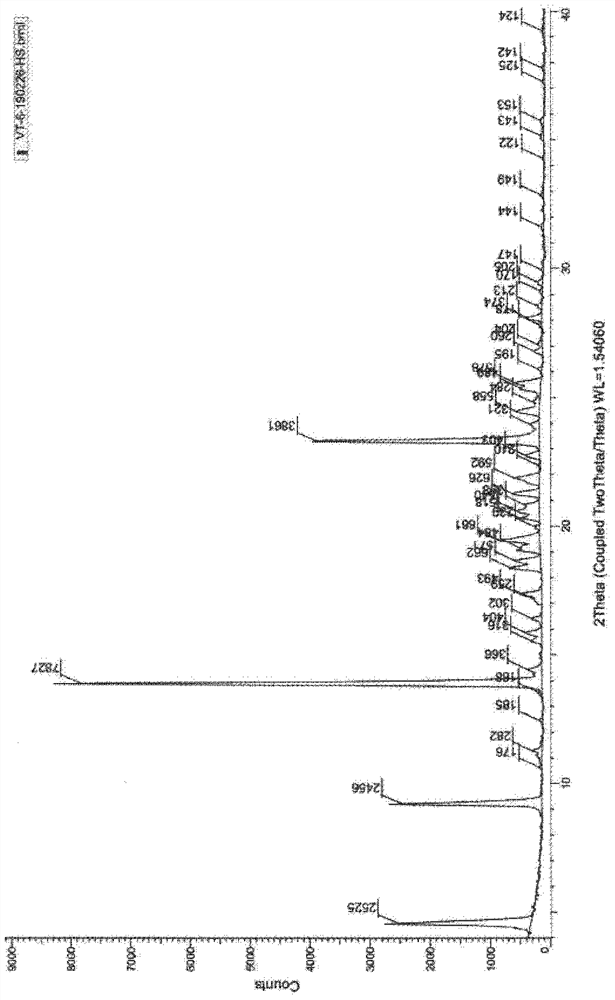
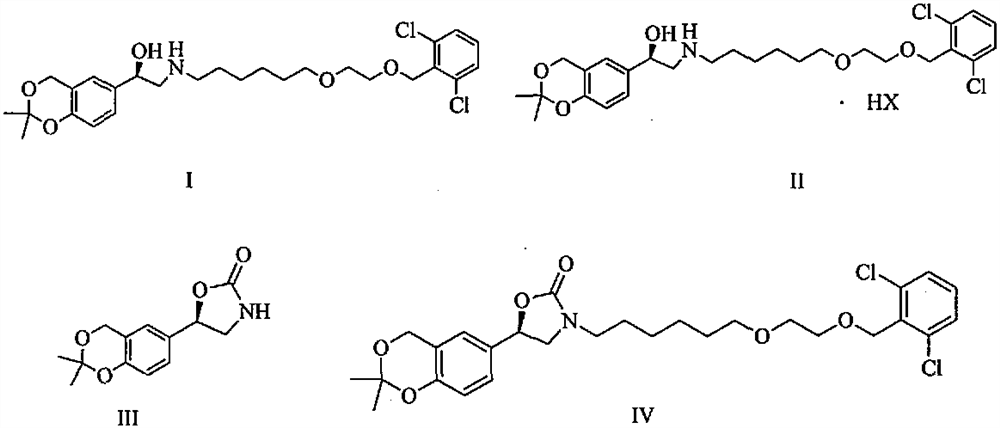
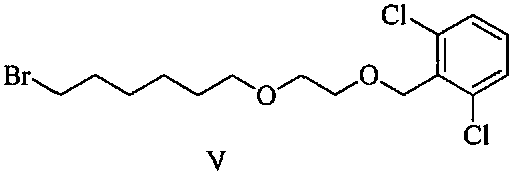
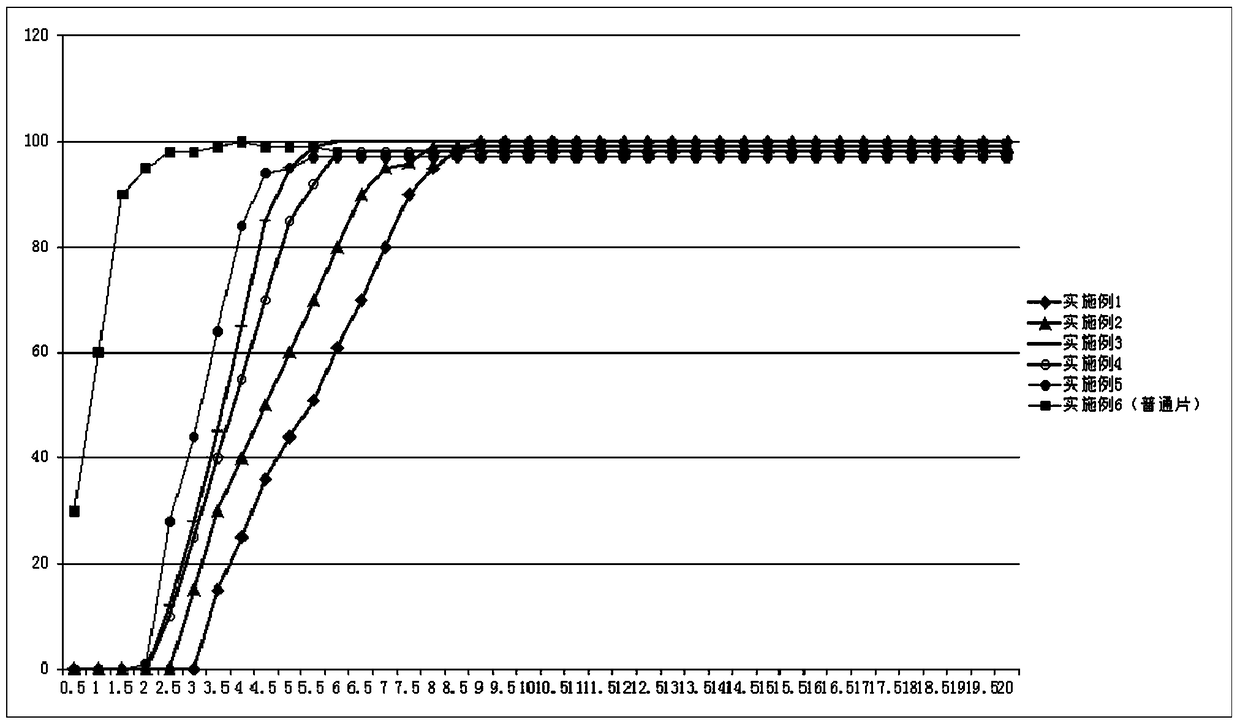
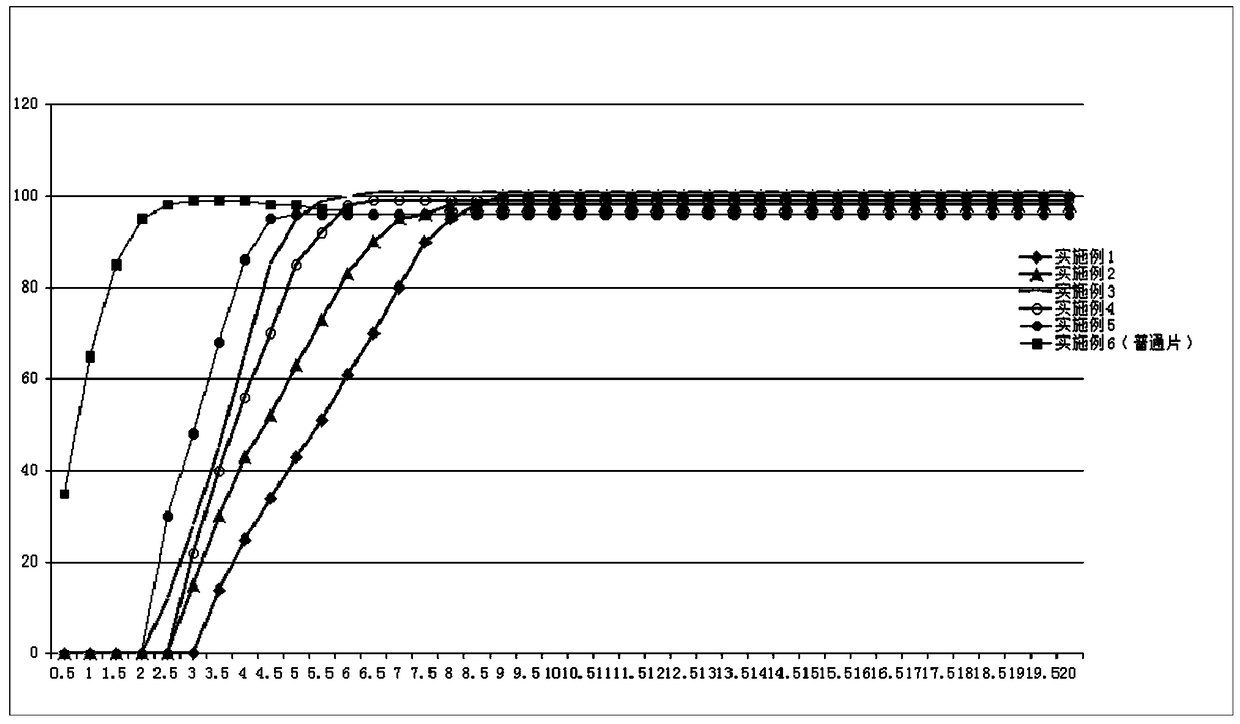
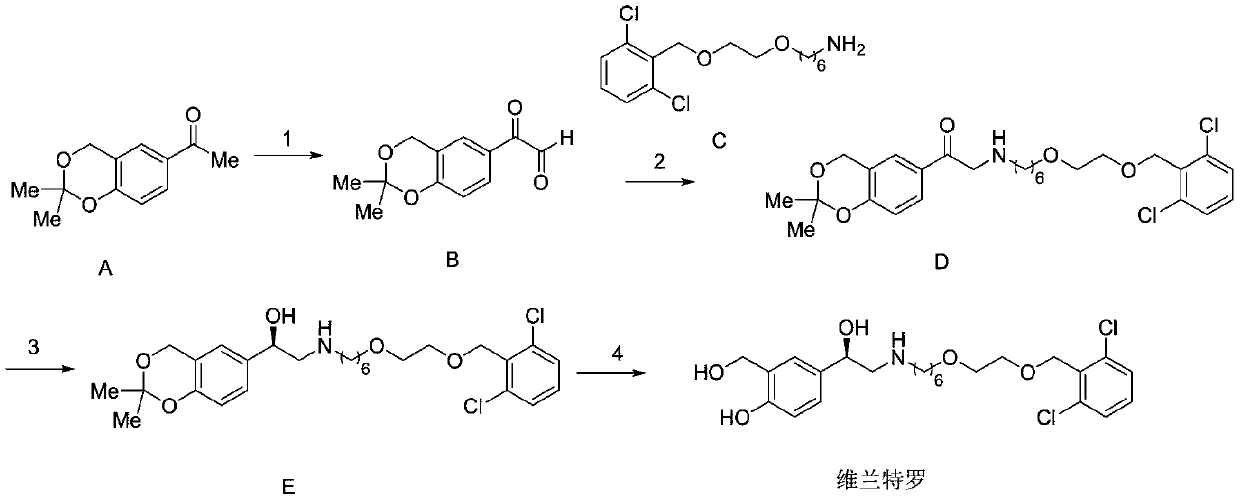
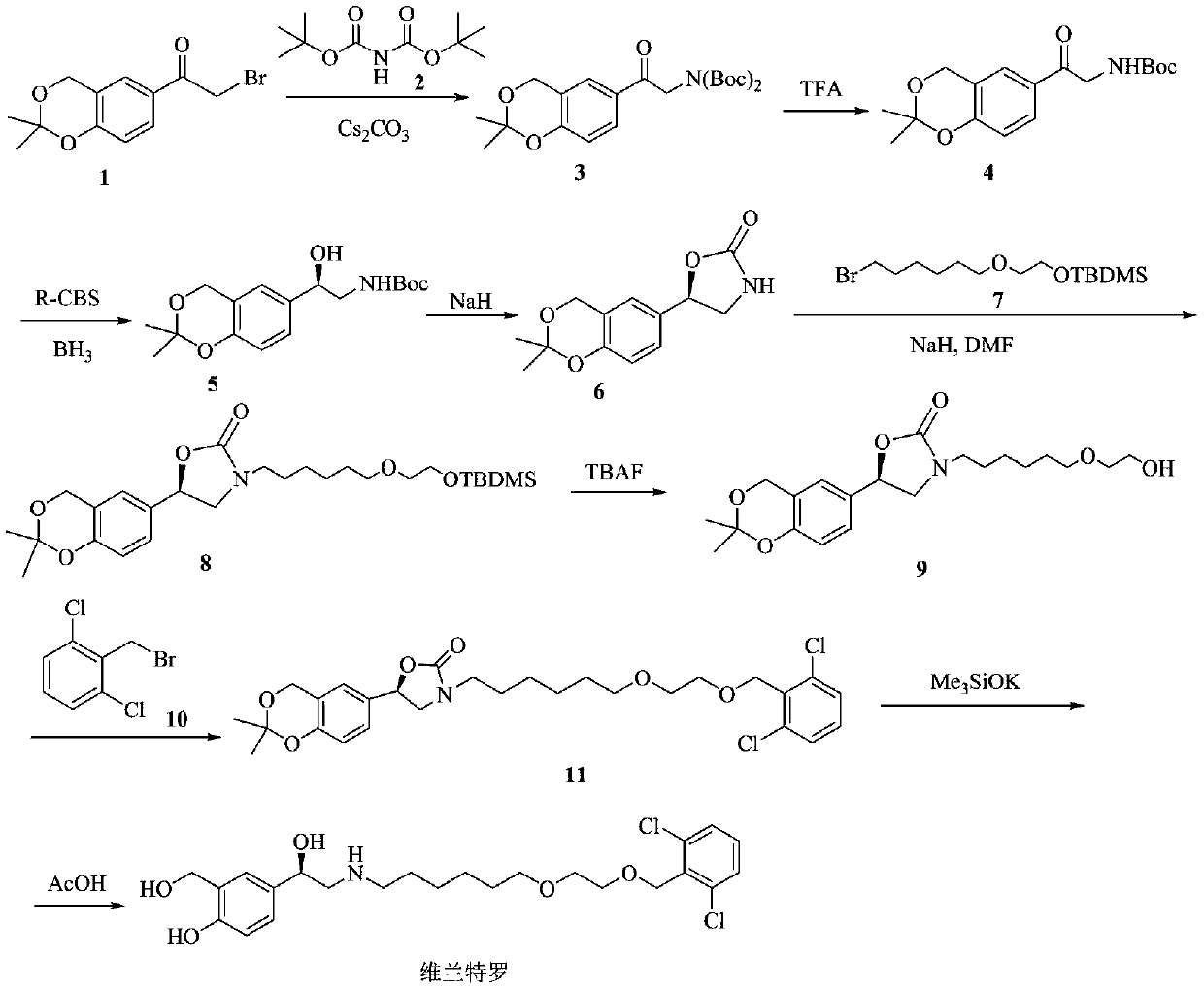
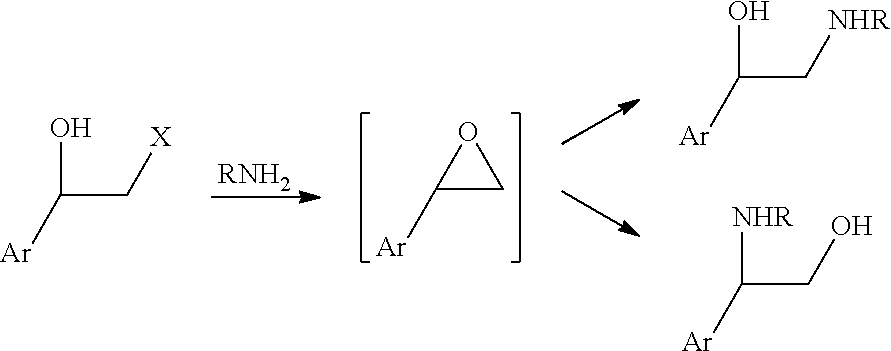Patents
Literature
33 results about "Vilanterol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
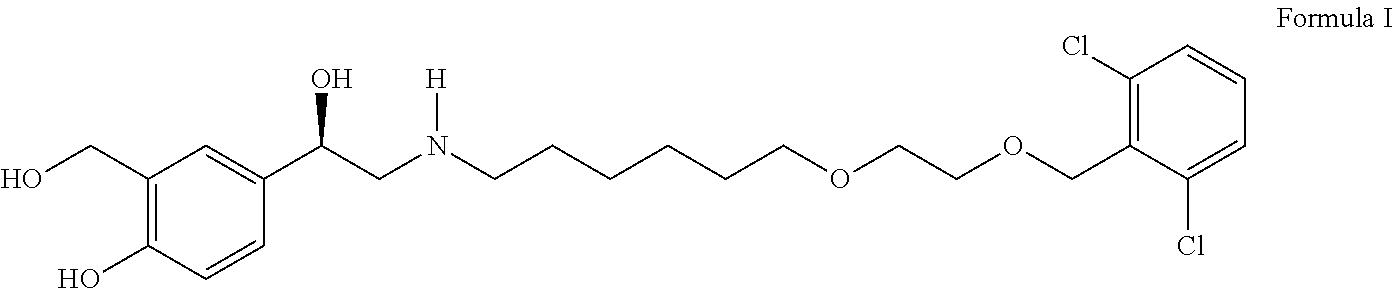
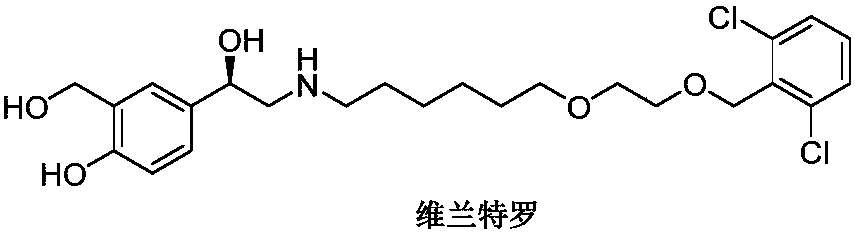
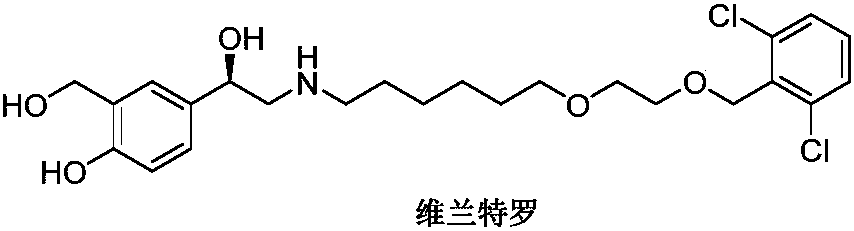
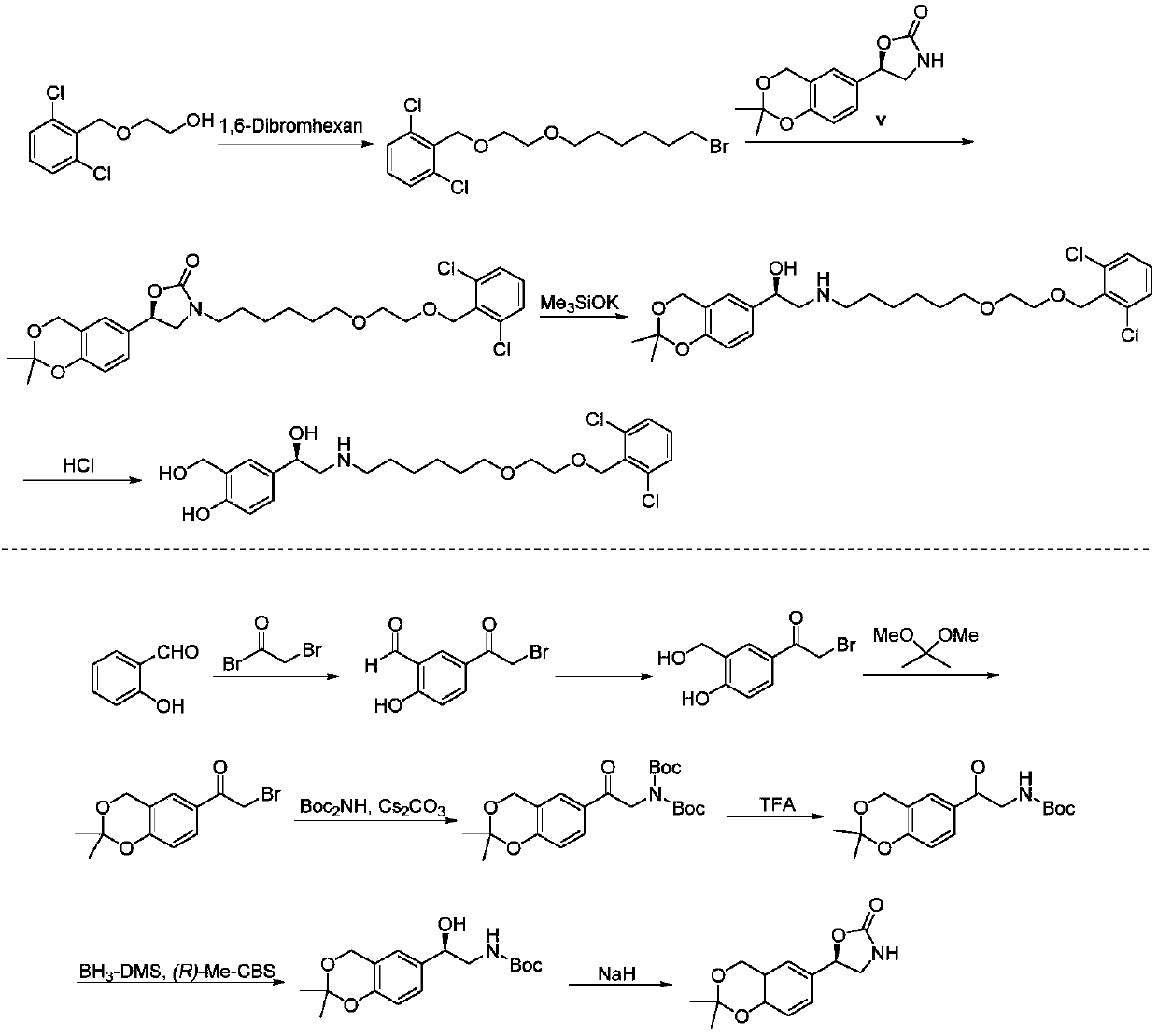
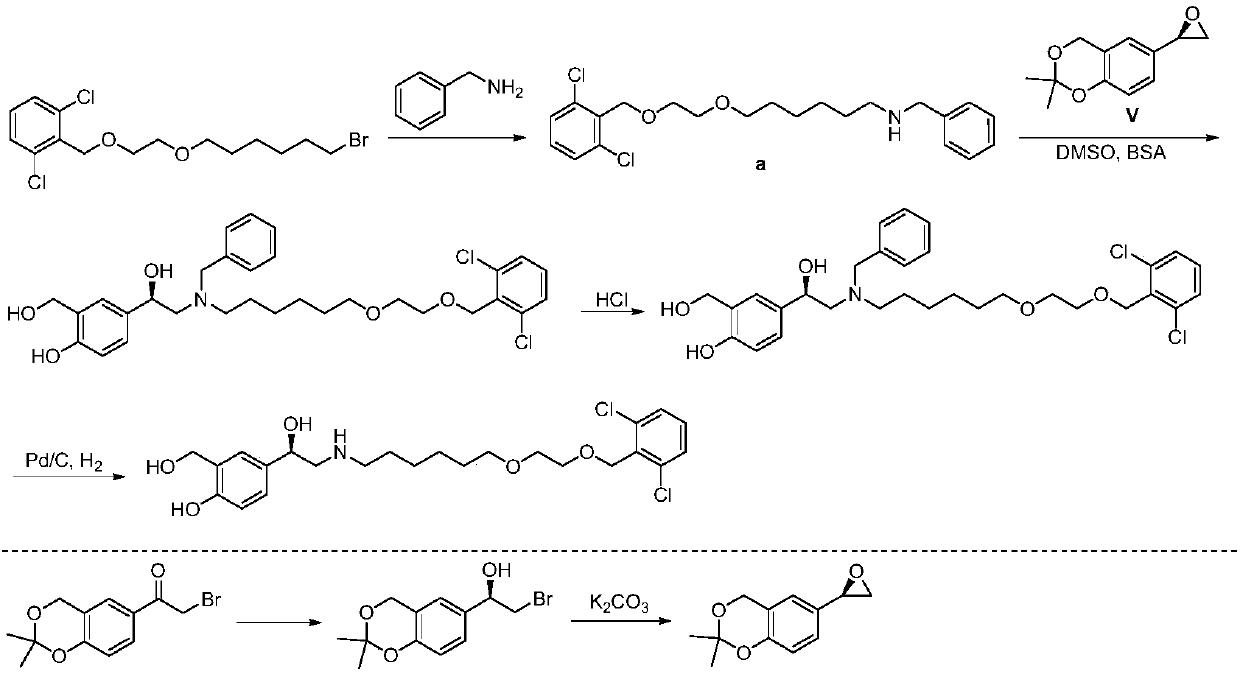
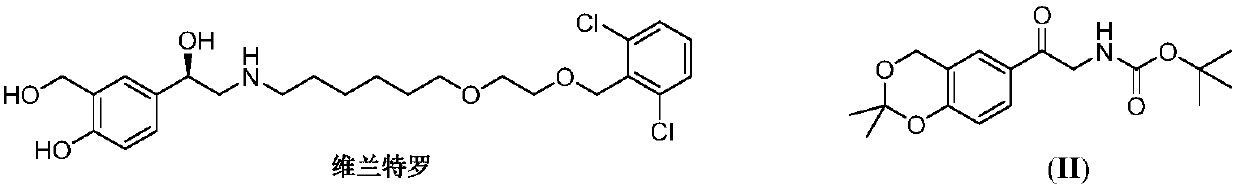
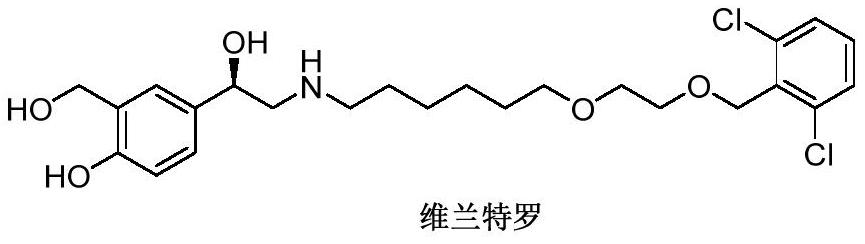
