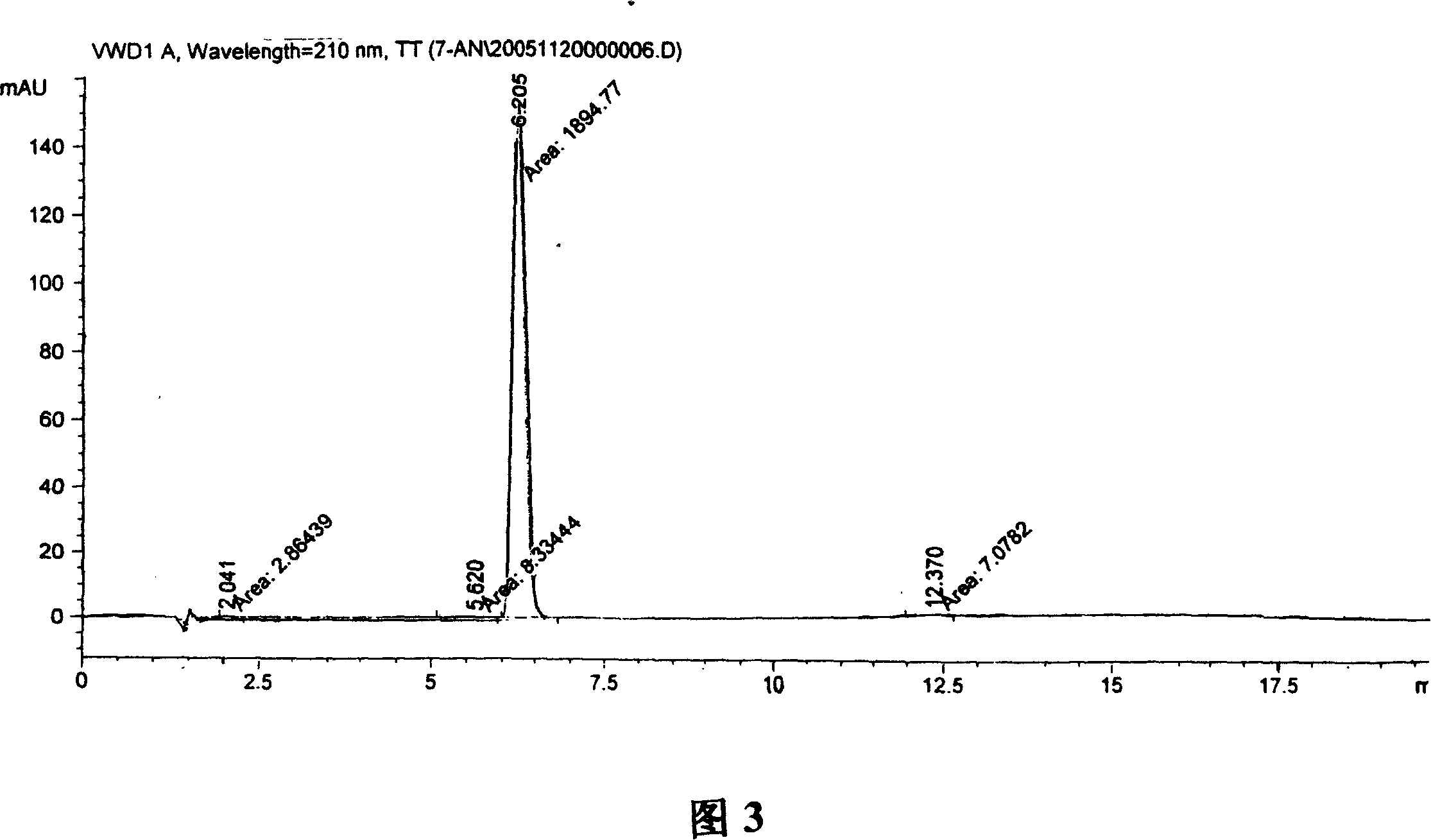
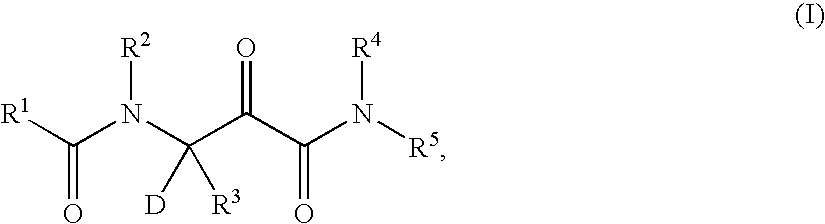
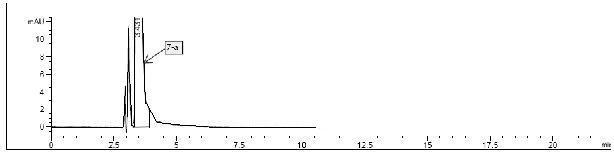
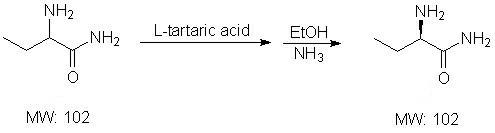
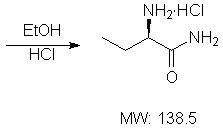
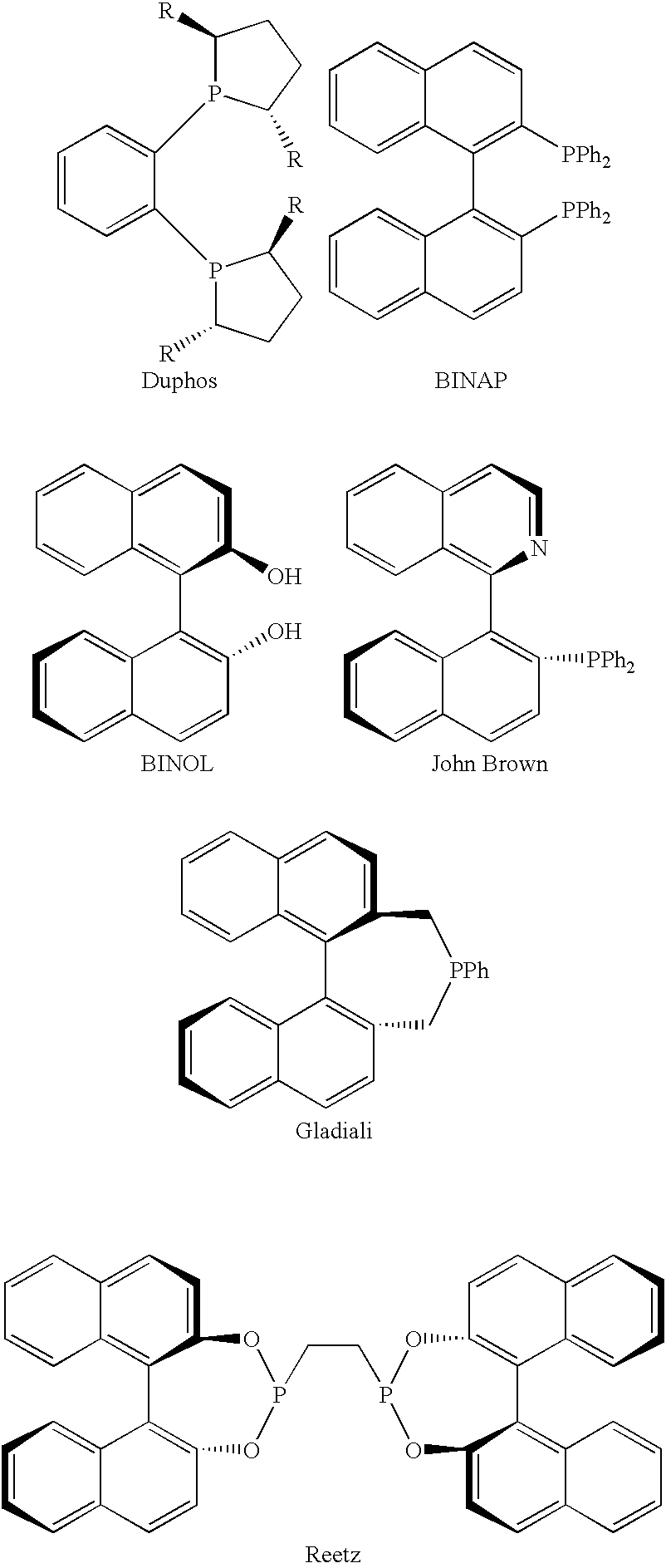
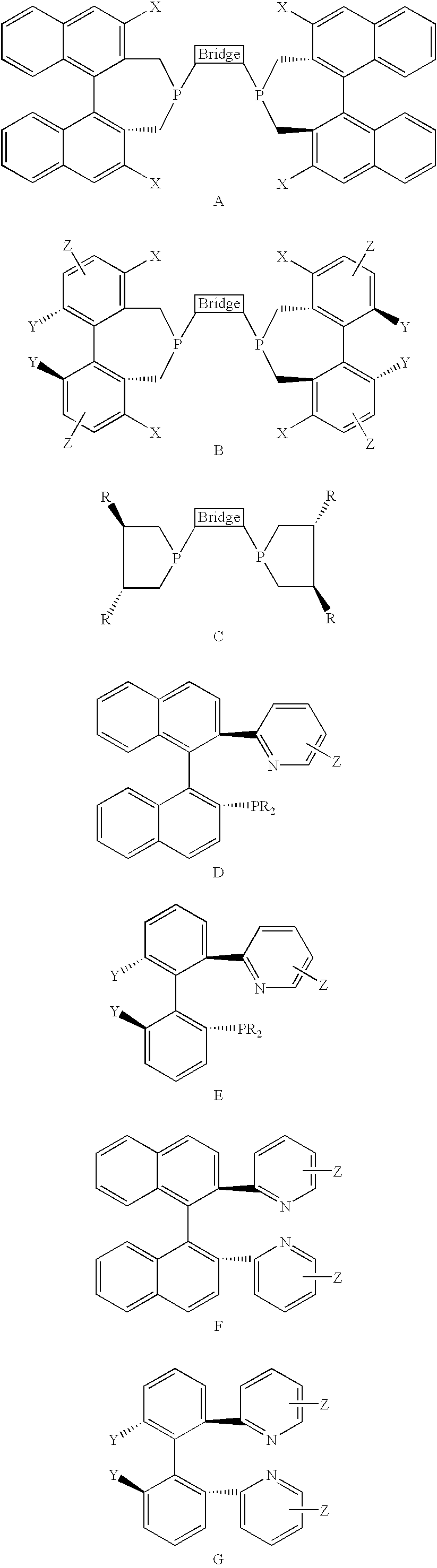
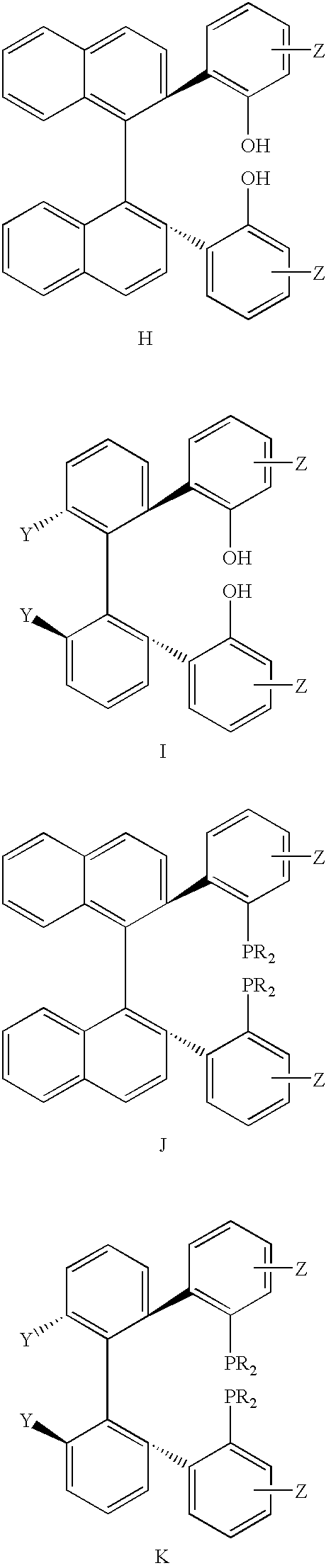
