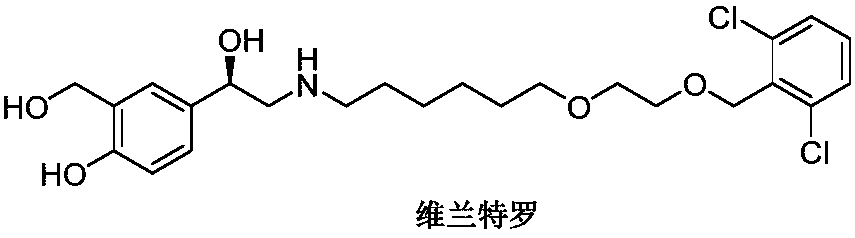
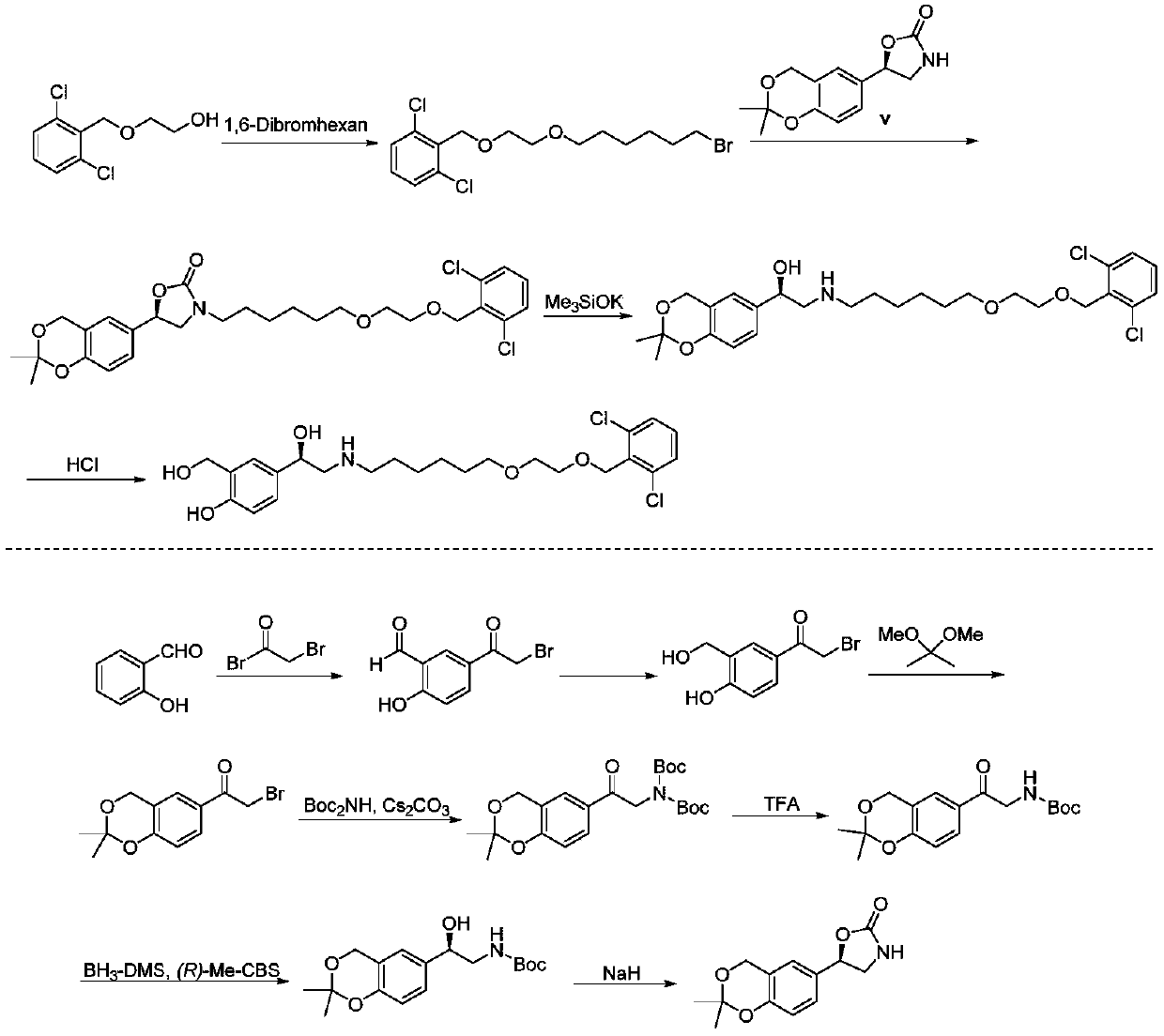
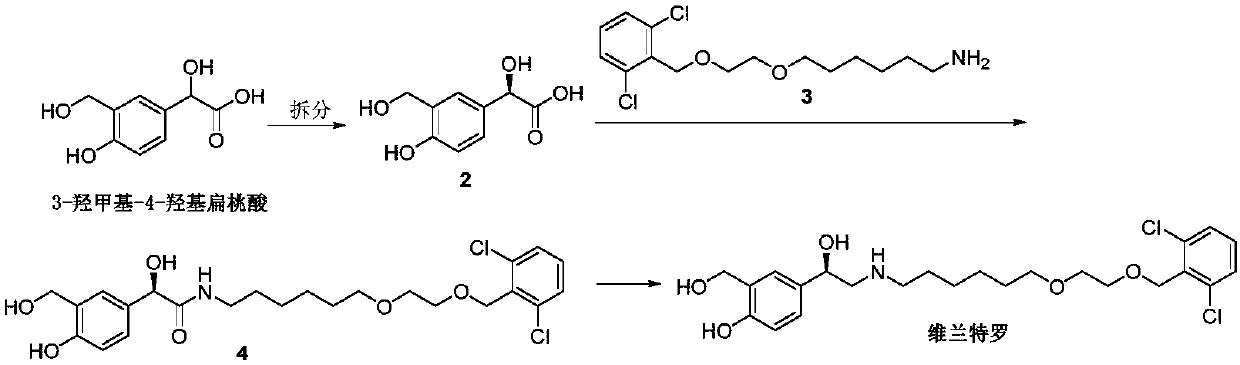
Synthesizing method of vilanterol intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of reducing production costs and reducing the steps of vilanterol synthesis, and achieve the effects of reducing production costs, shortening synthetic routes, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 4-hydroxymandelic acid (16.8g), paraformaldehyde (9g) into the there-necked flask, add glacial acetic acid (85mL), FeCl 3 (0.8g), reacted at 80°C for 4h, the reaction in TLC was completed, cooled to room temperature, concentrated the solvent in vacuo, added water (100mL) to the residue, stirred for half an hour, filtered to obtain a solid, and the crude product was recrystallized from ethanol to obtain 13.5 g of 4-hydroxy-3-formylmandelic acid, yield 68%, purity 98%.
[0019] Note: 4-Hydroxymandelic acid was purchased from Jiangxi Keyuan Biopharmaceutical Co., Ltd., and the purity of the raw material was 98%.
Embodiment 2
[0021] Add 4-hydroxymandelic acid (50.4g), paraformaldehyde (27g) in the there-necked flask, add trifluoroacetic acid (250mL), ZnCl 2 (2g), reacted at 70°C for 4h, the reaction in TLC was completed, cooled to room temperature, concentrated the solvent in vacuo, added water (200mL) to the residue, stirred for half an hour, filtered to obtain a solid, and the crude product was recrystallized from ethanol to obtain 4 -Hydroxy-3-formylmandelic acid 41g, yield 70%, purity 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com