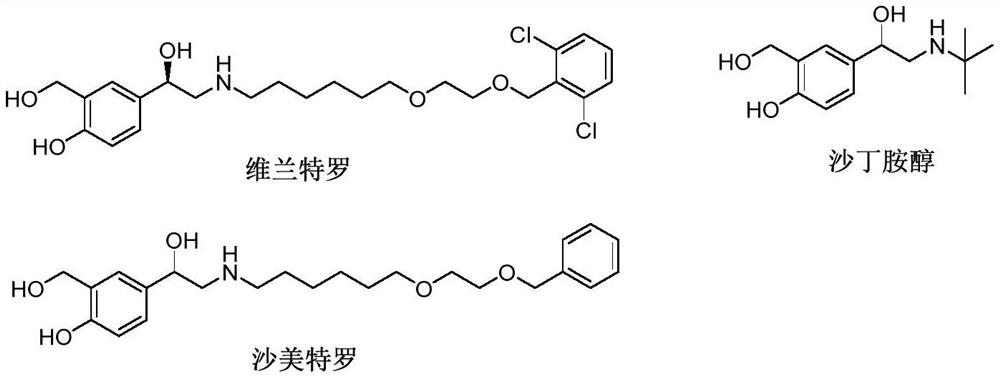
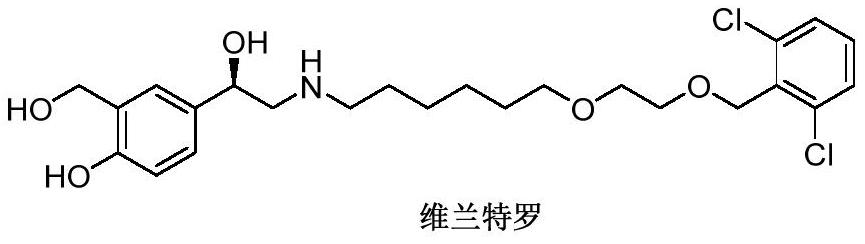
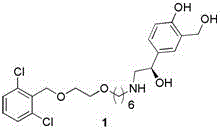
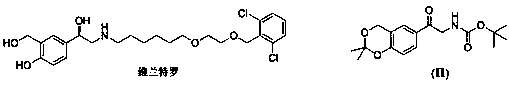
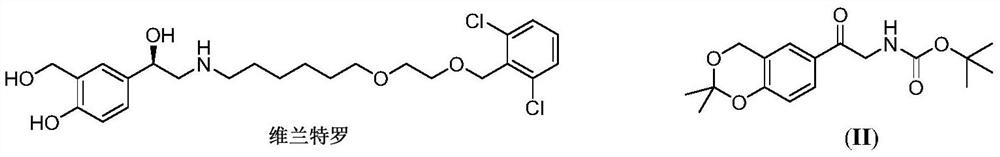
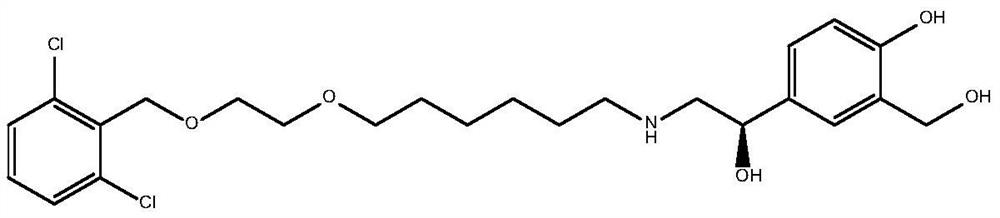
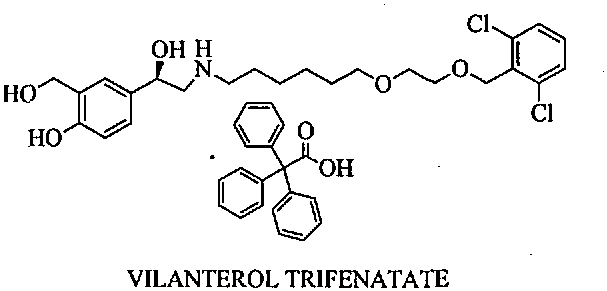
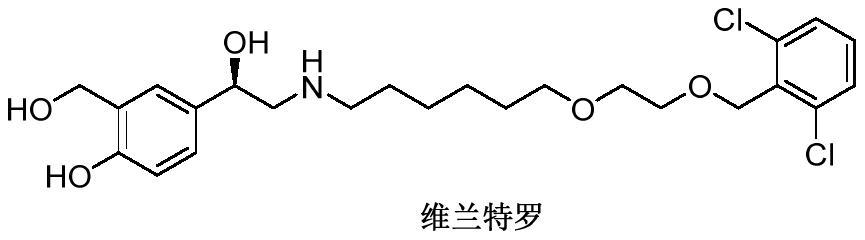
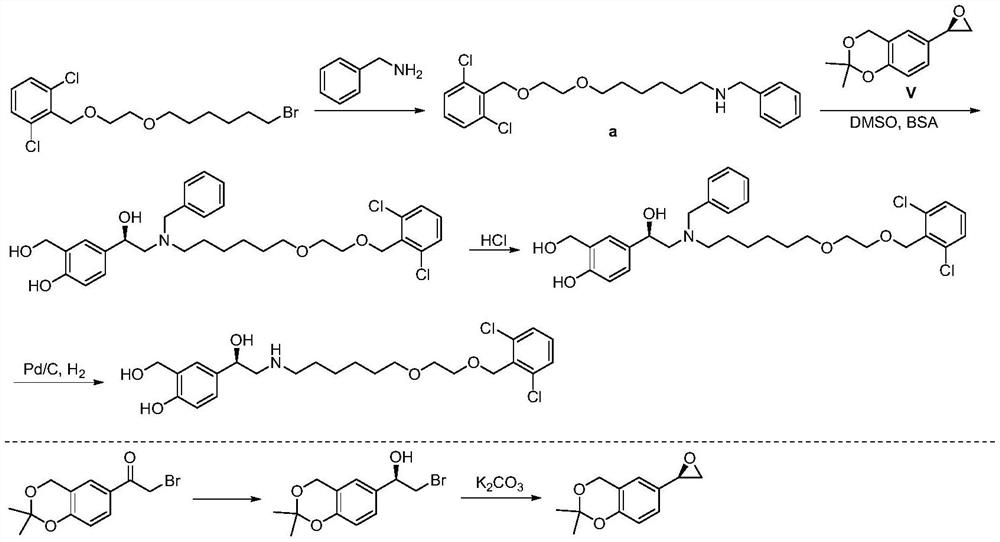
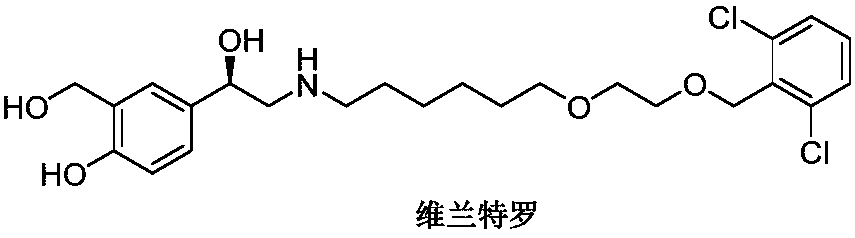
Vilanterol (INN, USAN) is an ultra-long-acting β₂ adrenoreceptor agonist (ultra-LABA), which was approved in May 2013 in combination with fluticasone furoate for sale as Breo Ellipta by GlaxoSmithKline for the treatment of chronic obstructive pulmonary disease (COPD).