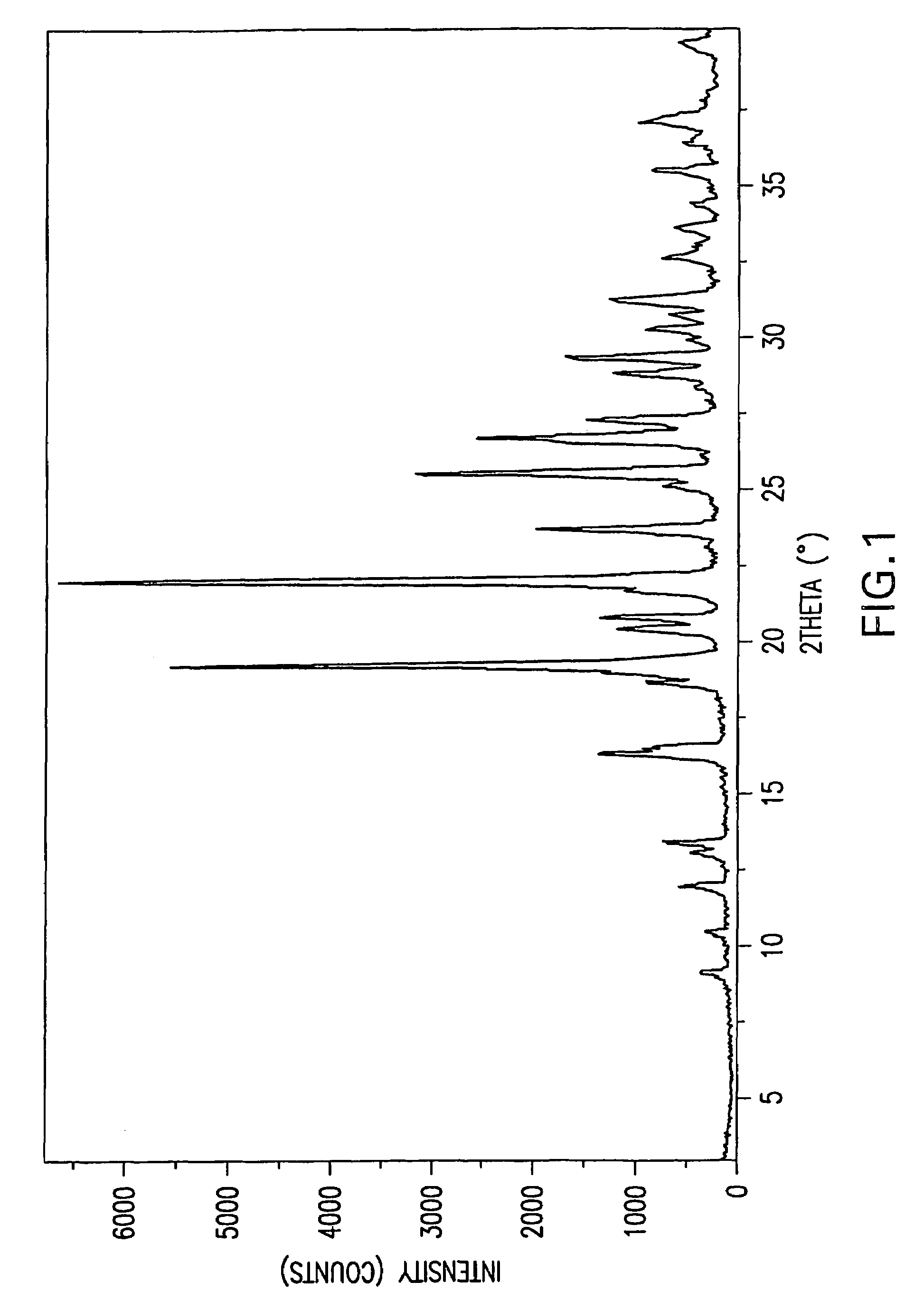
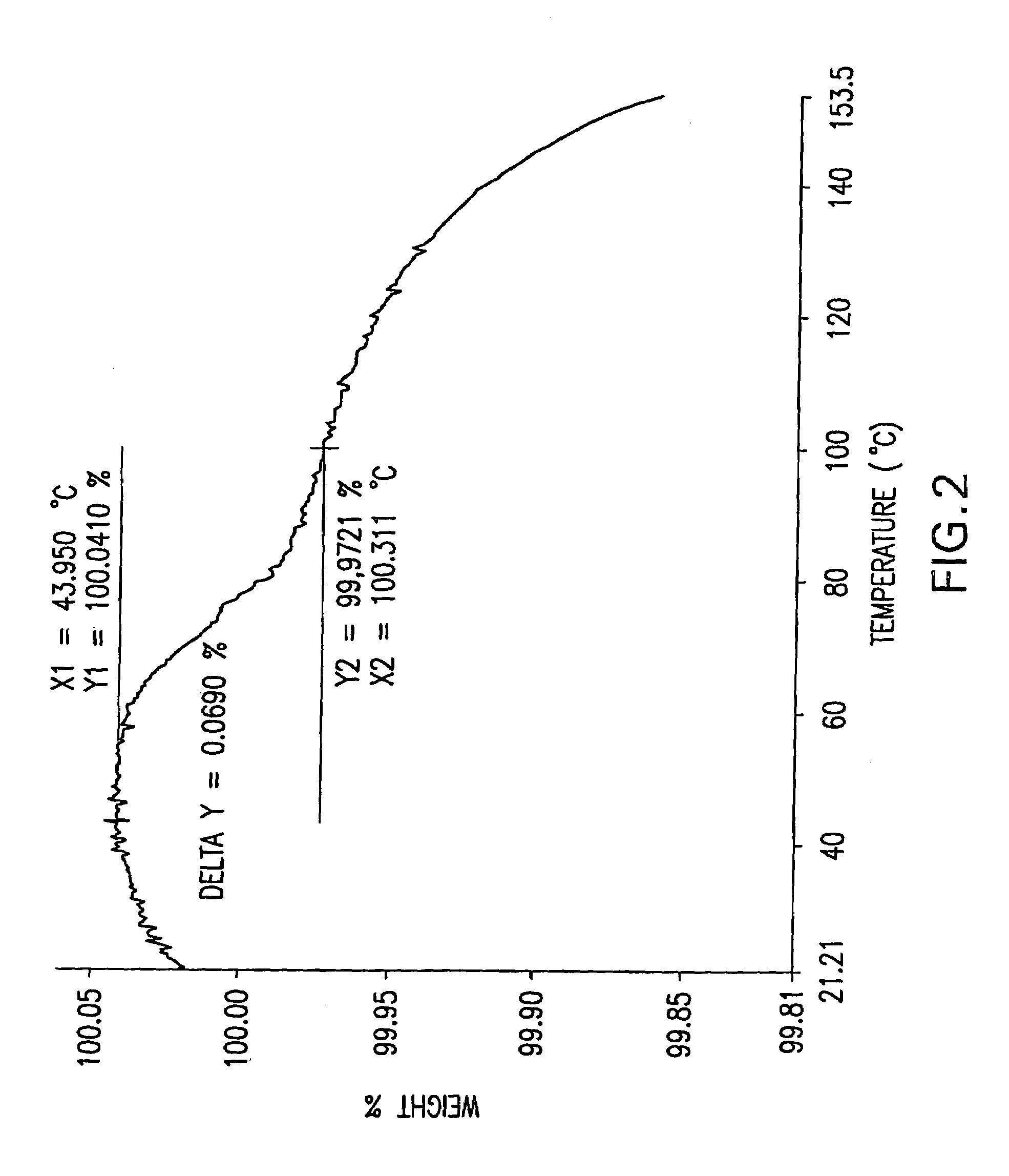
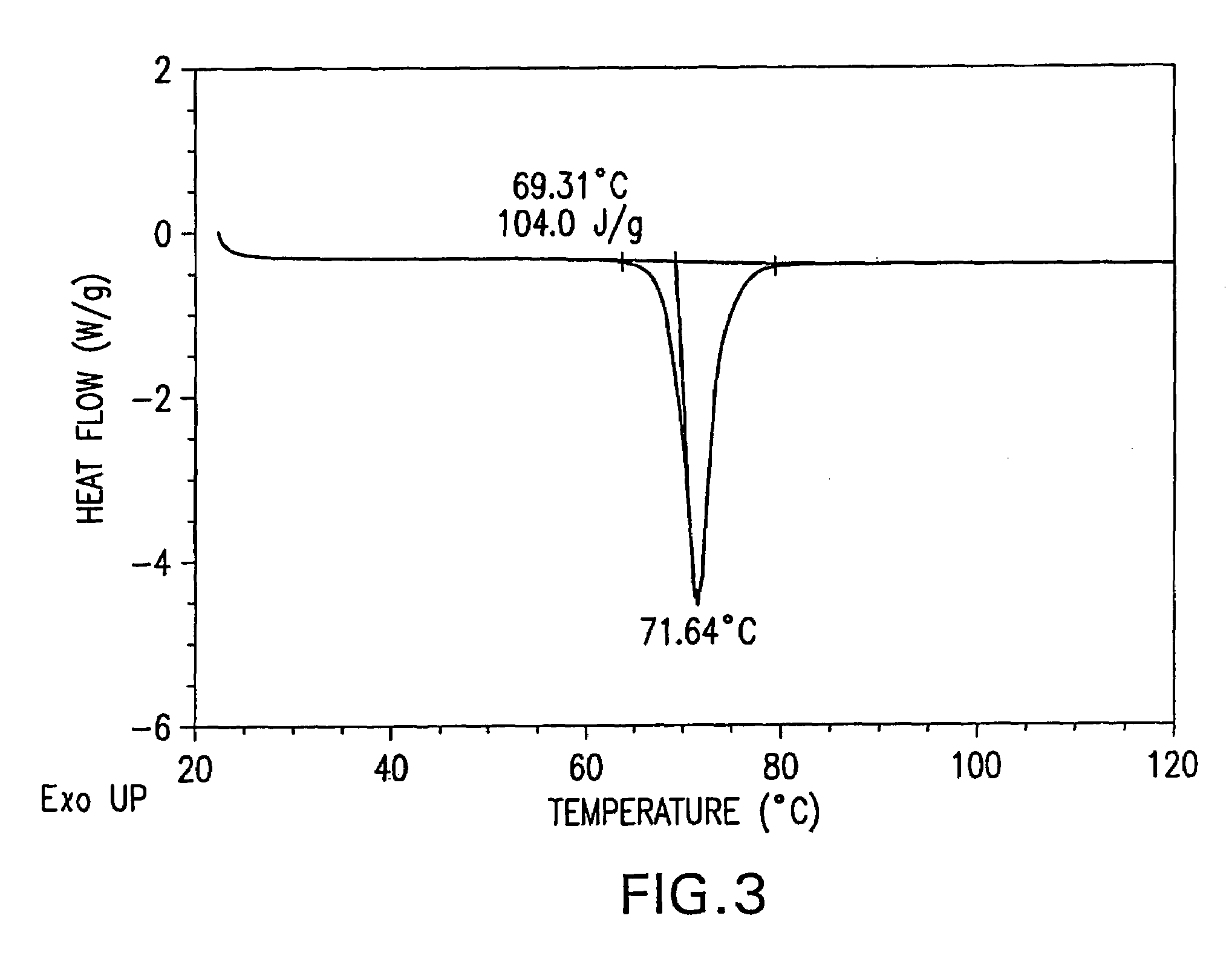
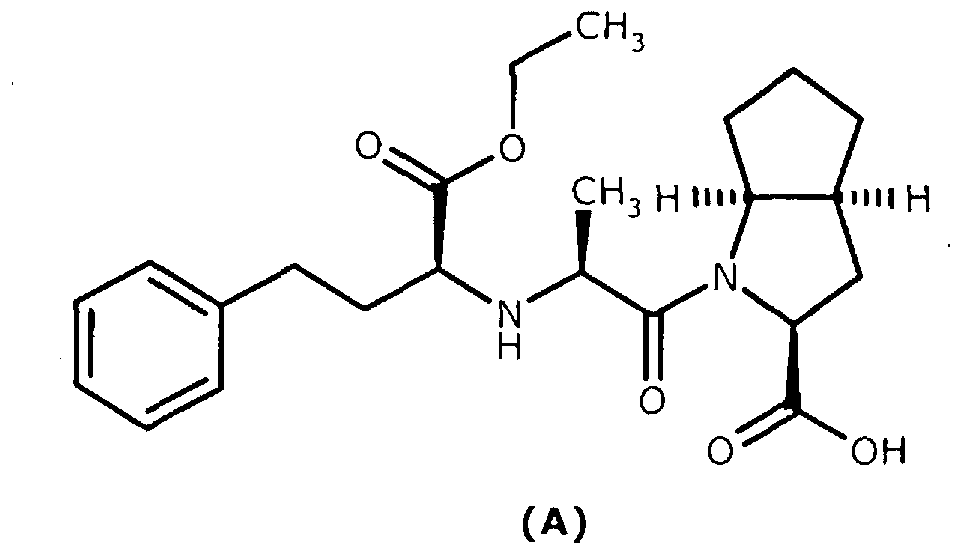
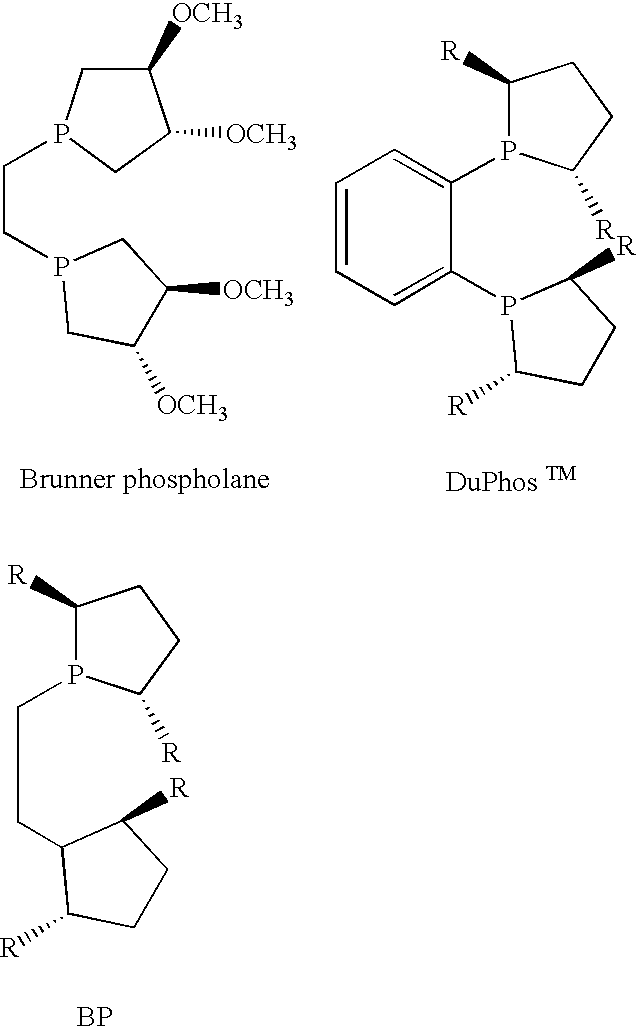
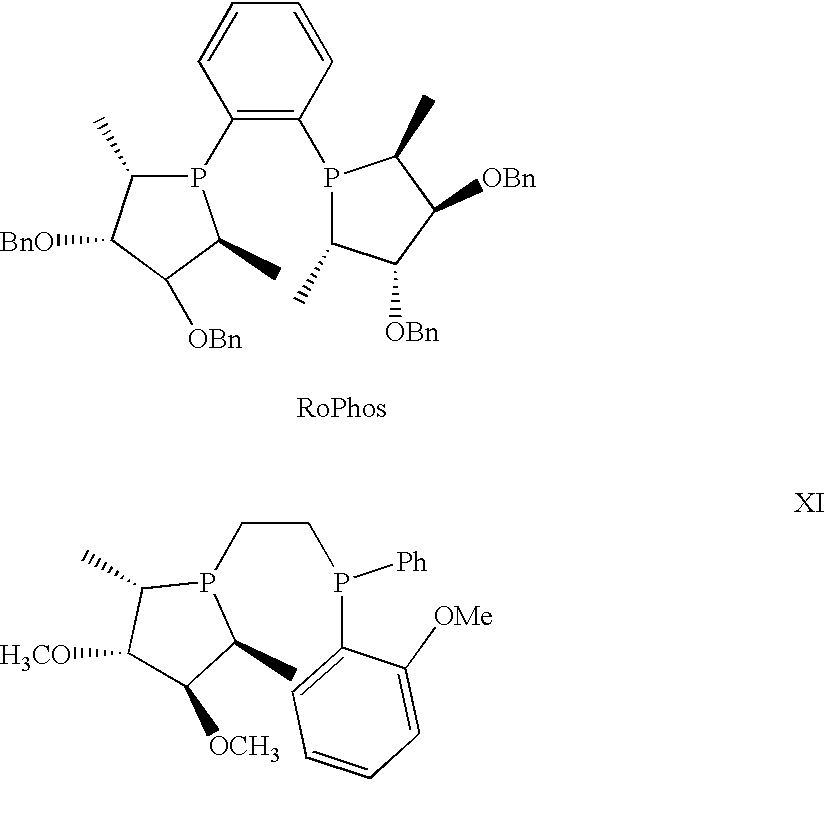
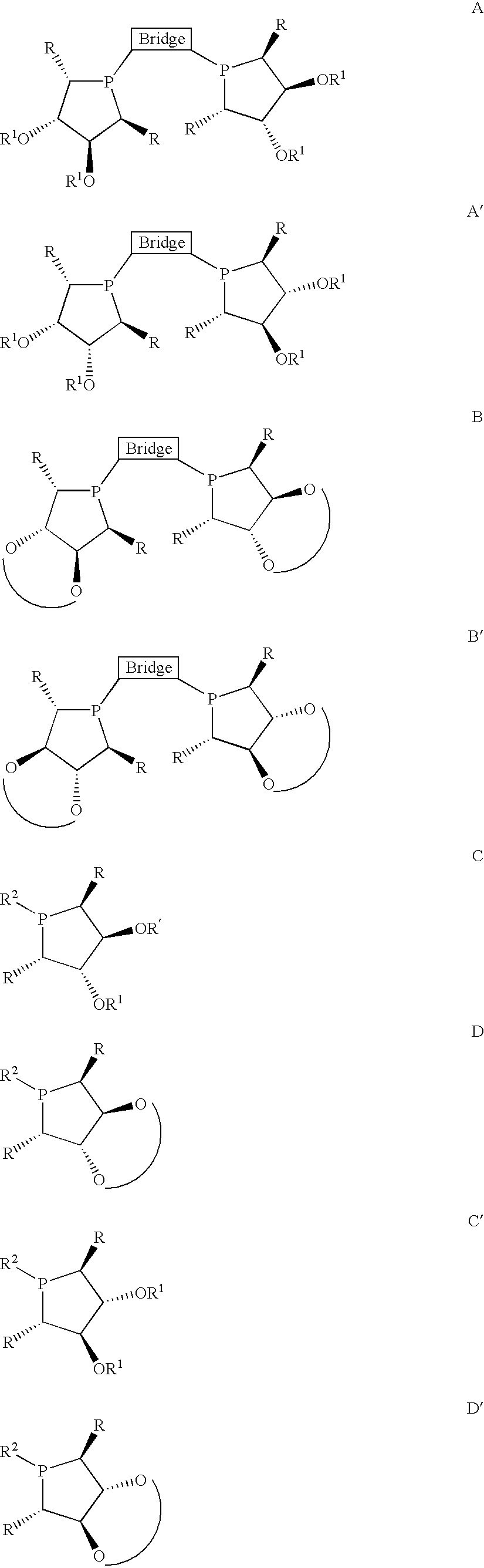
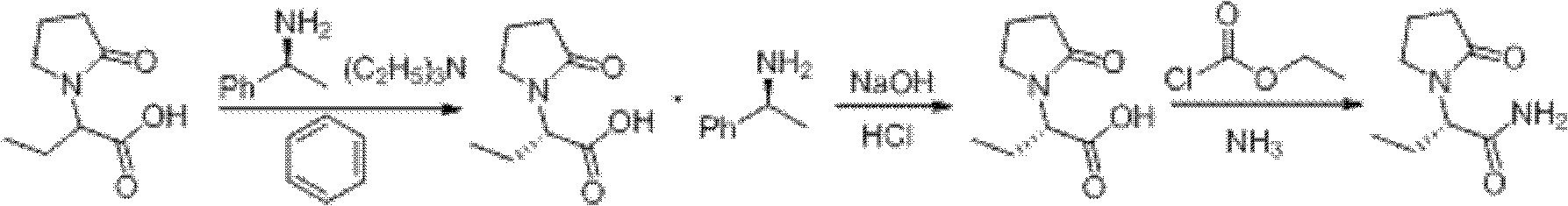
