Preparation method and application of vilanterol EP impurity 2
An impurity and reaction technology, which is applied in the field of preparation of vilanterol impurities, can solve problems such as the inability to provide a basis for quality control of vilanterol, and achieve the effects of high yield, few steps and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
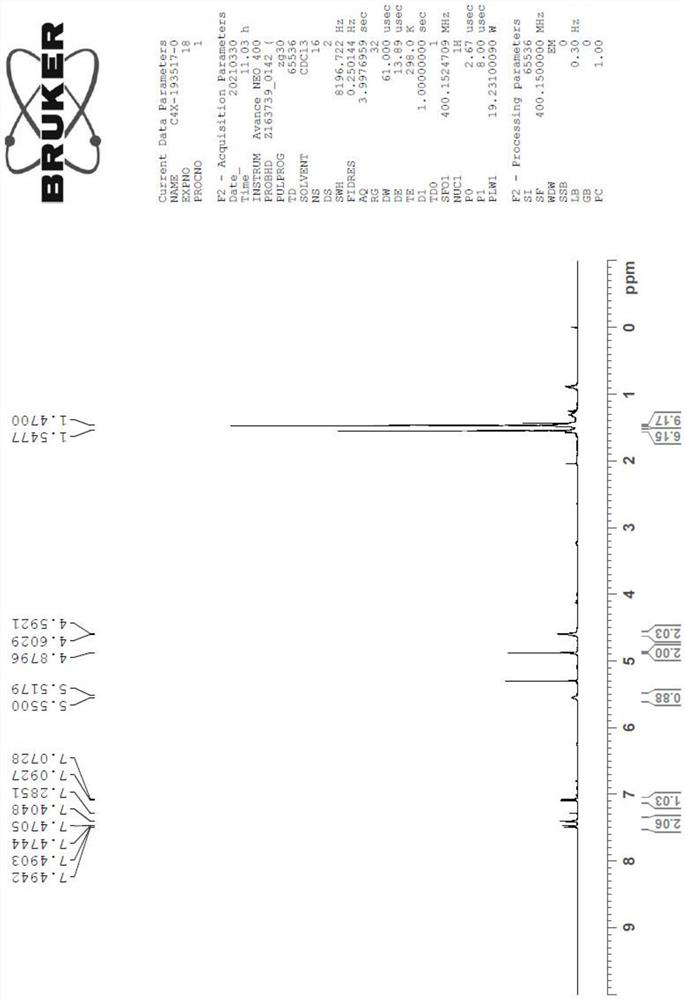
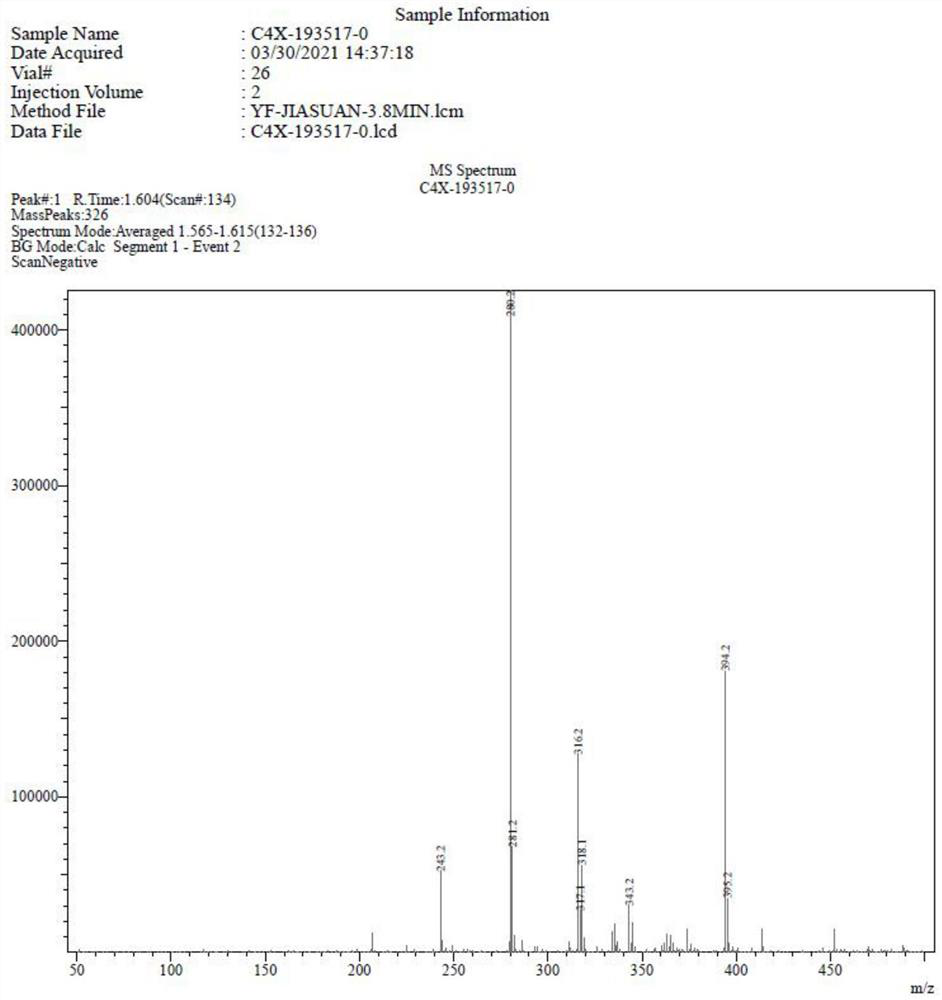
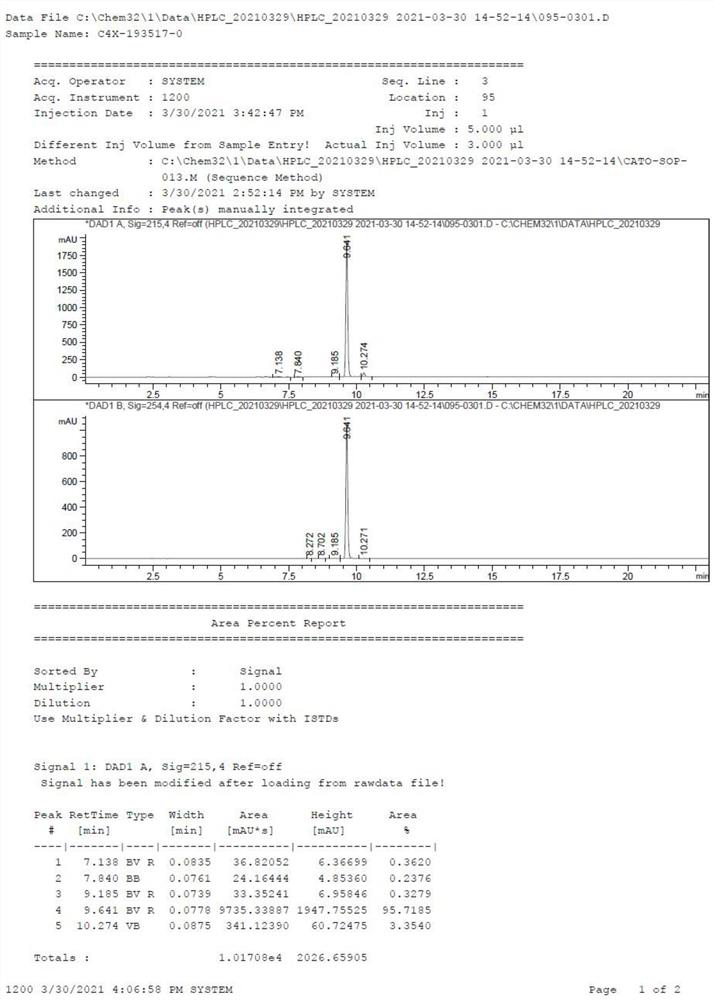
Image
Examples
Embodiment 1
[0062] Get 1 equivalent of the structural compound shown in formula I, dissolve in the acetonitrile of 10 times the volume of the structural compound shown in the formula I, the concentration of the structural compound shown in the formula I is 0.05~0.7 mol / liter, add p-toluenesulfonic acid 0.1 equivalent, without 2 equivalents of sodium sulfate in water, 1.5 equivalents of 2,2-dimethoxypropane, heated to 75°C under stirring, reacted for 1 hour, monitored the completion of the reaction, terminated the reaction, rotary evaporated the organic solvent, purified by column chromatography, mobile phase Petroleum ether: ethyl acetate = 1:0.3 to obtain white crystals (yield 90%), which is the compound of formula II.
[0063] Take 1 equivalent of the structural compound shown in formula II, dissolve it in a tetrahydrofuran solution with 10 times the volume of the structural compound shown in formula II, the reaction concentration of the structural compound shown in formula II is 0.05-0....
Embodiment 2