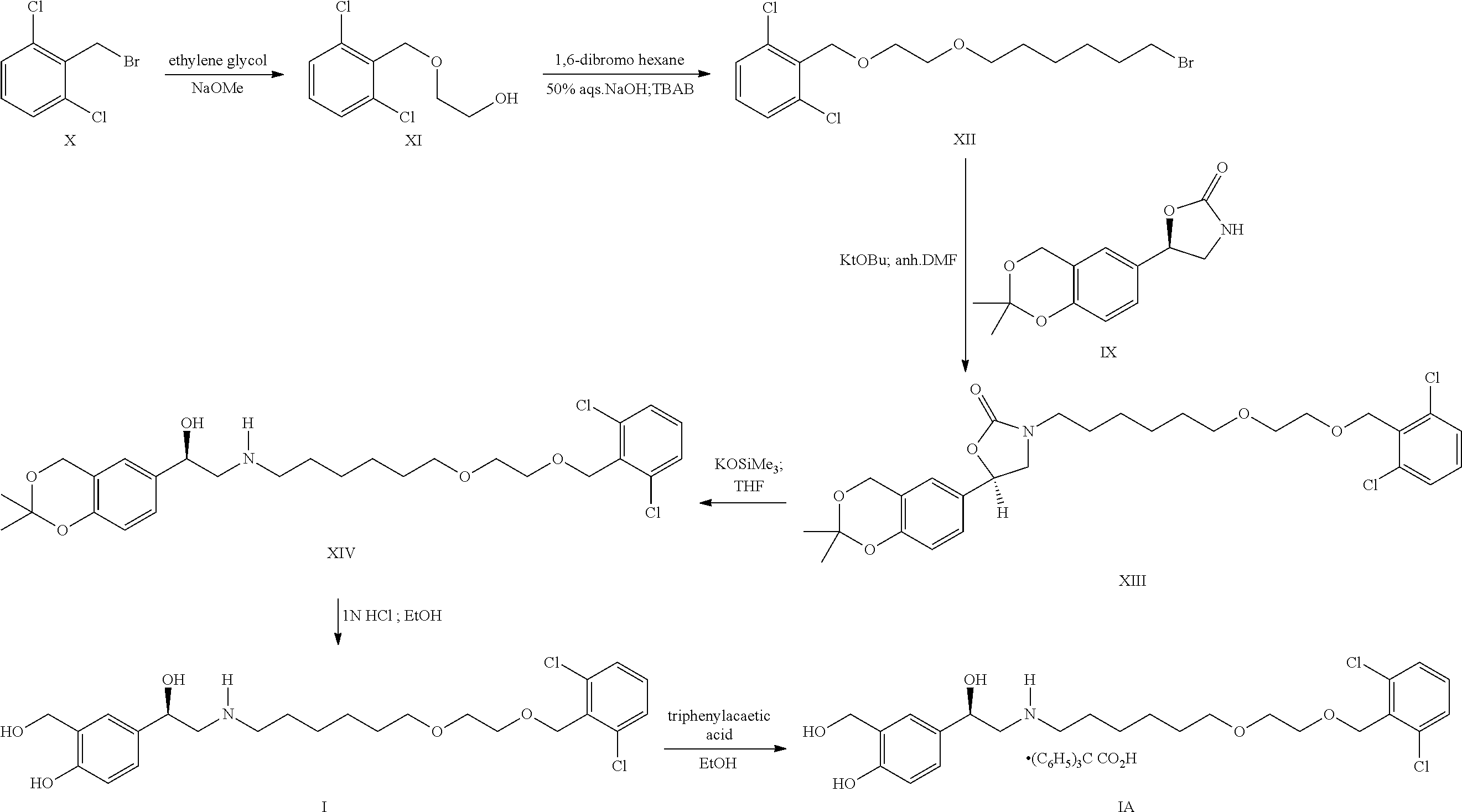
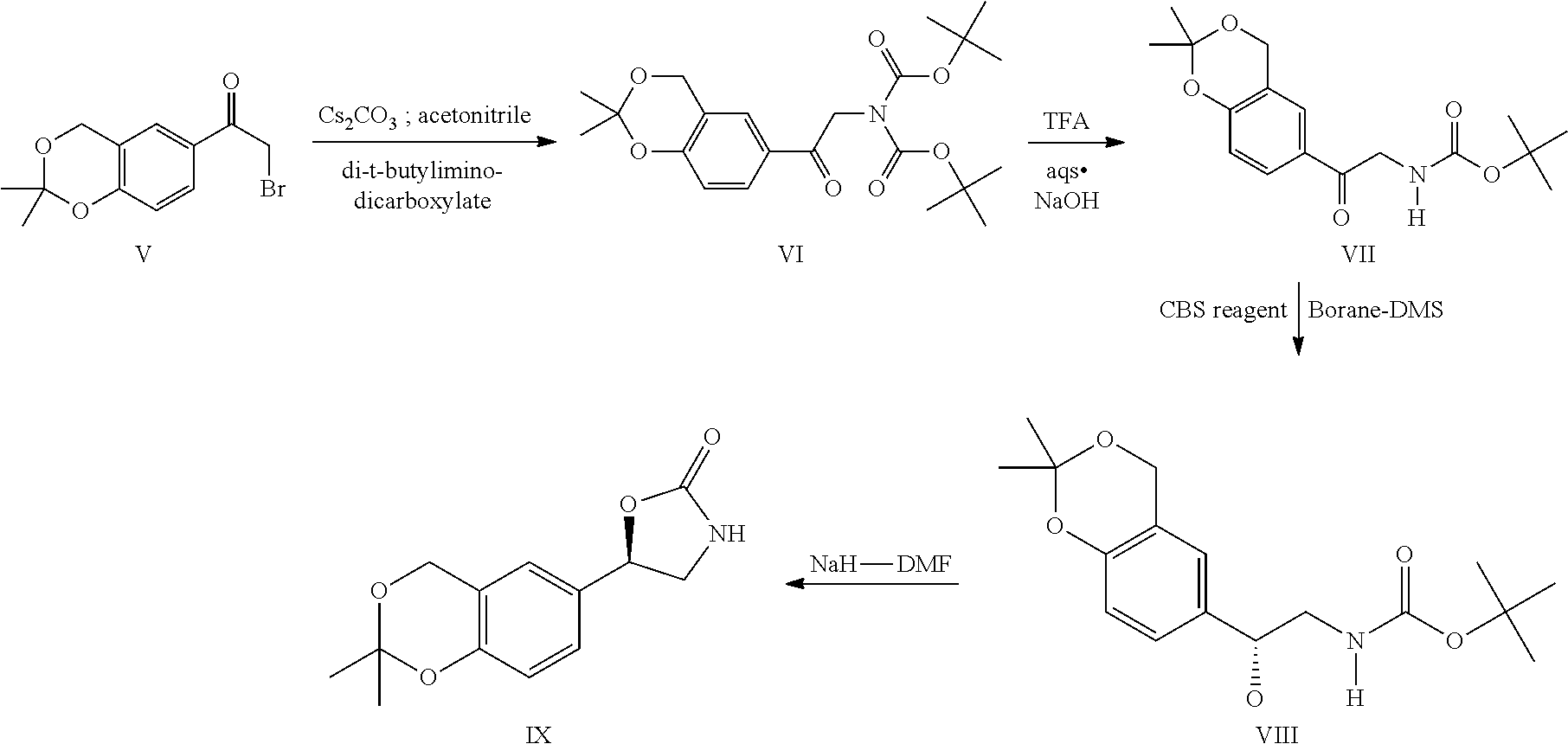
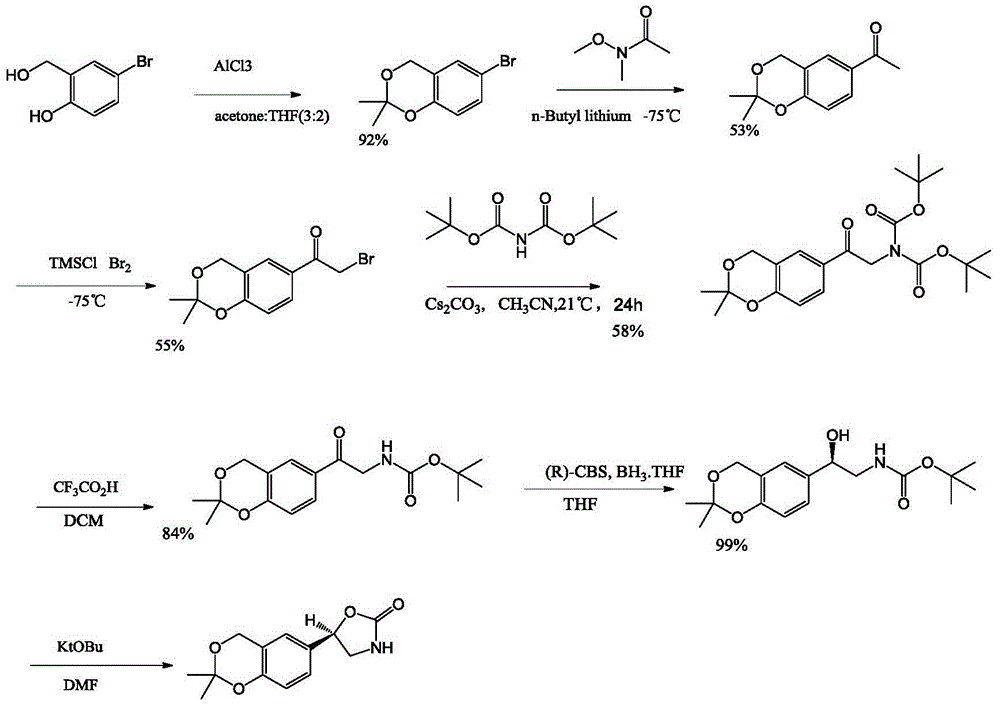
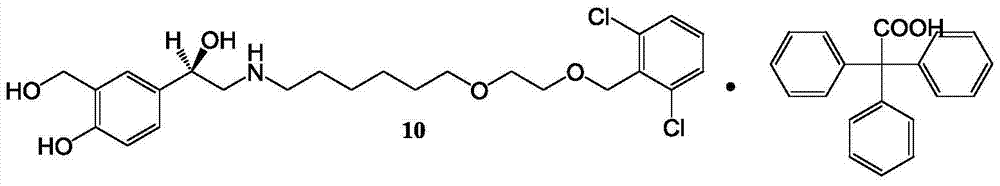
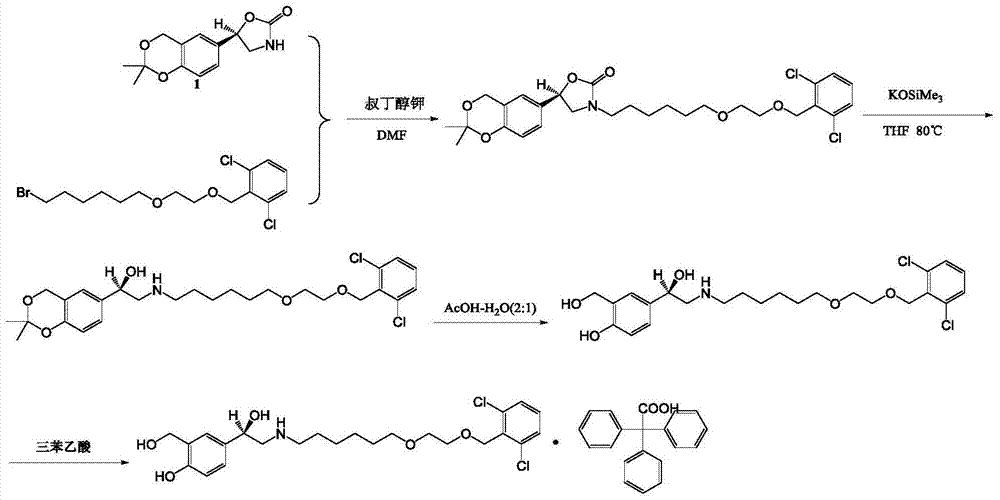
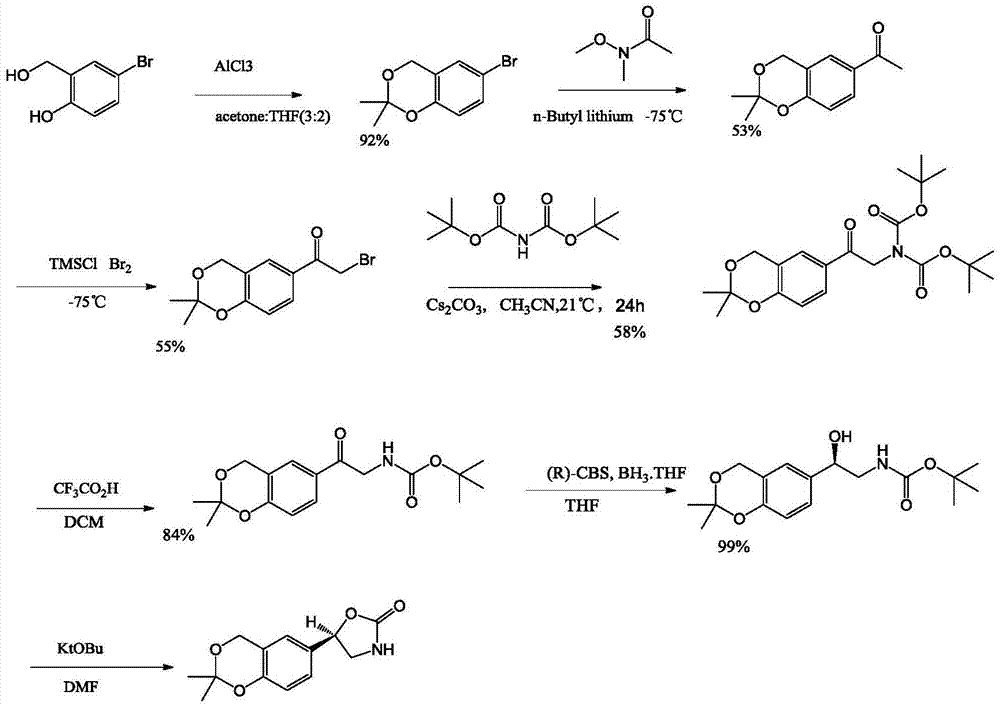
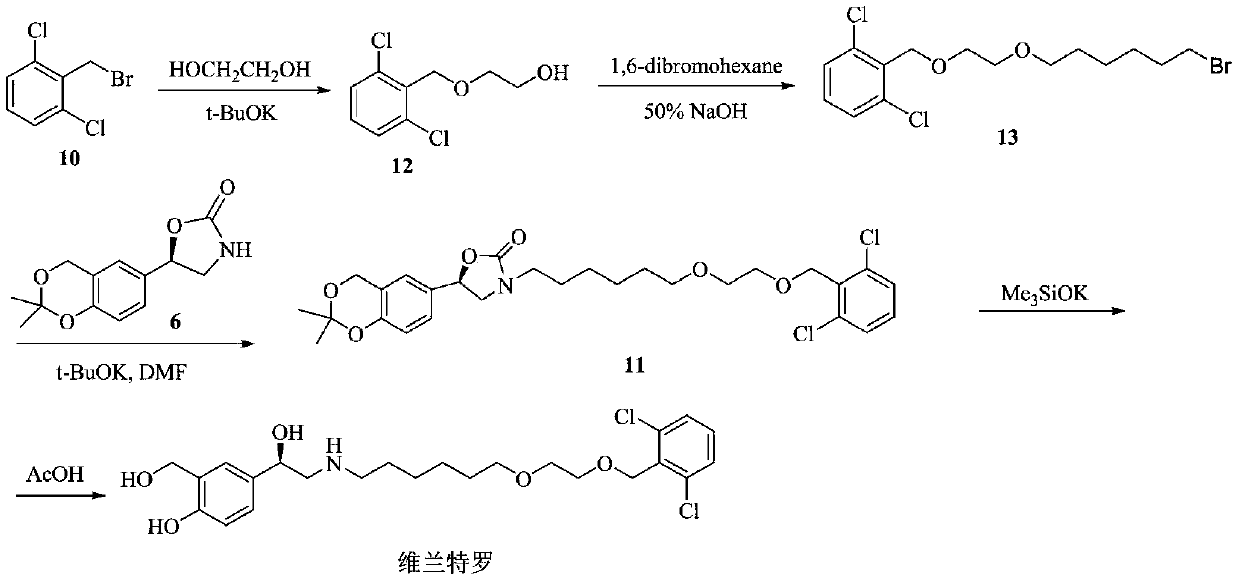
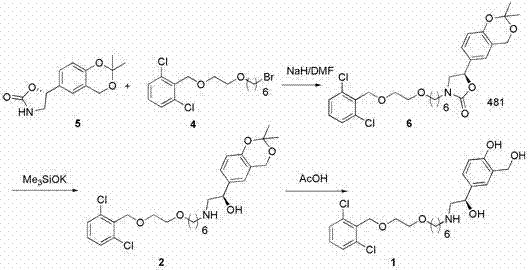
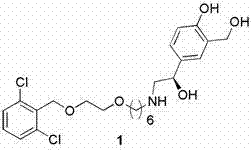
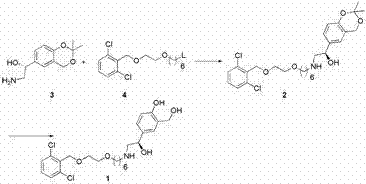
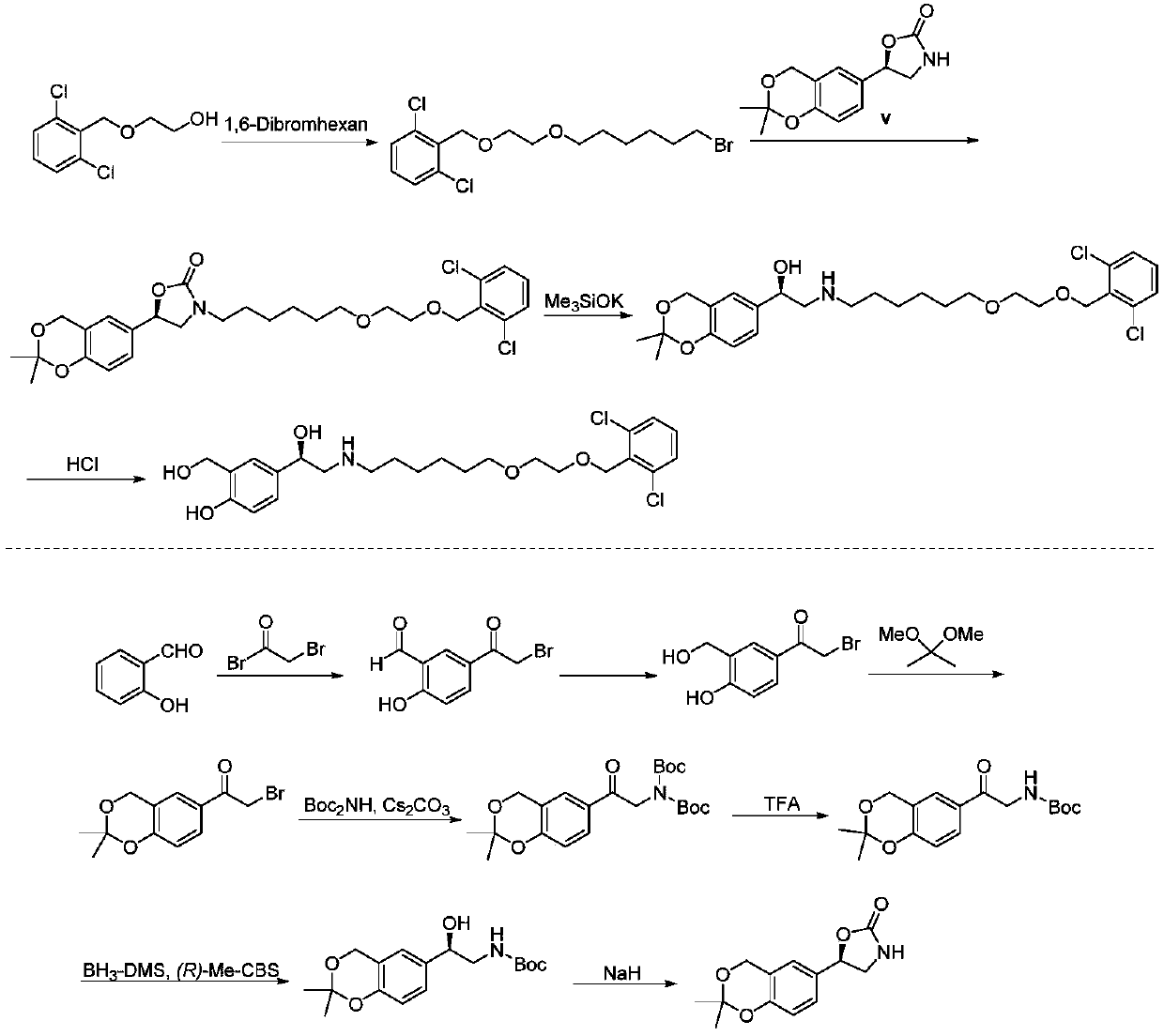
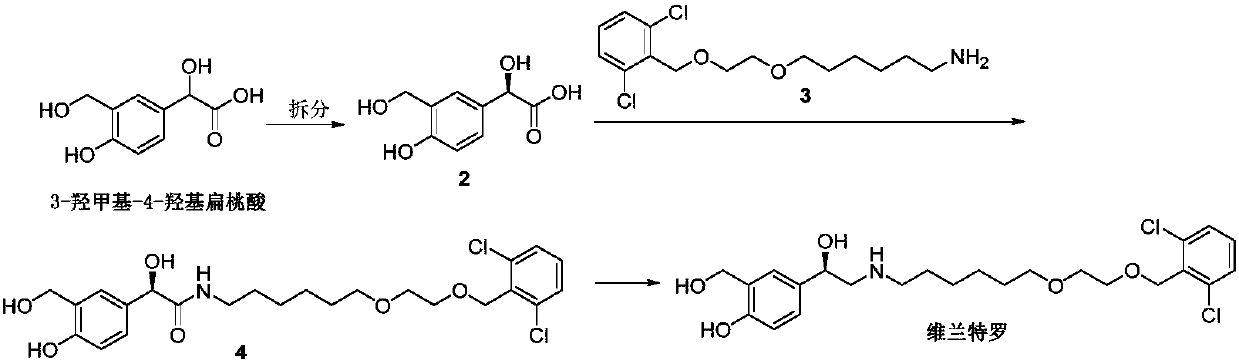
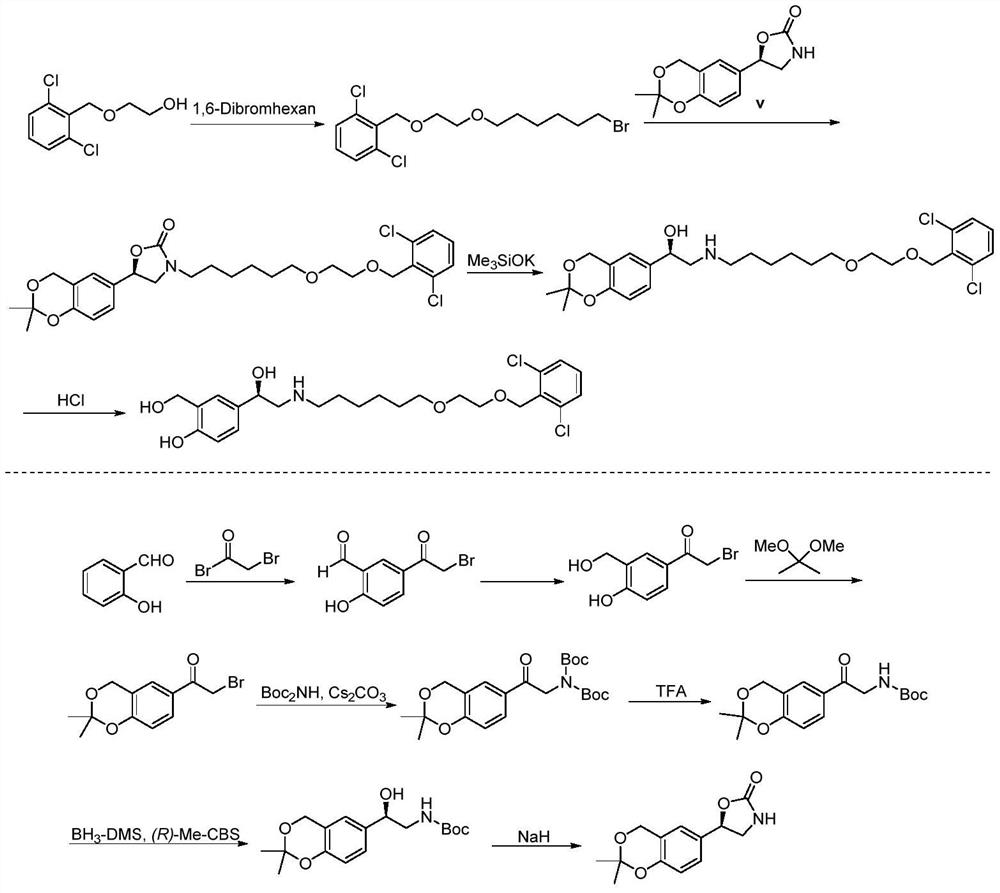
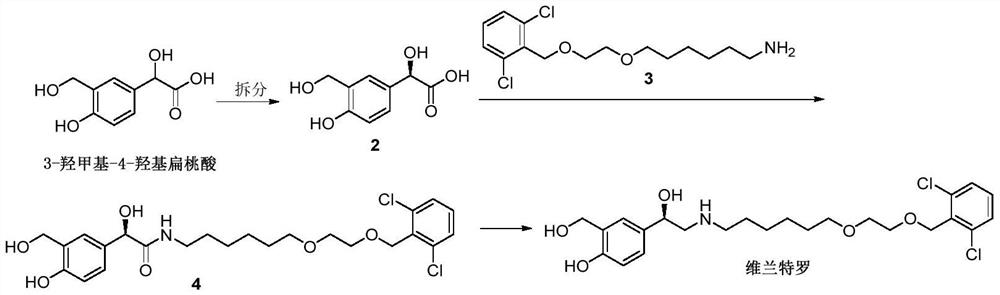
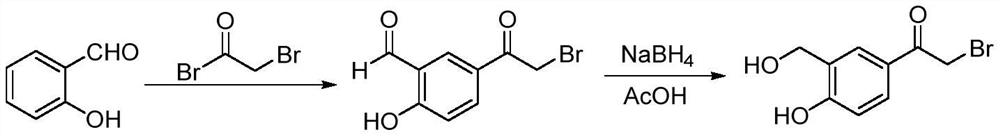
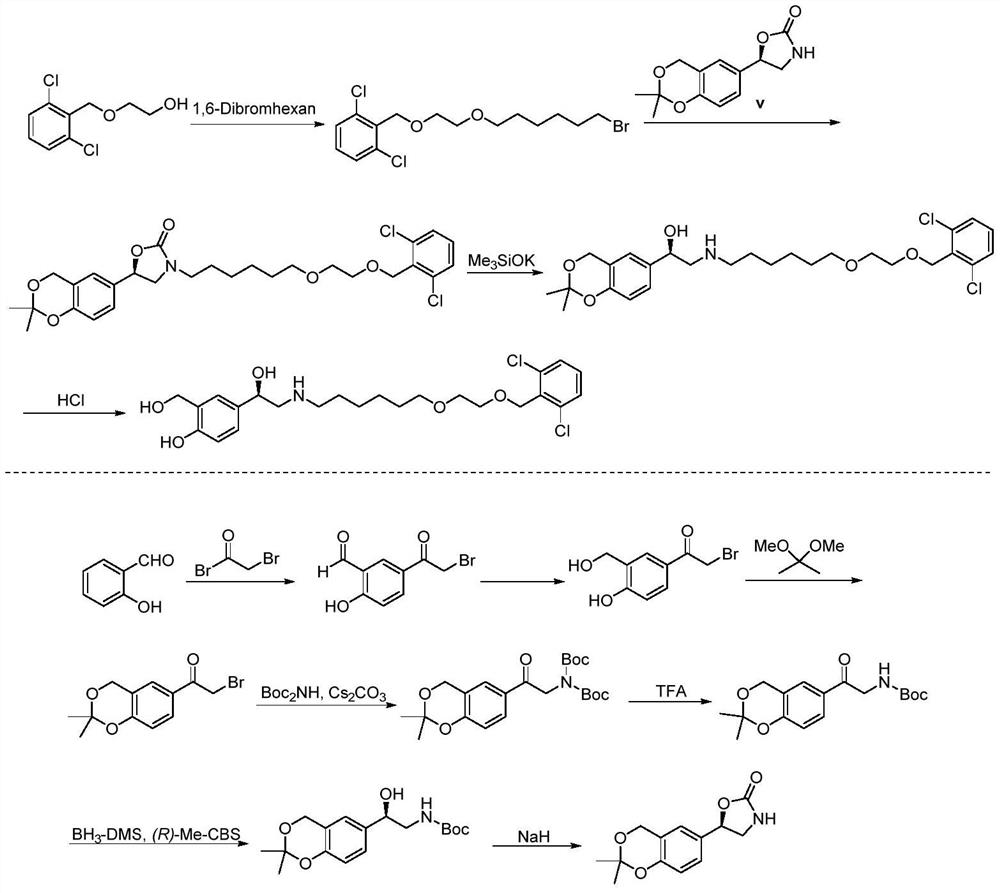
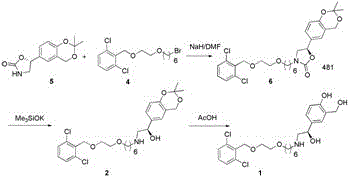
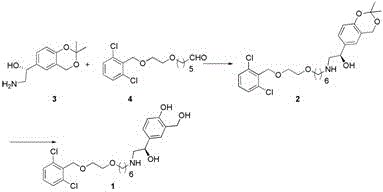
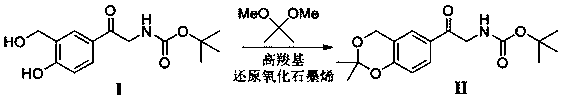
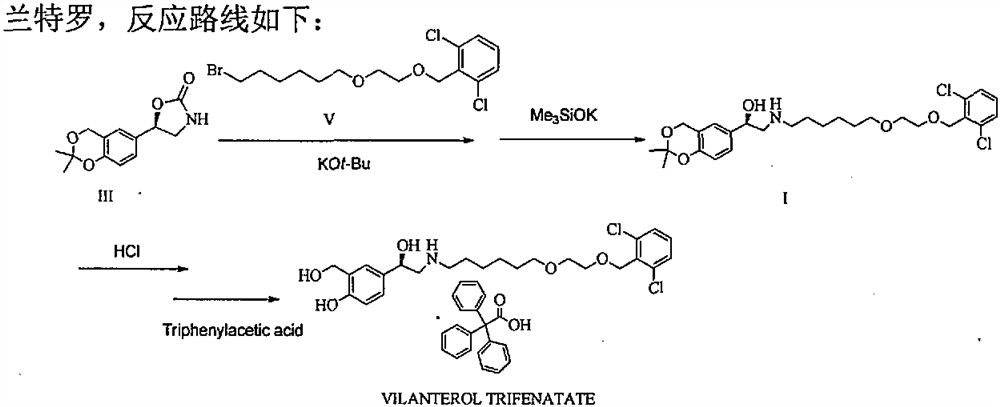
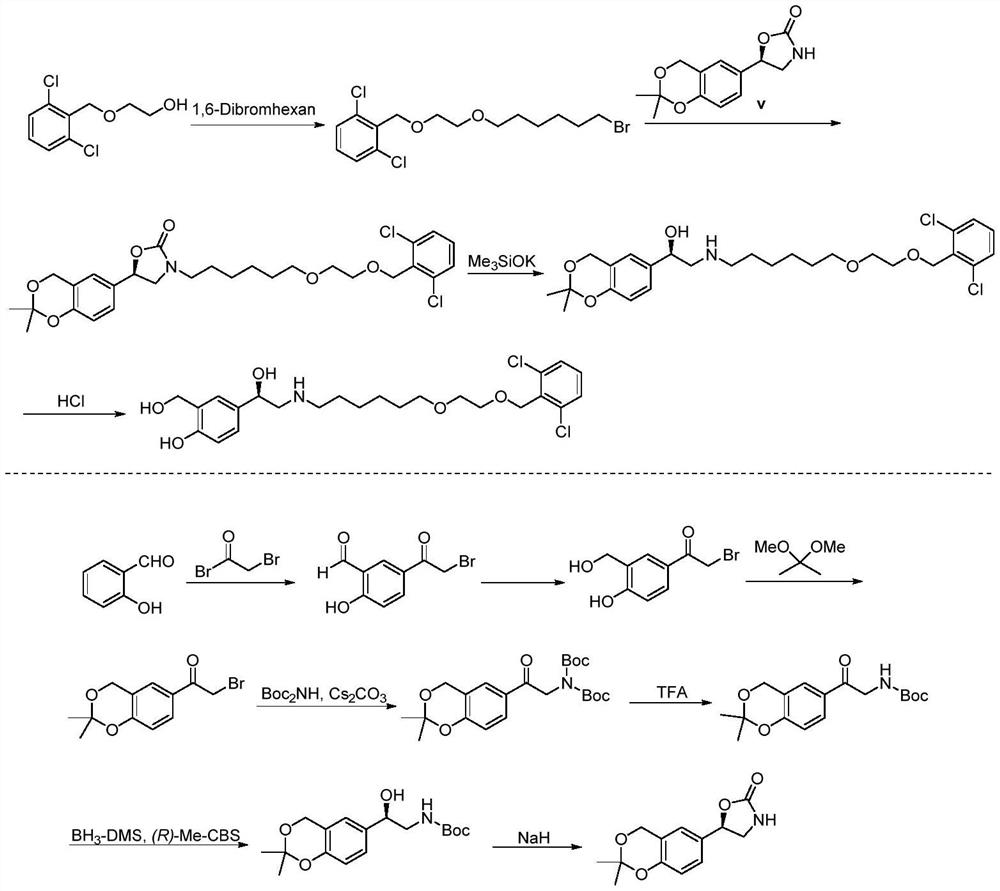
Process for the preparation of vilanterol and intermediates thereof
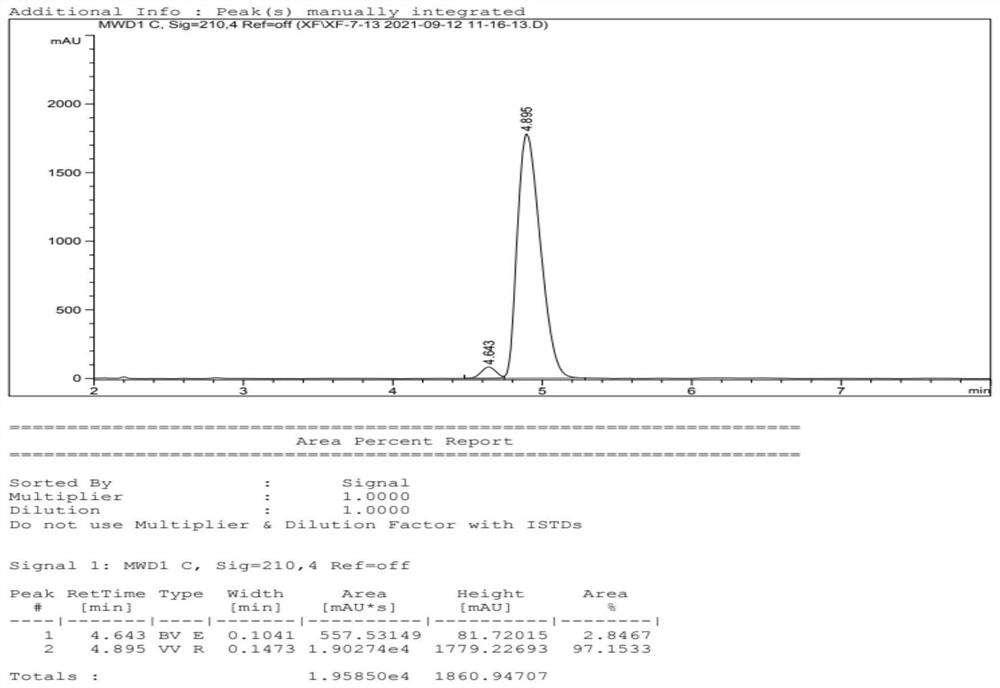
InactiveUS20150239862A1High yieldHigh purityOrganic compound preparationCarboxylic acid salt preparationVilanterolPhotochemistry
An improved process for the preparation of vilanterol and pharmaceutically acceptable salts thereof is disclosed. More specifically the improved process for preparing intermediates for the preparation of vilanterol is disclosed.
