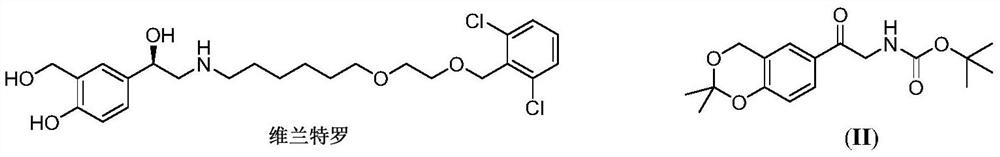
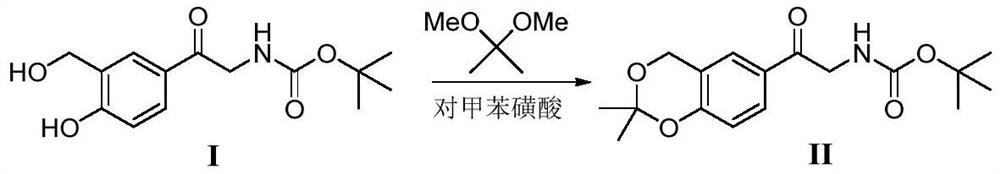
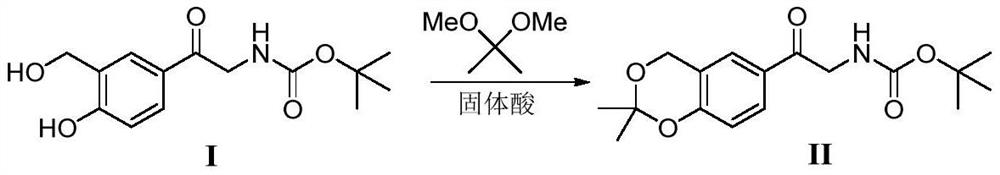
A kind of method of solid acid catalysis synthetic vilanterol intermediate
A solid acid catalysis and intermediate technology, applied in organic chemistry, chemical recycling, etc., can solve problems such as poor color, dark product color, and low yield, and achieve the effects of reducing environmental protection pressure, increasing yield, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 28.1g of intermediate (I), 2,2-dimethoxypropane (20.8g), SO 4 2- / ZrO 2 (1.4g), add chloroform (140mL), heat up to 60 ℃, react for 2 hours, and the reaction is completed by TLC. It was cooled to room temperature, filtered, and the filtrate was concentrated in vacuo to obtain a crude product. The crude product was recrystallized from isopropyl ether to obtain 29.2 g of a white solid with a yield of 91% and a purity of 99%.
Embodiment 2
[0022] 28.1g of intermediate (I), 2,2-dimethoxypropane (20.8g), SO 4 2- / TiO 2 (1.4 g), 1,2-dichloroethane (140 mL) was added, the temperature was raised to 55° C., and the reaction was carried out for 3 hours, and the reaction was completed under TLC control. It was cooled to room temperature, filtered, and the filtrate was concentrated in vacuo to obtain a crude product. The crude product was recrystallized with isopropyl ether to obtain 29.5 g of a white solid with a yield of 92% and a purity of 99%.
Embodiment 3
[0024] 28.1g of intermediate (I), 2,2-dimethoxypropane (20.8g), PO were added to the 500mL three-necked flask 4 3- / TiO 2 (1.4g), acetone (140mL) was added, the temperature was raised to 50°C, and the reaction was carried out for 3 hours, and the reaction was completed under TLC control. It was cooled to room temperature, filtered, and the filtrate was concentrated in vacuo to obtain a crude product. The crude product was recrystallized from isopropyl ether to obtain 29.2 g of a white solid with a yield of 91% and a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com