[0066] Take 1 equivalent of the structural compound shown in formula I, dissolve it in acetone with 10 times the volume of the structural compound shown in formula I, the concentration of the structural compound shown in formula I is 0.05~0.7 mol / liter, add 0.1 equivalent of p-toluenesulfonic acid, no 2.5 equivalents of sodium sulfate water, 2 equivalents of 2,2-dimethoxypropane. Under stirring, the temperature was raised to 75°C, and the reaction was carried out for 1 hour. After monitoring the completion of the reaction, the reaction was terminated, the organic solvent was evaporated to dryness, and purified by column chromatography. The mobile phase was petroleum ether:ethyl acetate=1:0.3, and white crystals were obtained (yield 87%), which is the structure compound shown in formula II.
[0067] Take 1 equivalent of the structural compound shown in formula II, dissolve it in a tetrahydrofuran solution with 10 times the volume of the structural compound shown in formula II, ...
Embodiment 3
[0069] Take 1 equivalent of the structural compound shown in formula I and dissolve it in dioxane with 10 times the volume of the structural compound shown in formula I. The concentration of the structural compound shown in formula I is 0.05-0.7 mol / liter, and add 0.09 equivalent of p-toluenesulfonic acid , 3.5 equivalents of anhydrous sodium sulfate, 2.5 equivalents of 2,2-dimethoxypropane, heated to 75°C under stirring, reacted for 1 hour, and monitored the completion of the reaction, terminated the reaction, rotary evaporated the organic solvent, and purified by column chromatography. The mobile phase was petroleum ether: ethyl acetate = 1:0.3, and white crystals (yield 85%) were obtained, which were compounds of the formula II.
[0070] Take 1 equivalent of the structural compound shown in formula II, dissolve it in a tetrahydrofuran solution with 10 times the volume of the structural compound shown in formula II, the reaction concentration of the structural compound shown ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com