Method for synthesizing vilanterol intermediate and salt thereof
An intermediate and system technology, applied in the field of pharmaceutical chemical synthesis, can solve problems such as high production cost and environmental pollution, and achieve the effects of reducing costs, reducing environmental pollution, and cheap and easy-to-obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
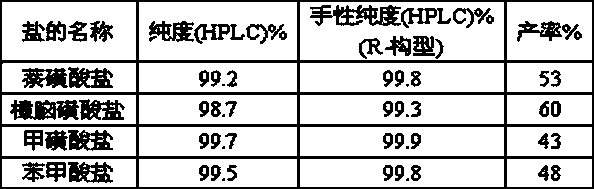
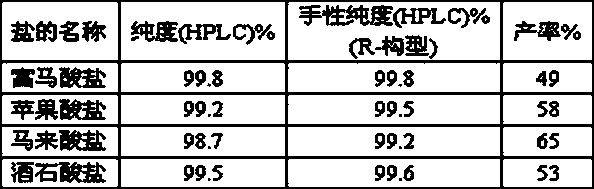
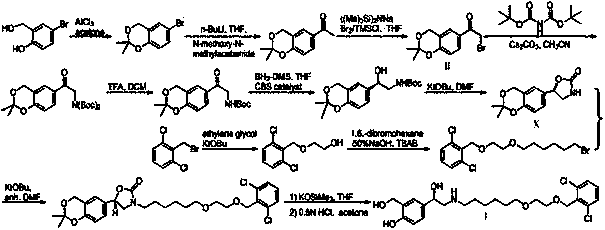
Image
Examples
Embodiment 1
[0023] (R)-1-(2,2-Dimethyl-4H-benzo[d][1,3]dioxan-6-yl)-2-((S)-1-phenylethyl Preparation steps of amino)ethanol and its salts 1): Preparation of 2,2-dimethyl-6-oxirane-4H-benzo[d][1,3]dioxane (compound of formula IV)
[0024] Add 12.8 grams of 2-bromo-1-(2,2-dimethyl-4H-1,3-benzodioxine-6-yl)ethanone (compound of formula II) in the three-neck round bottom flask and 100 milliliters of methanol, stirred to dissolve and then cooled to -10°C, then slowly added 2.4 grams of sodium borohydride, and reacted at room temperature for 90 minutes after the addition was complete. Add 50 milliliters of ammonium chloride aqueous solution to the reaction solution to quench, stir for 10 minutes and concentrate to remove most of the methanol, then add 50 milliliters of dichloromethane for extraction, the aqueous phase is extracted 3 times with 50 milliliters of dichloromethane, and the organic phases are combined . The organic phase was washed once with 20 ml of distilled water and once with ...
Embodiment 2
[0034] (R)-1-(2,2-Dimethyl-4H-benzo[d][1,3]dioxan-6-yl)-2-((S)-2-methoxy- Preparation of 1-phenylethylamino)ethanol and its salts
[0035] Step 1): (R)-1-(2,2-Dimethyl-4H-benzo[d][1,3]dioxan-6-yl)-2-((S)-2- Preparation of methoxy-1-phenylethylamino)ethanol
[0036] The preparation method of compound IV is the same as step 1) of Example 1.
[0037]Add 8.24 grams of epoxy compound IV to 50 milliliters of acetonitrile solvent, stir to dissolve it, then slowly add 9.06 grams of S-2-methoxyl-1-phenylethylamine, stir and react at 80°C for 6 hours, and use TLC method After monitoring the completion of the reaction, the reaction solution was concentrated. Add 30 mL of saturated aqueous sodium bicarbonate, extract with ethyl acetate (3 x 30 mL), and dry the organic phase over anhydrous sodium sulfate, filter, and concentrate to give (R)-1-(2,2-dimethyl- 4H-benzo[d][1,3]dioxane-6-yl)-2-((S)-2-methoxy-1-phenylethylamino)ethanol crude product 9.8 grams, yield The rate is 68%. The cr...
Embodiment 3
[0045] (R)-2-(Benzyl((S)-1-phenylethyl)amino)-1-(2,2-dimethyl-4H-benzo[d][1,3]dioxahexa Preparation of cyclo-6-yl)ethanol and its salts
[0046] Step 1): (R)-2-(Benzyl((S)-1-phenylethyl)amino)-1-(2,2-dimethyl-4H-benzo[d][1,3 ] the preparation of dioxane-6-yl) ethanol
[0047] The preparation method of compound IV is the same as step 1) of Example 1.
[0048] Add 8.24 grams of epoxy compound IV to 50 milliliters of tetrahydrofuran solvent, stir to dissolve it, then slowly add 10.97 grams of S-(-)-N-benzyl-1-phenethylamine, reflux for 4 hours, and monitor the reaction with TLC completely. After the reaction solution is cooled, add 30 milliliters of saturated ammonium chloride aqueous solution, stir at room temperature for 10 minutes, then add 3 grams of sodium chloride, continue to stir for 30 minutes, and leave to stand for layering, and the aqueous phase is extracted with ethyl acetate (3 × 30 ml), the organic phase was dried over anhydrous sodium sulfate, filtered, and co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com