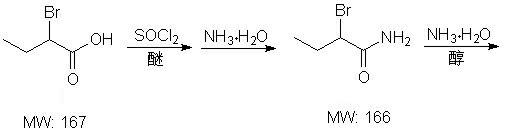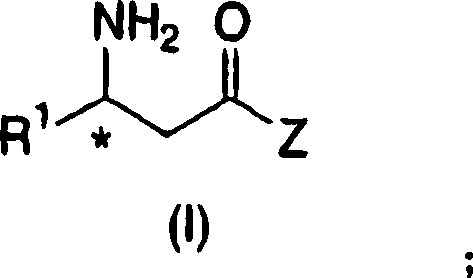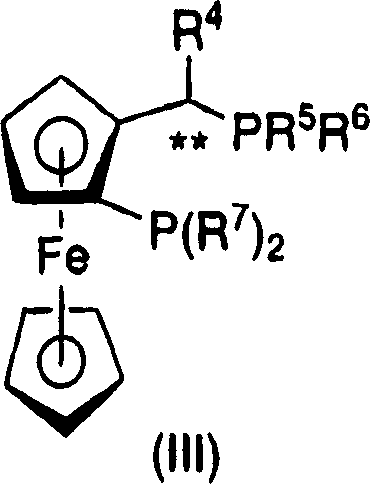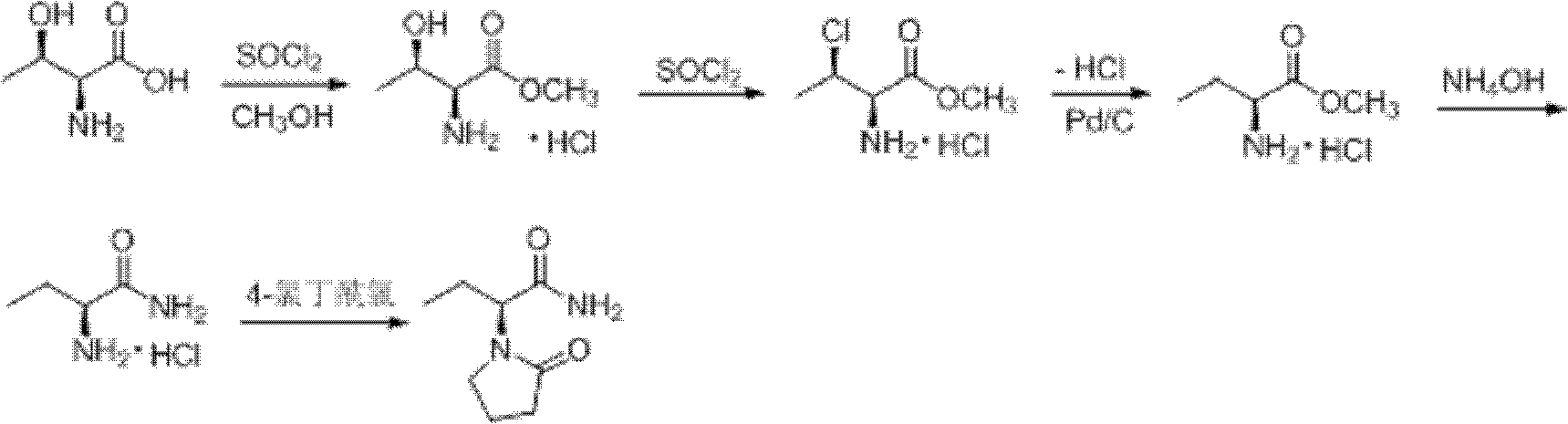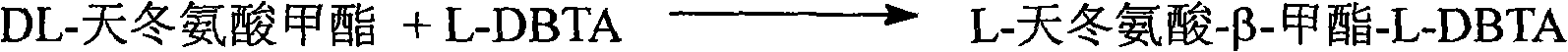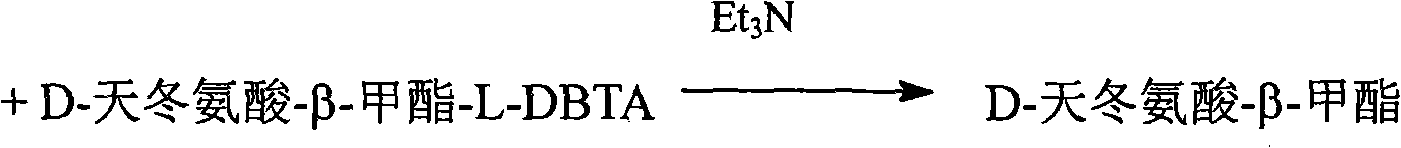Patents
Literature
330results about "Carboxylic acid amides optical isomer preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