Owner:CHEMAGIS
Dry powder inhalation medicine composition and preparation method thereof
InactiveCN105982880AAvoid stimulationImproving In Vitro Assay ParametersPowder deliveryPharmaceutical non-active ingredientsIndacaterolAdditive ingredient
The invention provides a dry powder inhalation medicine composition and a preparation method thereof. The composition is prepared from a coating agent with a specific particle size characteristic, lactose monohydrate with a specific particle size characteristic for a carrier and a micronized medicinal active ingredient, wherein the coating agent is an inhaling magnesium stearate or a mixture of the inhaling magnesium stearate and micronized lactose monohydrate; and the medicinal active ingredient is selected from at least one of glycopyrronium bromide, umeclidinium, indacaterol, formoterol, vilanterol, fluticasone and pharmaceutically available salt of the active ingredients. The preparation method comprises the following steps of sufficiently mixing and coating the coating agent and the lactose monohydrate, and uniformly mixing with the micronized medicinal active ingredients.
Owner:SICHUAN HAISCO PHARMA CO LTD
Vilanterol intermediate, preparation method and application thereof
ActiveCN105646285AHigh yieldReduce yieldCarbamic acid derivatives preparationOrganic compound preparationBenzeneCarboxylic acid
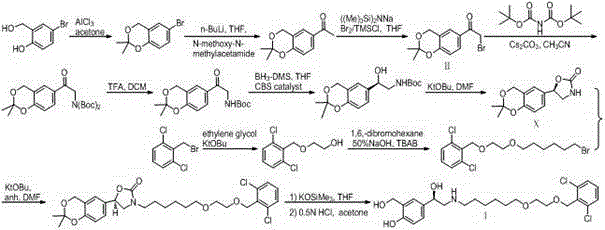
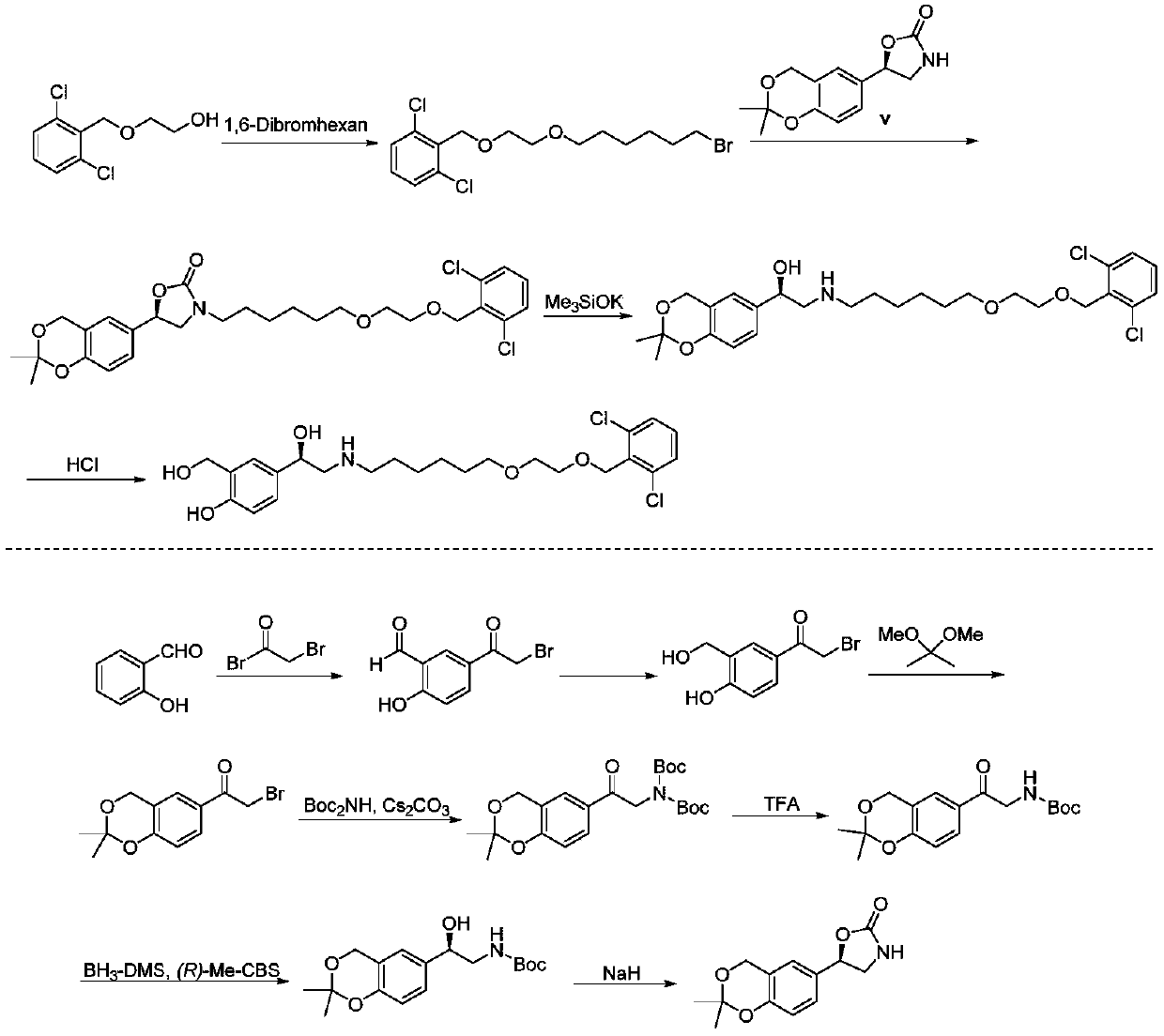
The invention provides a new Vilanterol intermediate compound 2. According to the invention, primary amino is introduced into a compound 5 through Delepine reaction, then di-tert-butyl dicarbonate is employed for amino protection, and the strategy greatly improves the yield and atom utilization rate. On the other hand, cheap and easily available urotropin is adopted in the invention to lower the cost, thus being easy for industrial large-scale production. Compared with the prior art, the yield of the method involved in the invention is increased to 65% by three-step reaction, also use of expensive di-tert-butyl iminodicarboxylate and cesium carbonate is avoided, the cost is reduced, the operation is simple, and the conditions are mild. Therefore, the method is suitable for industrial preparation of Vilanterol and its key intermediate (5R)-5-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-1, 3 oxazolidine-2-one. (formula of compound 2).