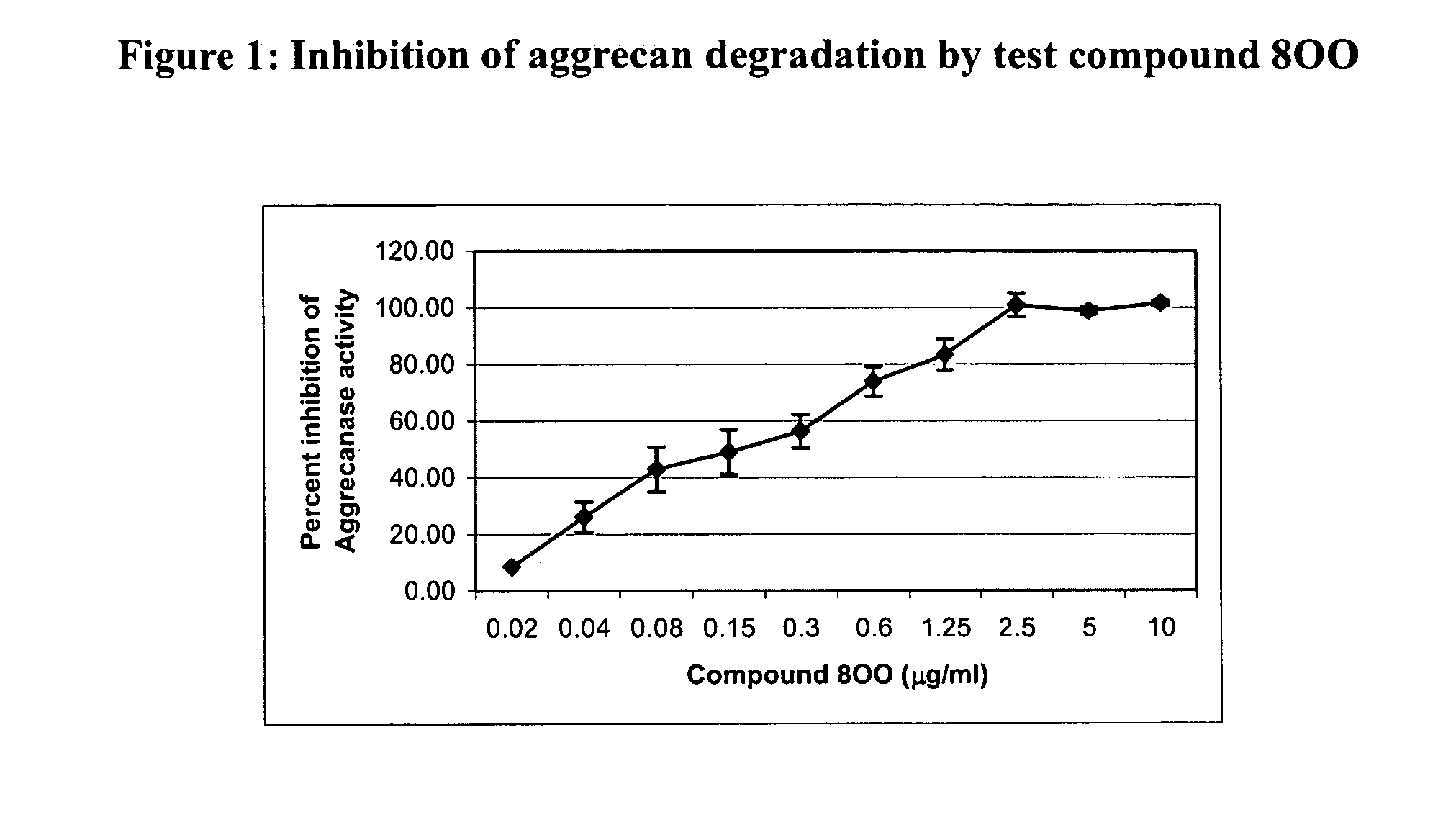
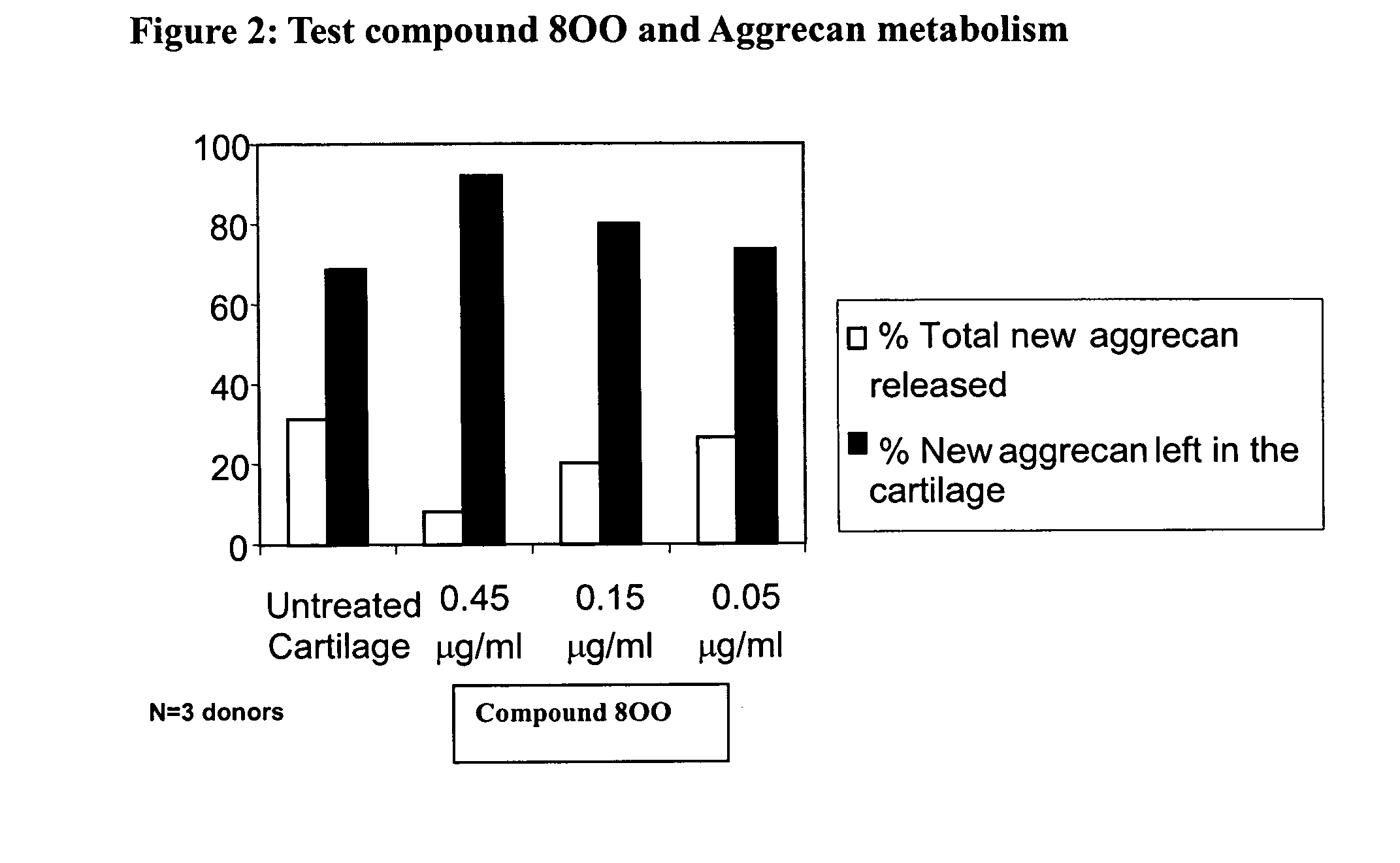
Glutamate aggrecanase inhibitors
Owner:WYETH
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
ActiveUS7468459B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleEnantiomer
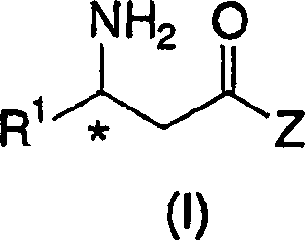
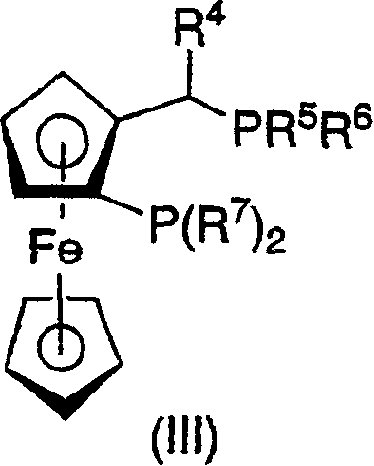
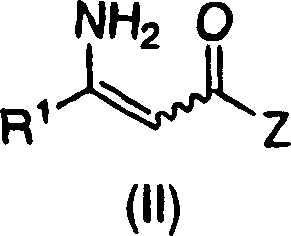
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives which are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of a prochiral beta amino acrylic acid derivative substrate in the presence of a transition metal precursor complexed with a chiral ferrocenyl diphosphine ligand.
Owner:MERCK SHARP & DOHME LLC
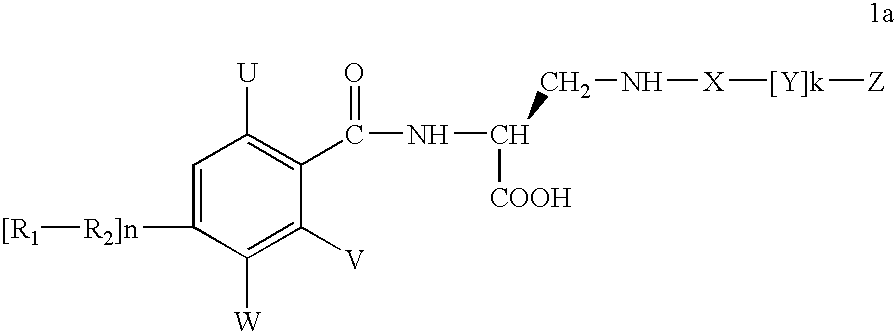
Diaminopropionic acid derivatives
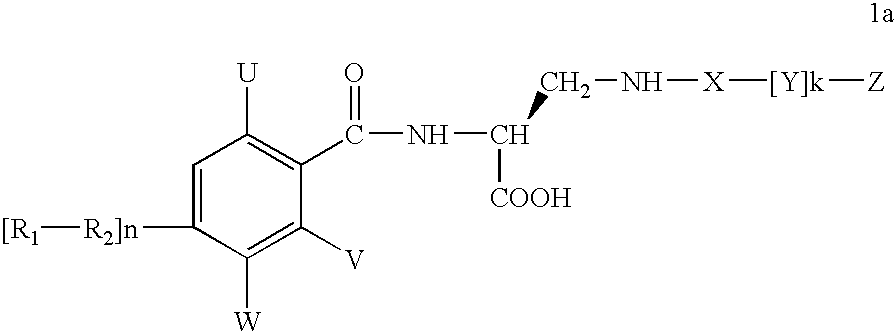
InactiveUS7217728B2Organic active ingredientsPeptide/protein ingredientsReperfusion injuryAcid derivative
A compound of formula 1awhich is useful for treating reperfusion injury, and salts, prodrugs, and related compounds.
Owner:F HOFFMANN LA ROCHE INC
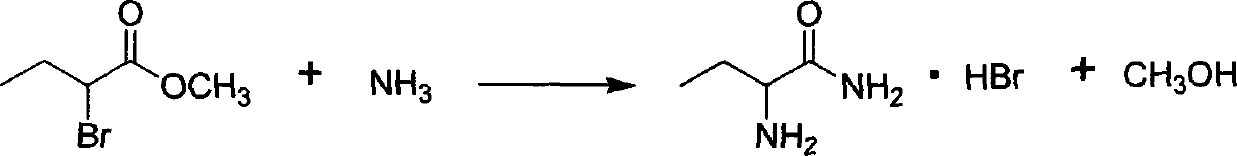
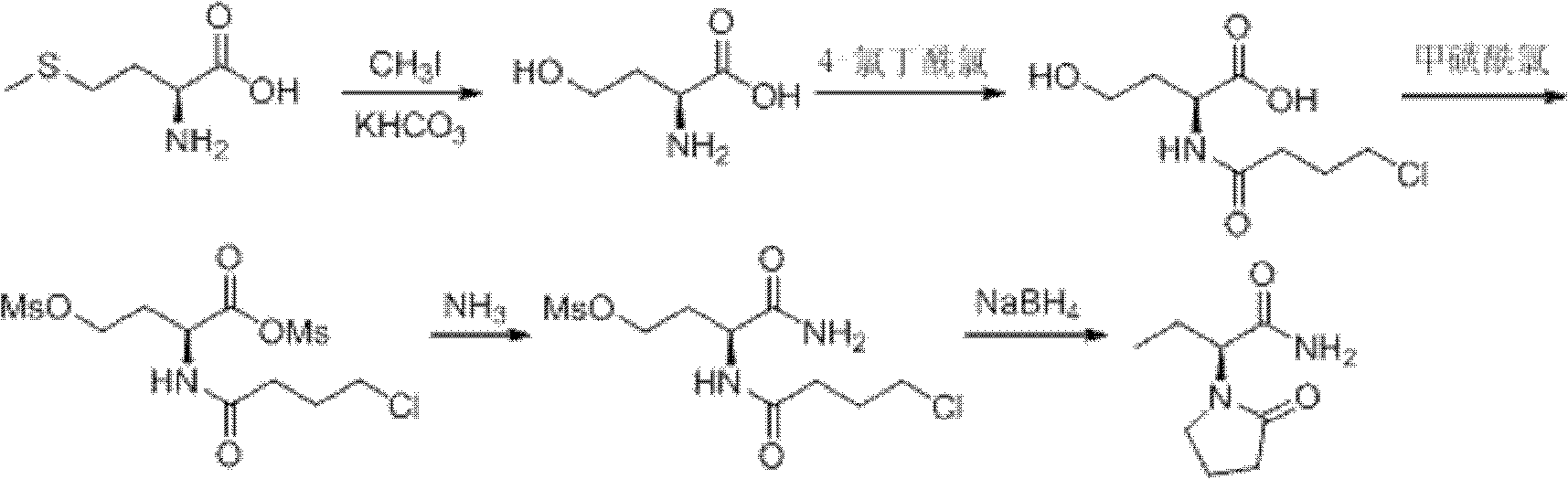
Synthesis, split and racemization of chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504AReduce dosageLow costOrganic compound preparationCarboxylic acid amides optical isomer preparationButyramidePyrrolidine
The invention discloses a new synthesizing technique of chiral drug (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetamide ( left Piracetam) intermediate (S)-(+)-2-aminobutanamide hydrochlorate, which comprises the following steps: adopting 2-brobutyrate as initial raw material; aminating; esterifying; ammonolyzing; detaching; looping; obtaining the object compound; making mixed rotary free alkaline (+-)-2-aminobutanamide; adopting half-quantum resolution method to connect chemical detaching salt to evolve salt; removing the detaching agent through alkalization; obtaining the (S)-(+)-2-aminobutanamide hydrochlorate with optical activity; using the mother liquor to make the product. The invention improves the receiving rate and saves the cost of raw material with simply technical operation and low cost, which resolves the resolved mother liquor after racemic action again to reduce the pollution of environment, therefore fitting for industrialized manufacturing.
Owner:ABA CHEM CORP
Deuterated hepatitis C protease inhibitors
InactiveUS20070225297A1Improve concentrationImprove bioavailabilityOrganic active ingredientsOrganic compound preparationVirus inhibitorsMedicinal chemistry
Owner:VERTEX PHARMA INC
Process for the preparation of chiral beta amino acid derivatives by asymmetric hydrogenation
InactiveCN1761642AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMetalloleAsymmetric hydrogenation
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives which are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of a prochiral beta amino acrylic acid derivative substrate in the presence of a transition metal precursor complexed with a chiral ferrocenyl diphosphine ligand.