Owner:SHANGHAI INST OF PHARMA IND +1
Method for synthesizing vilanterol intermediate and salt thereof
The invention provides a method for preparing a vilanterol intermediate (formula V) and a salt (formula VII) thereof, belonging to the field of chemical drug synthesis. The method comprises the steps of carrying out ring opening reaction on an epoxy compound 2,2-dimethyl-6-ethylene oxide-4H-benzo[d][1,3]dioxane and an amine chiral auxiliary to prepare a chiral compound V; then, separating the compound V from a mixture in a way of forming a crystal salt with an acid. A reagent used in the method provided by the invention is cheap and easily available, and hypertoxic chiral oxazaborolidine is prevented from being used, so that the cost is reduced, and the environment pollution is reduced.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
A kind of vilanterol intermediate and its preparation method and application
ActiveCN105646285BHigh yieldReduce yieldCarbamic acid derivatives preparationOrganic compound preparationHexamethylenetetramineCarboxylic acid
The invention provides a new Vilanterol intermediate compound 2. According to the invention, primary amino is introduced into a compound 5 through Delepine reaction, then di-tert-butyl dicarbonate is employed for amino protection, and the strategy greatly improves the yield and atom utilization rate. On the other hand, cheap and easily available urotropin is adopted in the invention to lower the cost, thus being easy for industrial large-scale production. Compared with the prior art, the yield of the method involved in the invention is increased to 65% by three-step reaction, also use of expensive di-tert-butyl iminodicarboxylate and cesium carbonate is avoided, the cost is reduced, the operation is simple, and the conditions are mild. Therefore, the method is suitable for industrial preparation of Vilanterol and its key intermediate (5R)-5-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-1, 3 oxazolidine-2-one. (formula of compound 2).
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
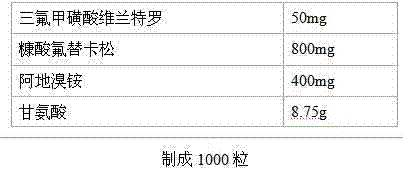
Vilanterol trifluoromethanesulfonate-containing pharmaceutical preparation
InactiveCN103933049AReduce the number of acute exacerbationsImprove lung functionOrganic active ingredientsAntipyreticAnticholinergic DrugsGlucocorticoid
The invention discloses a vilanterol trifluoromethanesulfonate-containing pharmaceutical preparation, which comprises the following main active ingredients: long-acting beta2 receptor agonist vilanterol trifluoromethanesulfonate, glucocorticoid drug fluticasone furoate and long-acting anticholinergic drug aclidinium bromide, or physiologically acceptable salts of the fluticasone furoate and aclidinium bromide, thus composing a compound preparation. The vilanterol trifluoromethanesulfonate has the effect of continuously dilating blood vessels for 24 hours; the fluticasone furoate is fast-acting potent glucocorticoid, and the duration time of the drug is longer than that of other glucocorticoids; the aclidinium bromide is long-acting cholinergic receptor antagonist, and combines with beta2 receptor agonist so as to play roles of synergism and reduction of adverse drug reaction. The vilanterol trifluoromethanesulfonate, the fluticasone furoate and the aclidinium bromide are combined for use to prepare an inhalable compound preparation which can be taken once daily due to 24-hour acting. The vilanterol trifluoromethanesulfonate-containing pharmaceutical preparation can enhance the curative effect, solves the compliance problem of patients with coexisting chronic obstructive pulmonary disease and asthma, thus being appreciable in application prospects.
Owner:SHANGHAI NEW ASIA PHARMA +1
Preparation method of vilanterol and salt thereof
ActiveCN111807973ACost of protectionQuality assuranceOrganic compound preparationAmino-hyroxy compound preparationButanedioic acidCombinatorial chemistry
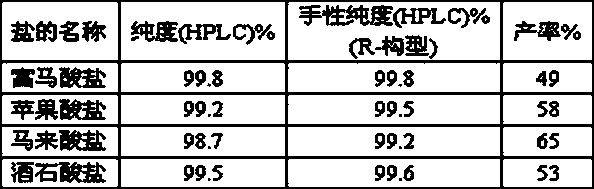
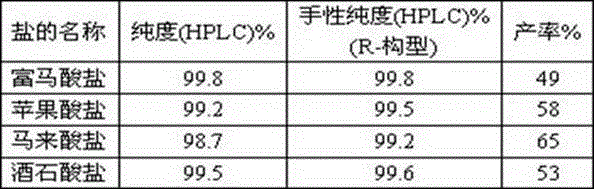
The invention relates to a preparation method of vilanterol and a salt thereof. The preparation method comprises the following steps: in a solvent, reacting a mixture containing a compound shown as aformula I with succinic acid to obtain a compound shown as a formula II-1; and subjecting the obtained compound shown as the formula II-1 to reacting and conversion to obtain vilanterol and the salt thereof. The preparation method of the vilanterol and the salt thereof has the advantages of higher yield, high purity, easiness in refining, simplicity and convenience in operation and suitability forindustrialization.
Owner:SHANGHAI ANOVENT PHARMA CO LTD
Pulse-controlled release tablet containing fluticasone furoate and vilanterol and preparation method thereof
InactiveCN105796522BSolve the problem of not being able to float and suspend in the stomach contentsAvoid untimely release drugsOrganic active ingredientsRespiratory disorderGastric fluidDissolution
The invention provides a pulsatile controlled-release tablet containing fluticasone furoate and vilanterol. The pulsatile controlled-release tablet can reach plasma concentration at midnight and early morning, so the purpose of chrono-chemotherapy is achieved. The pulsatile controlled-release tablet comprises a floating layer and a tablet core. The floating layer uses hydrophilic high molecules and fatty alcohol as adjuvant materials, so the floating layer can retain for a certain period of time in the stomach; when the tablet contacts with gastric juice, the surface of the floating layer hydrates to form gel, so the size of the tablet becomes larger; when the gel is smaller than the concentration of the gastric juice, the tablet is allowed to float above contents in the stomach; and the tablet core appears along with dissolution of the skeleton of the floating layer, so drugs are released from the tablet core.
Owner:HYBIO PHARMA
Preparation method of vilanterol
ActiveCN111377822AFew synthetic stepsRaw materials are easy to getOrganic compound preparationBulk chemical productionCompound aPtru catalyst
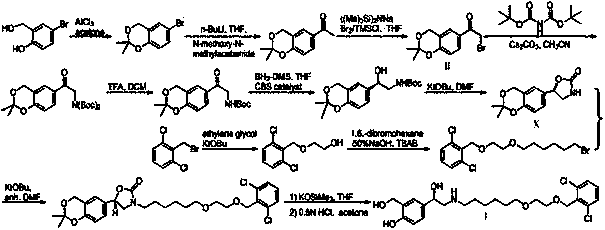
The invention provides a preparation method of vilanterol, which comprises the following steps: 1) oxidation reaction: reacting a compound A with an oxidant to obtain a compound B; wherein the oxidantis selenium dioxide; 2) reductive amination reaction: carrying out condensation reaction on the compound B and a compound C to generate an imine intermediate, and carrying out a reaction on the imineintermediate under the action of a reducing agent to obtain a compound D; 3) reduction reaction: carrying out a reaction on the compound D with a chiral catalyst and a reducing agent to obtain a compound E, and 4) ring-opening reaction: performing deprotection ring-opening on the compound E under an acidic condition to obtain vilanterol. The method is advantaged in that the initial raw materialsare easy to obtain, the process is suitable for industrial production, the production yield is high and quality is stable.
Owner:TIANJIN PHARMA GROUP CORP
New synthesis method of long-acting beta2 receptor agonist vilanterol
InactiveCN104744270AMild reaction conditionsRaw materials are easy to getOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsAgonist
The present invention discloses a new synthesis method of a long-acting beta2 receptor agonist vilanterol, and provides a completely-new synthesis method of a long-acting beta2 receptor agonist vilanterol. According to the method, the idea that the Schiff base is firstly produced and then reduction is performed is adopted to effectively avoid disadvantages of harsh reaction conditions (such as anhydrous reaction), expensive reagents and the like of the existing process, and the method has characteristics of easily available raw materials, short route, high yield, and market competitiveness.
Owner:成都伊诺达博医药科技有限公司
Preparation method of vilanterol intermediate
ActiveCN109678701ARaw materials are easy to getMild reaction conditionsOrganic compound preparationCarboxylic compound preparationFormylation reactionDrugs synthesis
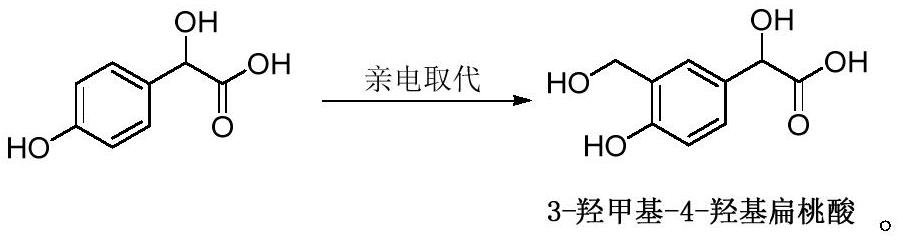
The invention discloses a preparation method of a vilanterol intermediate and belongs to the field of drug synthesis. The method is characterized in that 4-hydroxy-mandelic acid is taken as a raw material and is subjected to formylation reaction to obtain 4-hydroxy-3-formyl mandelic acid; an aldehyde group is reduced to obtain 3-hydroxymethyl-4-hydroxyphenylglycolic acid. The invention provides anovel intermediate of vilanterol; the raw material of the intermediate is easy to obtain; synthesis reaction conditions are mild and the operation is simple; in addition, by using the intermediate tosynthesize the vilanterol, a synthetic route is greatly shortened, and the production cost is reduced; the preparation method is suitable for industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
Method for preparing Vilanterol
ActiveCN109574860AReduce usageIncrease profitOrganic compound preparationCarboxylic acid amides preparationGlyoxylic acidDrugs synthesis
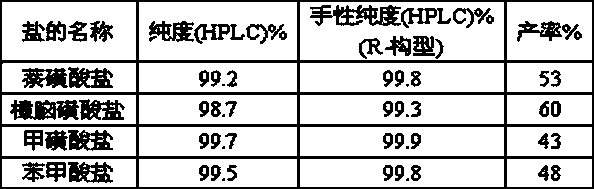
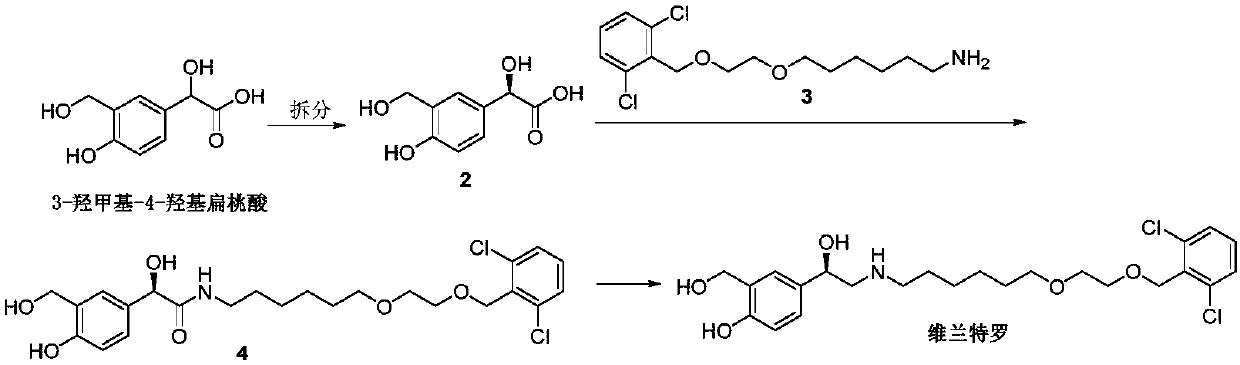
The invention discloses a method for preparing Vilanterol, and belongs to the field of drug synthesis. The method includes the steps: (1) performing addition reaction on salicyloyl and glyoxylic acidto generate an intermediate 1; (2) performing chiral resolution on the intermediate 1 to obtain a chiral intermediate 2; (3) performing acylation reaction on the intermediate 2 and an intermediate 3 to generate an intermediate 4; (4) reducing the intermediate 4 to obtain the Vilanterol. The intermediate 3 is obtained by performing Darebin reaction on an intermediate 5. Raw materials are easily obtained, a synthetic route is short, production cost is reduced, reaction conditions are mild, and the method is simple in operation and suitable for industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
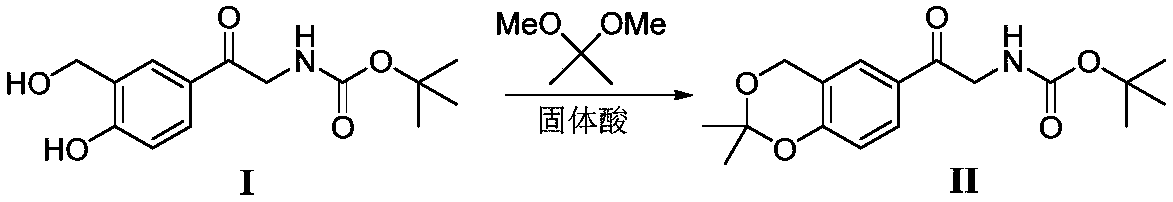
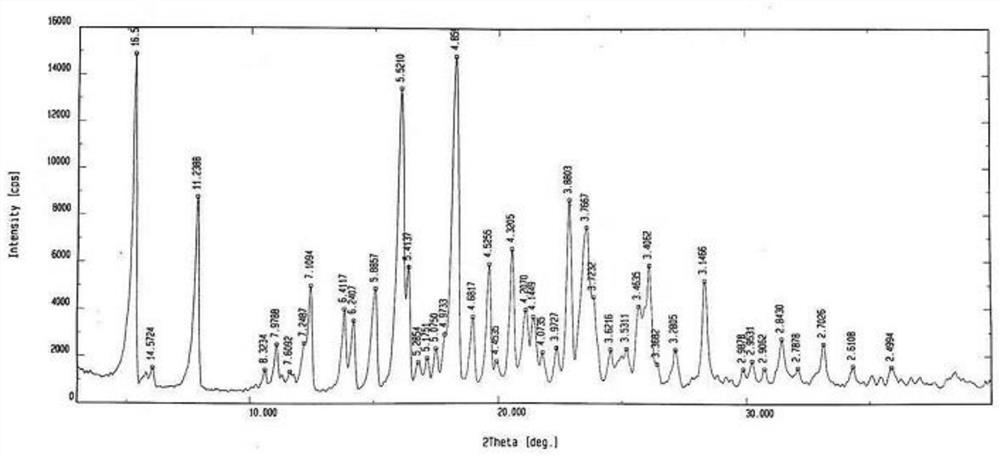
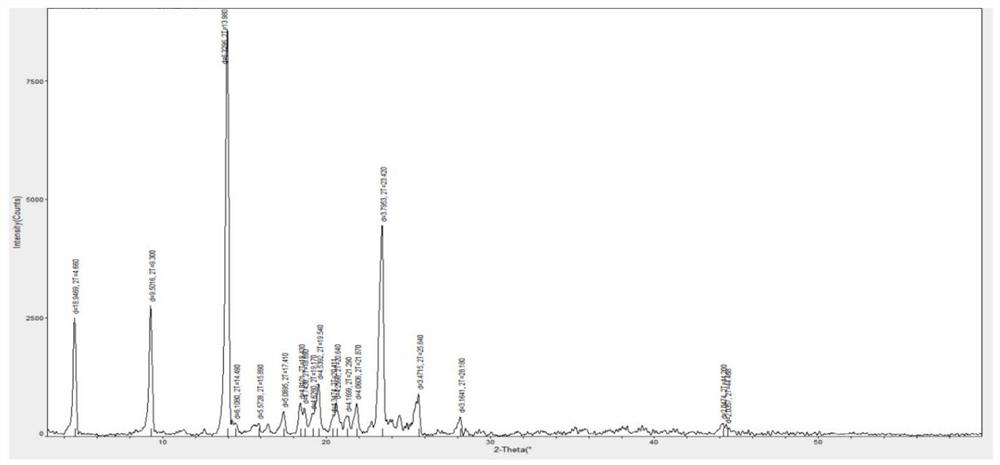
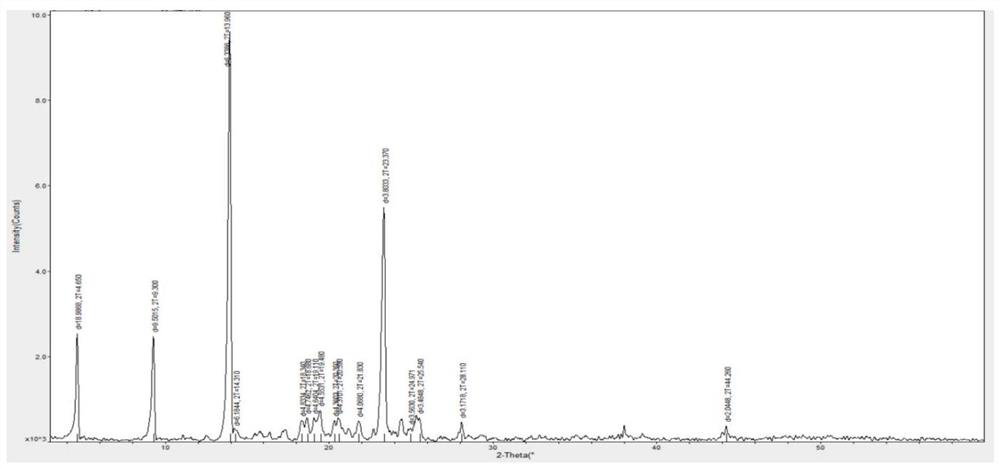
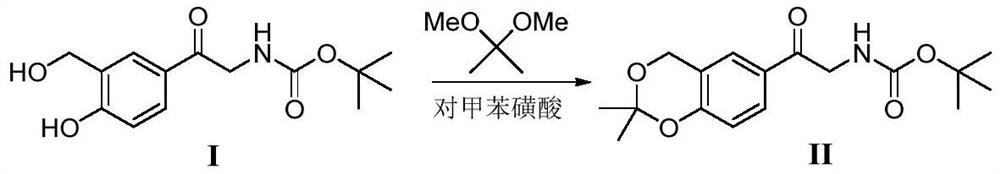
Method for synthesizing Vilanterol midbody by virtue of solid acid catalysis
ActiveCN109574982ARecyclingSolve the disadvantages of strong organic acidsOrganic chemistryChemical recyclingState of artBenzene
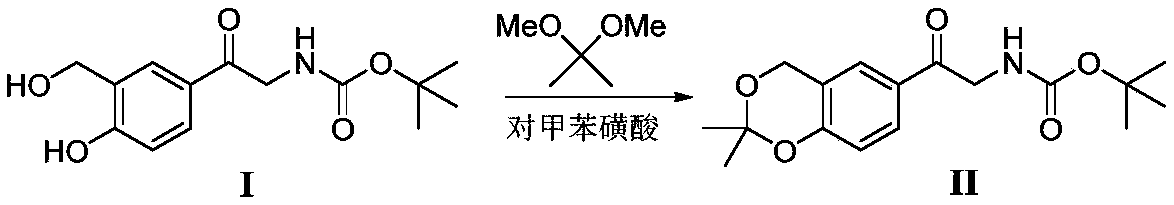
The invention discloses a method for synthesizing Vilanterol midbody 2-(2,2-dimethyl-4H-1,3-benzodiazepine-6-group)-2-carbonyl ethyl carbamate tert-butyl ester (II) by virtue of solid acid catalysis,which belongs to the technical field of organic catalysis. According to the method, the solid acid is used to catalyze the synthesis of Vilanterol midbody (II),so that the defect in the prior art by adopting organic strong acid can be solved, the recycling of the catalyst is realized, and the pressure of environmental protection can be alleviated. The solid acid catalyzed synthesis route of the invention is environment-friendly and meets the requirements of modern pollution-free industrialized production.
Owner:ANHUI DEXINJIA BIOPHARM
Vilanterol intermediate as well as preparation method and application thereof
PendingCN113121492AImprove quality controlQuality assuranceOrganic compound preparationOrganic chemistry methodsChemical synthesisChemical compound
The invention provides a vilanterol intermediate as well as a preparation method and application thereof, and relates to the technical field of chemical synthesis. According to the vilanterol intermediate, the compound shown in the formula I is salt of the compound shown in the formula II, the solid compound shown in the formula I is obtained from the oily compound shown in the formula II, the purity reaches up to 99.0% or above, when the compound shown in the formula I is used for preparing vilanterol and pharmaceutically acceptable salt thereof, purification through tedious separation methods such as column chromatography is not needed, materials are easy to measure accurately, the quality control of the vilanterol intermediate is facilitated, various defects caused by the fact that the compound shown in the formula II is an oily substance are effectively overcome, and the quality of vilanterol and pharmaceutically acceptable salt thereof can be better guaranteed.
Owner:TIANJIN PHARMA GROUP CORP
A kind of preparation method of vilanterol intermediate
ActiveCN109678701BRaw materials are easy to getMild reaction conditionsOrganic compound preparationCarboxylic compound preparationFormylation reactionBiochemical engineering
The invention discloses a preparation method of a vilanterol intermediate, belonging to the field of drug synthesis. The method uses 4-hydroxymandelic acid as a raw material to obtain 4-hydroxy-3-formylmandelic acid through formylation reaction, and then reduces the aldehyde group to obtain 3-hydroxymethyl-4-hydroxymandelic acid. The invention provides a new intermediate of vilanterol, the intermediate raw material is easy to get, the synthesis reaction conditions are mild, and the operation is simple, and the synthesis of vilanterol by the intermediate greatly shortens the synthetic route and reduces the production cost. cost, suitable for industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
A kind of method for synthesizing vilanterol intermediate in mixed solvent
ActiveCN109574817BHigh selectivityHigh yieldOrganic compound preparationCarbonyl compound preparationDrugs synthesisKetone
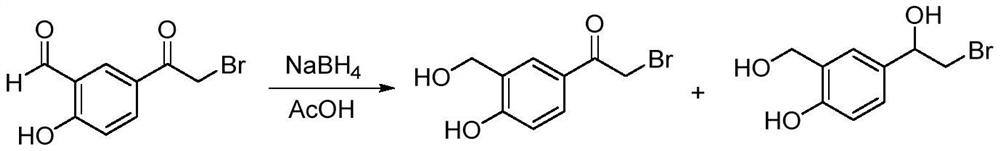
The invention discloses a method for synthesizing an intermediate of vilanterol in a mixed solvent, belonging to the field of drug synthesis. The method obtains 2-bromo-1-[4-hydroxy-3-(hydroxymethyl)phenyl]ethan-1-ketone by reducing 5-bromoacetyl-2-hydroxybenzaldehyde with sodium borohydride. The invention reduces in a mixed solvent, improves the selectivity and yield of the reaction, has simple aftertreatment, and is suitable for industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
Pulsatile controlled-release tablet containing fluticasone furoate and vilanterol and preparation method thereof
InactiveCN105796522ASolve the problem of not being able to float and suspend in the stomach contentsAvoid untimely release drugsOrganic active ingredientsRespiratory disorderDissolutionControlled Release Tablet
The invention provides a pulsatile controlled-release tablet containing fluticasone furoate and vilanterol. The pulsatile controlled-release tablet can reach plasma concentration at midnight and early morning, so the purpose of chrono-chemotherapy is achieved. The pulsatile controlled-release tablet comprises a floating layer and a tablet core. The floating layer uses hydrophilic high molecules and fatty alcohol as adjuvant materials, so the floating layer can retain for a certain period of time in the stomach; when the tablet contacts with gastric juice, the surface of the floating layer hydrates to form gel, so the size of the tablet becomes larger; when the gel is smaller than the concentration of the gastric juice, the tablet is allowed to float above contents in the stomach; and the tablet core appears along with dissolution of the skeleton of the floating layer, so drugs are released from the tablet core.
Owner:HYBIO PHARMA
A kind of synthetic method of vilanterol intermediate
ActiveCN109665959BRaw materials are easy to getMild reaction conditionsOrganic compound preparationCarboxylic compound preparationBiochemical engineeringDrugs synthesis
Owner:安徽德信佳济大新药技术有限公司
A new process for synthesizing vilanterol
ActiveCN104744271BOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsVilanterol
The present invention discloses a new method for synthesizing vilanterol. The present invention provides a completely-new vilanterol synthesis method, wherein the easily available starting raw material is adopted so as to effectively avoid disadvantages of harsh reaction conditions (such as anhydrous reaction), expensive reagents and the like of the existing process, and the method has characteristics of short route, high yield, and market competitiveness.
Owner:成都伊诺达博医药科技有限公司
Preparation method for synthesizing vilanterol intermediate by catalysis with modified graphene
PendingCN110423233AResolve heterogeneitySolve difficult to recyclePhysical/chemical process catalystsOrganic chemistrySynthesis methodsStrong acids
The invention discloses a method for synthesizing a vilanterol intermediate tert-butyl 2-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)-2-carbonylethylcarbamate by catalysis with modified reduced graphene oxide with a high carboxylation edge, and belongs to the technical field of organic catalysis. The method utilizes an acidic catalytic reaction of the relatively large amount of carboxyl groups at the edge of reduced graphene oxide to overcome the disadvantage that an adopted organic strong acid is difficult to recover in the prior art, solve the problem of heterogeneous phase and difficult recyclingapplication of a catalyst, and reduce environmental protection pressure. The catalytic synthesis method adopting graphene is environmentally friendly, and meets requirements of modern green industrial production.
Owner:UNIV OF JINAN
Method for synthesizing precursor of vilanterol intermediate
PendingCN114133373ALow priceFew reaction stepsOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsPtru catalystMethyl benzene
The invention relates to a method for synthesizing (1R)-1-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-2-nitroethanol, and the route of the method is as follows: the (1R)-1-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-2-nitroethanol synthesized by adopting the synthesis method is applied to preparation of (5R)-5-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-1, 2, 4-triazole-3-yl)-2-nitroethanol, and the (1R)-1-(2, 2-dimethyl-4H-1, 3-benzodioxin-6-yl)-2-nitroethanol is used as a raw material. The method can be used for preparing 2, 3-oxazolidine-2-ketone, reaction steps can be shortened, and the method has the advantages of being high in reaction conversion number, high in atom economy, suitable for industrial production and the like. Compared with the currently reported asymmetric Henry reaction synthesis route, the method has the advantages that the price of the catalyst required by the synthesis route is low, the molar yield of the alpha-nitroketone reduction step is greater than 85%, and the method has the characteristics of high asymmetric selectivity and easiness in production.
Owner:RAFFLES PHAMRMATECH CO LTD
A kind of method of synthesizing vilanterol intermediate and salt thereof
The invention provides a method for preparing vilanterol intermediate formula V and its salt formula VII, and belongs to the field of pharmaceutical chemical synthesis. The method utilizes epoxy compound 2,2-dimethyl-6-oxirane-4H-benzo[d][1,3]dioxane to undergo ring-opening reaction with amine chiral auxiliary agent to prepare chiral Compound V, which is then isolated from the mixture by forming a crystalline salt with an acid. The reagents used in the method of the invention are cheap and easy to obtain, avoid using highly toxic chiral oxazoboridine as a catalyst, reduce cost and reduce environmental pollution.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
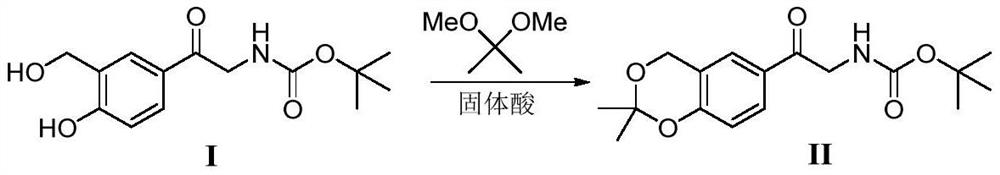
A kind of method of solid acid catalysis synthetic vilanterol intermediate
ActiveCN109574982BRecyclingSolve the disadvantages of strong organic acidsOrganic chemistryChemical recyclingBenzodiazepinePtru catalyst
The invention discloses a method for synthesizing Vilanterol midbody 2-(2,2-dimethyl-4H-1,3-benzodiazepine-6-group)-2-carbonyl ethyl carbamate tert-butyl ester (II) by virtue of solid acid catalysis,which belongs to the technical field of organic catalysis. According to the method, the solid acid is used to catalyze the synthesis of Vilanterol midbody (II),so that the defect in the prior art by adopting organic strong acid can be solved, the recycling of the catalyst is realized, and the pressure of environmental protection can be alleviated. The solid acid catalyzed synthesis route of the invention is environment-friendly and meets the requirements of modern pollution-free industrialized production.