Owner:MERCK SHARP & DOHME BV
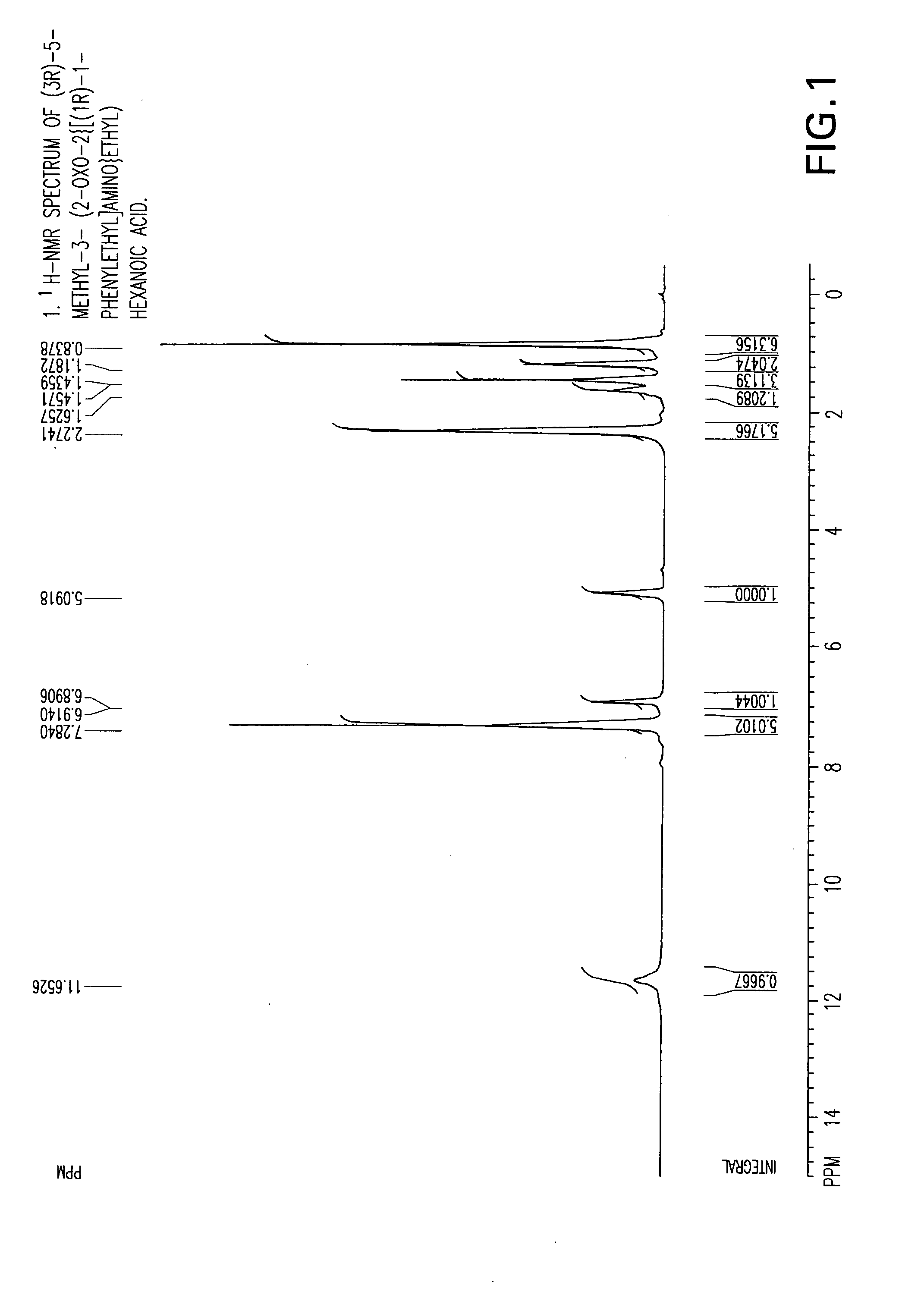
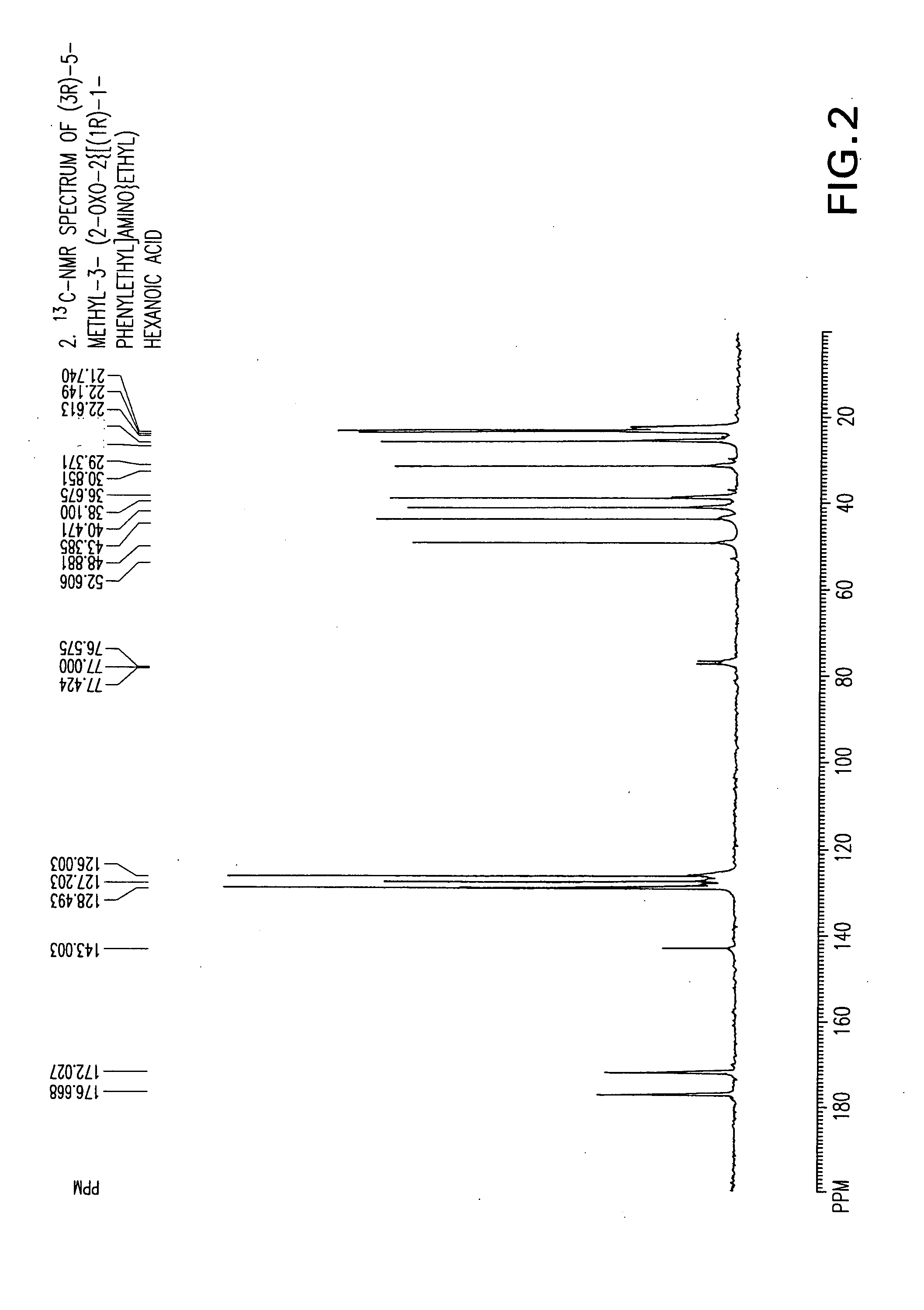