Owner:ANHUI DEXINJIA BIOPHARM
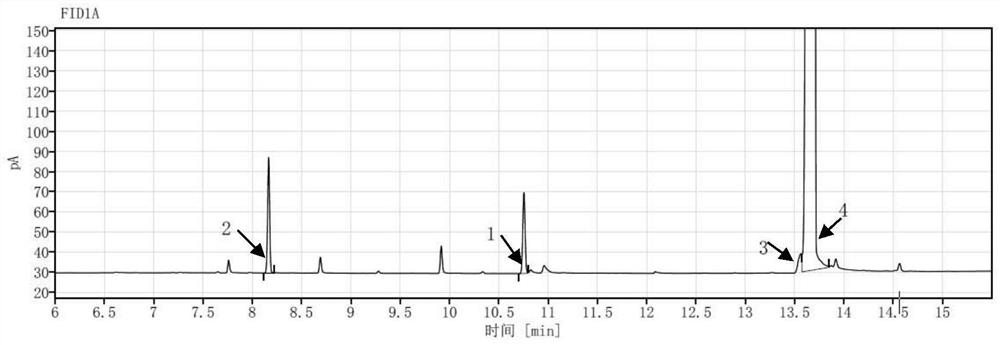
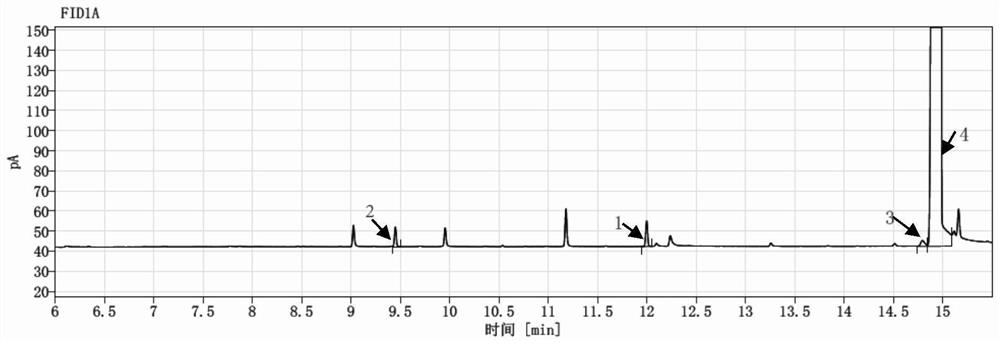
Method for simultaneously detecting various gene impurities of vilanterol triphenylacetate
PendingCN114441677AGuarantee product qualityEnsure medication safetyComponent separationTriphenylacetic acidGas liquid chromatographic
The invention discloses a method for detecting a genotoxic impurity containing bromohexyl in vilanterol triphenylacetate. The genotoxic impurity is selected from VL02imp4 of a structural formula I, VL02imp6 of a structural formula II and / or VL02imp8 of a structural formula III. The method is based on a gas chromatography-flame ionization detector, and the chromatographic conditions are as follows: 5% diphenyl-95% dimethyl polysiloxane is used as a capillary chromatographic column of a stationary liquid; the initial temperature is 35-45 DEG C, the temperature is increased to 315-325 DEG C at the speed of 13-18 DEG C / min, and the temperature is maintained The carrier gas is nitrogen, the flow velocity is 0.8-1.2 ml / min, the constant flow mode is adopted, and the split ratio is (8: 1)-(12: 1); the sample introduction temperature is 250 DEG C; the sample size is 1 microliter; the detection temperature is 315-325 DEG C, the flow rate of nitrogen is 25ml / min, the flow rate of hydrogen is 40ml / min, and the flow rate of air is 400ml / min.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
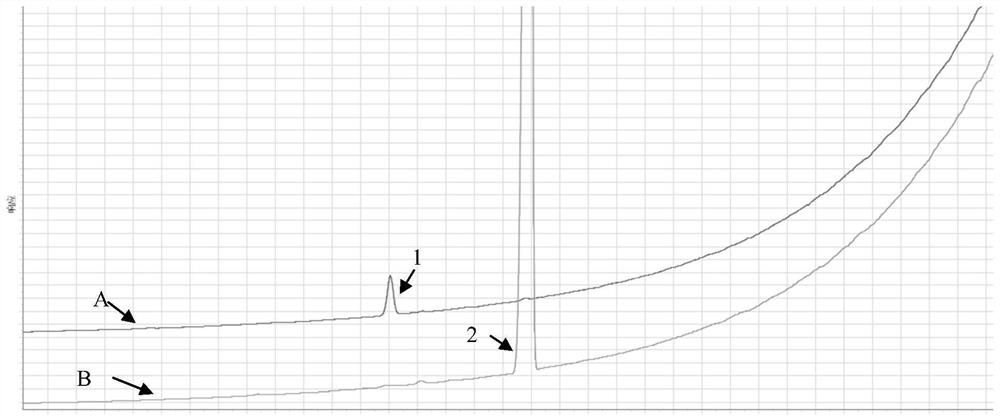
Preparation method and application of vilanterol EP impurity 2
PendingCN114315787AQuality improvementRaw materials are easy to getOrganic chemistryPreparing sample for investigationGlycineChemical compound
The invention discloses a preparation method and application of a vilanterol EP impurity 2, and the preparation method comprises the following steps: dissolving a compound with a structure as shown in a formula I in a first organic solvent, adding acid and a dewatering agent, and reacting with 2, 2-dimethoxypropane under a heating condition to obtain a compound with a structure as shown in a formula II; dissolving the compound with the structure as shown in the formula II in a second organic solvent, adding N-t-butyloxycarboryl-glycine-N '-methoxyl-N'-methyl amide, adding n-butyllithium at low temperature, and reacting for 1 hour to obtain a compound with the structure as shown in the formula III, namely the vilanterol EP impurity 2. The method has the advantages of easily available raw materials, few steps, high yield and low cost, and fills the technical blank of preparation of the vilanterol EP impurity 2. A cheap and high-quality impurity reference substance can be provided for quality research of vilanterol, and the method has important significance on safe medication of vilanterol.
Owner:广州佳途科技股份有限公司
Application of Vilanterol in preparation of anti-coronavirus medicine and medicine
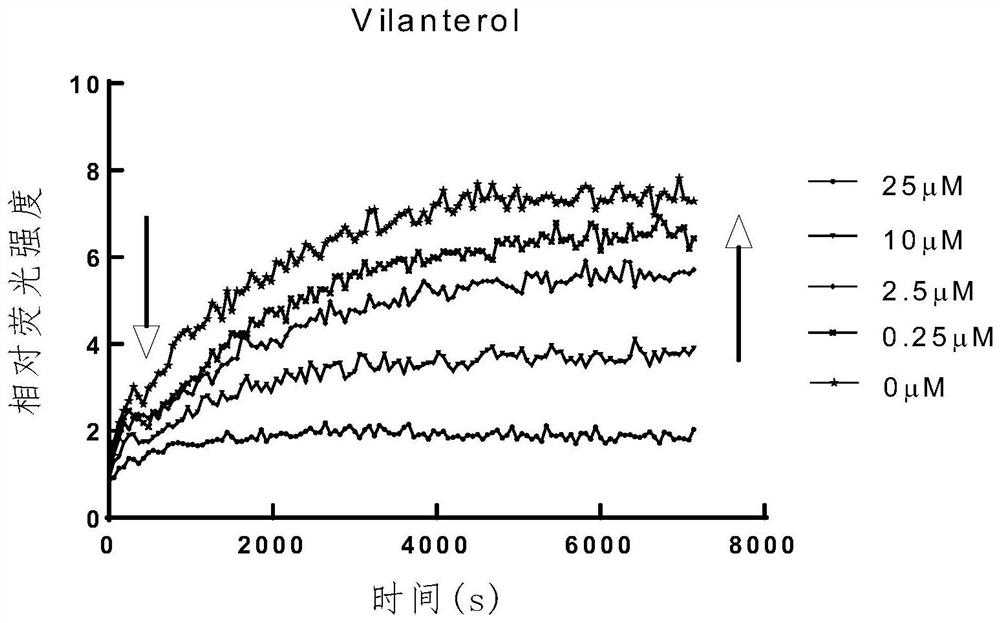
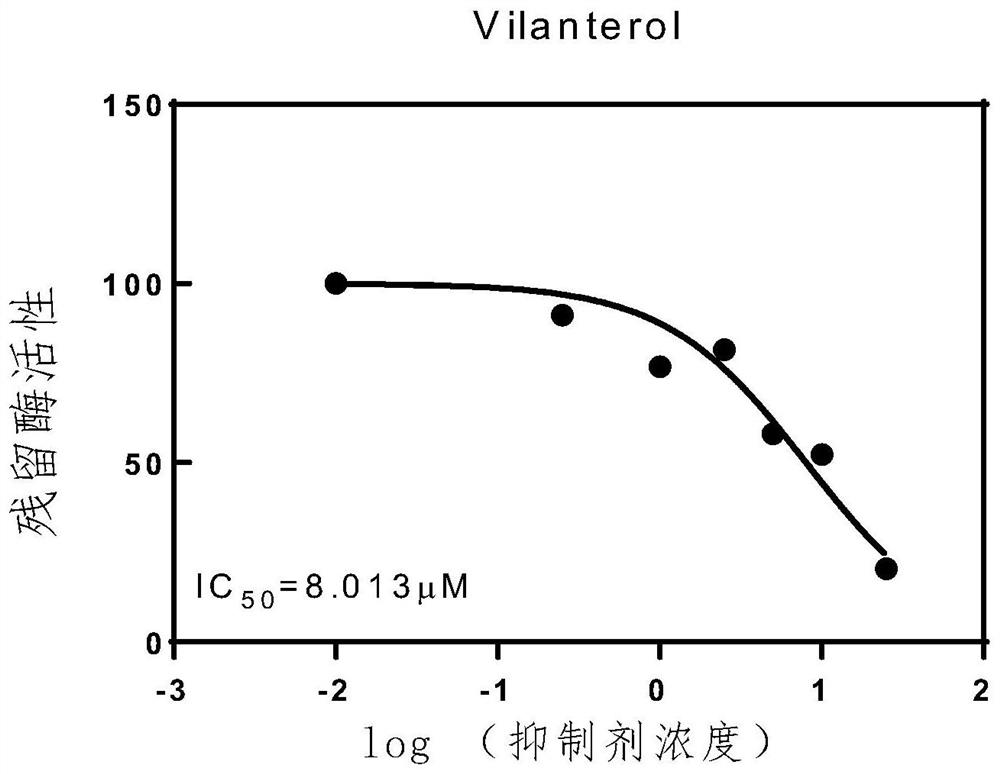
The invention belongs to the technical field of medicinal chemistry, and particularly relates to application of Vilanterol in preparation of a medicament for resisting coronavirus infection. According to the application, a new function of the Vilanterol is found, and the Vilanterol has a relatively strong inhibition effect on the enzymatic activity of target papaya-like protease Plpro of the coronavirus, especially novel coronavirus, and has the potential of being developed into a medicine for treating and preventing the coronavirus, especially resisting the novel coronavirus.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of vilanterol and salt thereof
ActiveCN111807973BCost of protectionQuality assuranceOrganic compound preparationAmino-hyroxy compound preparationButanedioic acidCombinatorial chemistry
The present invention relates to the preparation method of vilanterol and salt thereof, it comprises the following steps: in solvent, the mixture containing the compound of formula I is reacted with succinic acid to prepare the compound of formula II-1; The reaction transforms vilanterol and its salts. The preparation method of vilanterol and its salt of the present invention has higher yield, high purity, easy refining and simple operation, and is suitable for industrialization.
Owner:SHANGHAI ANOVENT PHARMA CO LTD
A kind of method for preparing vilanterol
ActiveCN109574860BReduce usageIncrease profitOrganic compound preparationCarboxylic acid amides preparationGlyoxylic acidDrugs synthesis
The invention discloses a method for preparing vilanterol, which belongs to the field of drug synthesis. The method comprises the following steps: (1) addition reaction of salicyl alcohol and glyoxylic acid to generate intermediate 1; (2) intermediate 1 undergoes chiral resolution to obtain chiral intermediate 2; (3) intermediate 2 and intermediate Intermediate 3 undergoes acylation reaction to generate intermediate 4, wherein intermediate 3 is obtained by Delbin reaction of intermediate 5; (4) intermediate 4 is reduced to obtain vilanterol. The invention has easy-to-obtain raw materials, short synthesis route, reduced production cost, mild reaction conditions, simple operation and is suitable for industrialized production.
Owner:ANHUI DEXINJIA BIOPHARM
Synthesizing method of vilanterol intermediate
ActiveCN109665959ARaw materials are easy to getMild reaction conditionsOrganic compound preparationCarboxylic compound preparation4-hydroxymandelic acidVilanterol
The invention discloses a synthesizing method of a vilanterol intermediate and belongs to the field of drug synthesis. According to the synthesizing method of the vilanterol intermediate, 4-hydroxymandelic acid serving as the raw material is prepared through electrophilic substitution reaction into 3-hydroxymethyl-4-hydroxymandelic acid. The prepared new vilanterol intermediate is simple in raw material acquisition and operation, and when applied to synthesizing vilanterol, can greatly shortens the synthetic route and reduce the production cost, thereby being applicable to industrial production.
Owner:安徽德信佳济大新药技术有限公司
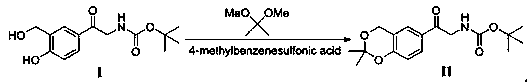
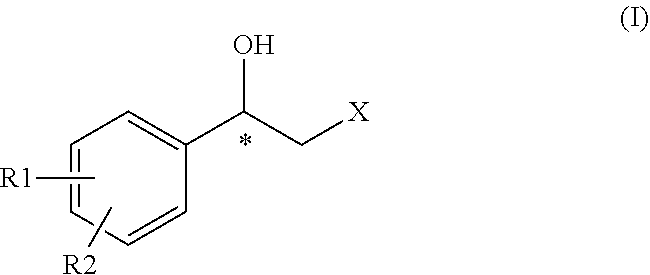
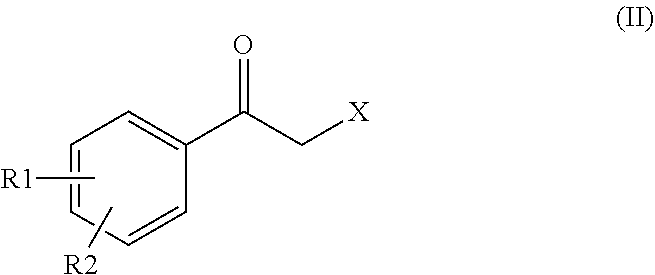
Process for preparing intermediates for the synthesis of optically active beta-amino alcohols by enzymatic reduction and novel synthesis intermediates
ActiveUS11053227B2High yieldHigh enantiomeric excessesOrganic chemistry methodsCarboxylic acid amides optical isomer preparationCombinatorial chemistryKetone
Subject-matter of the present invention is a process for preparing intermediates for the synthesis of optically active beta-amino alcohols by enzymatic reduction of the corresponding beta-amino ketones. Subject-matter of the invention are also said novel synthesis intermediates and the use thereof in the preparation of active pharmaceutical ingredients, among which vilanterol and the salts thereof.
Owner:OLON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com