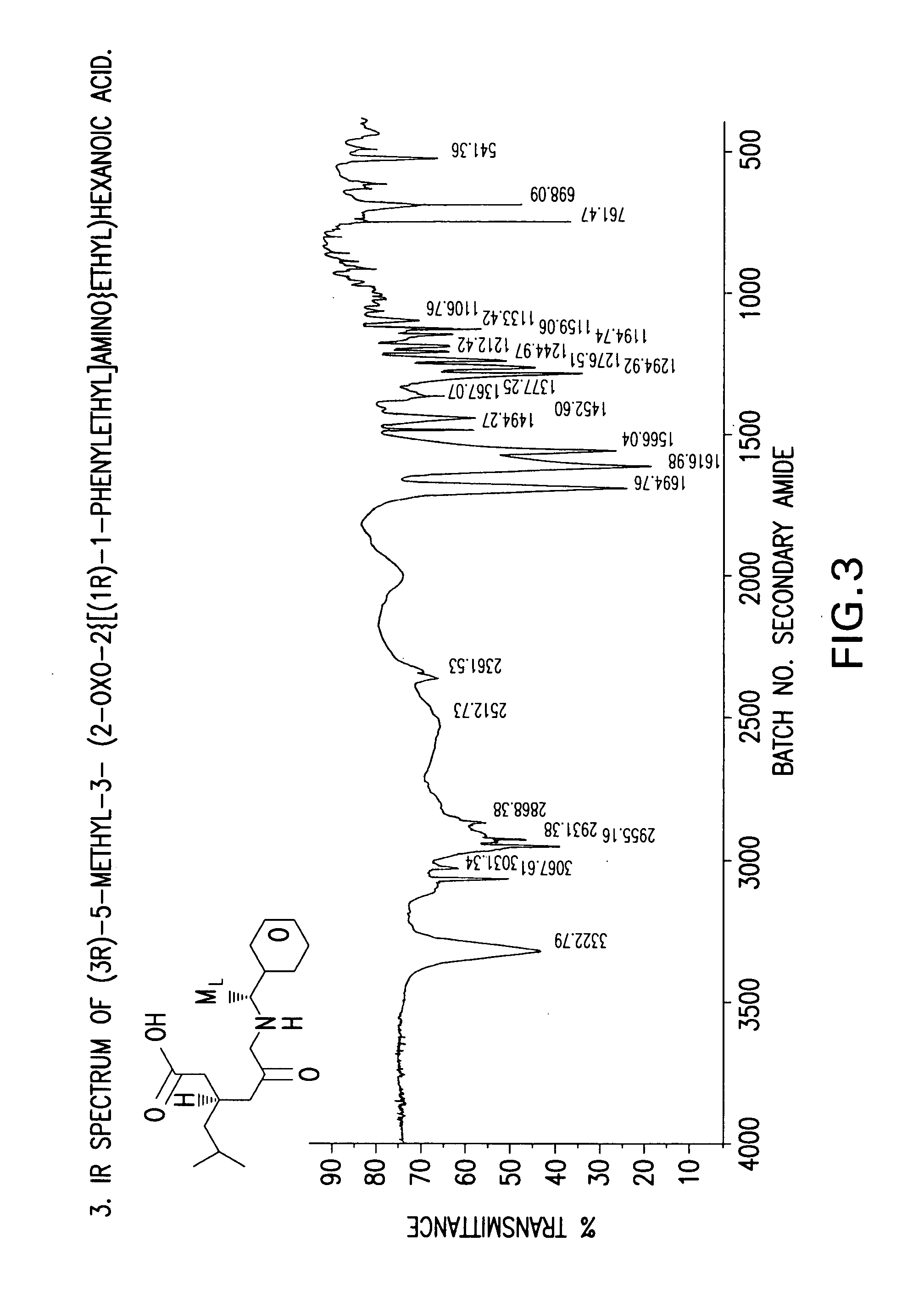
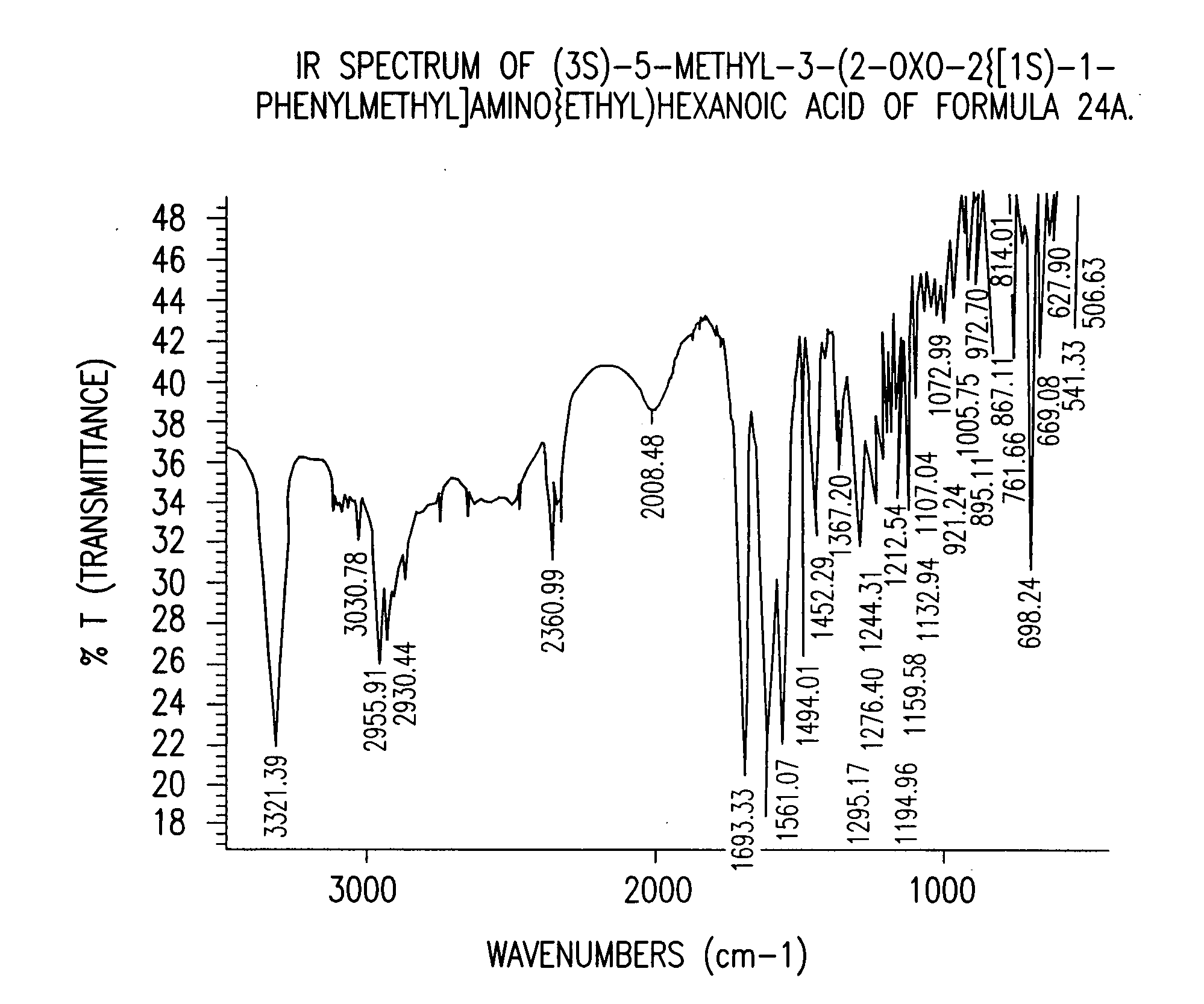
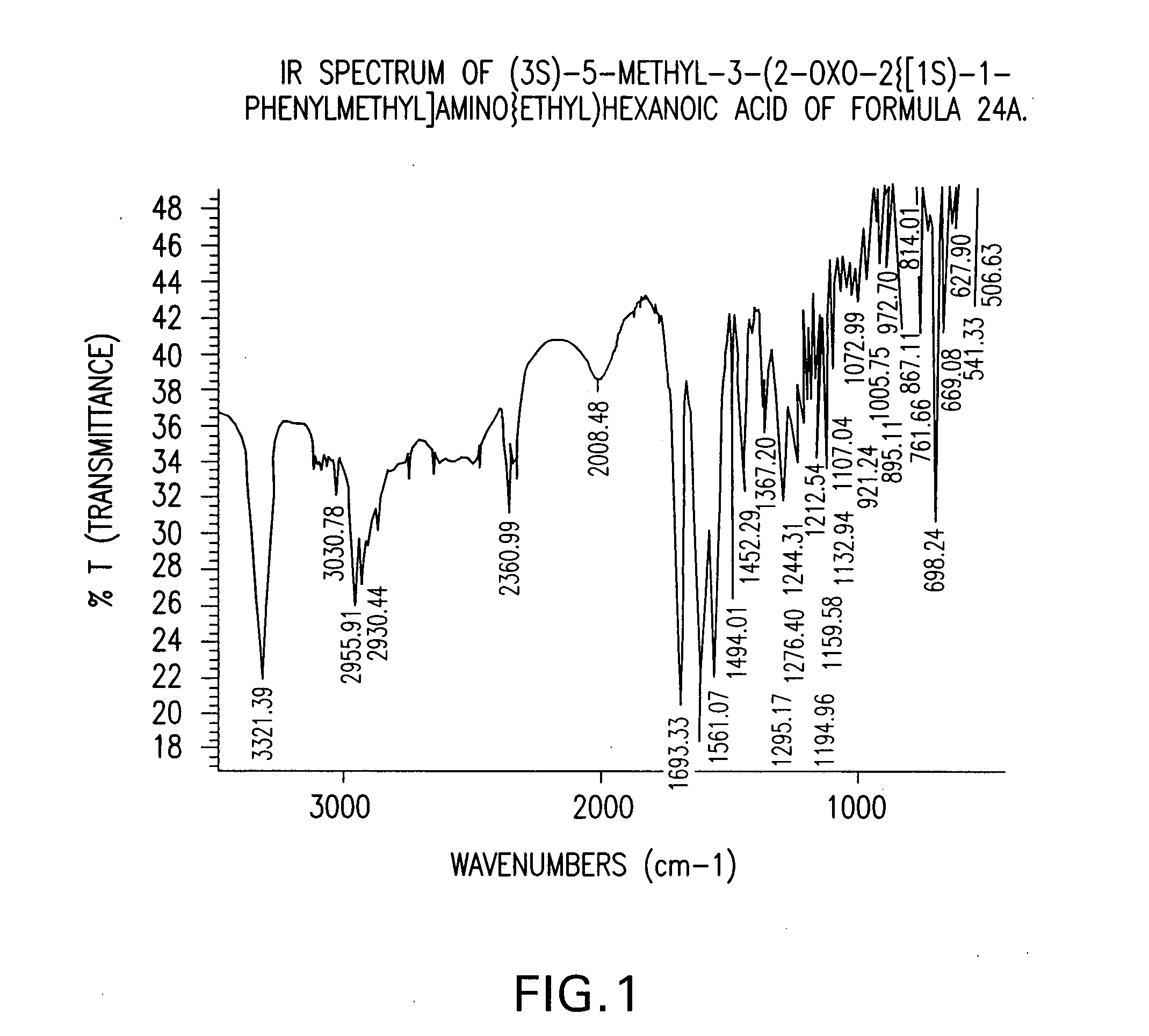
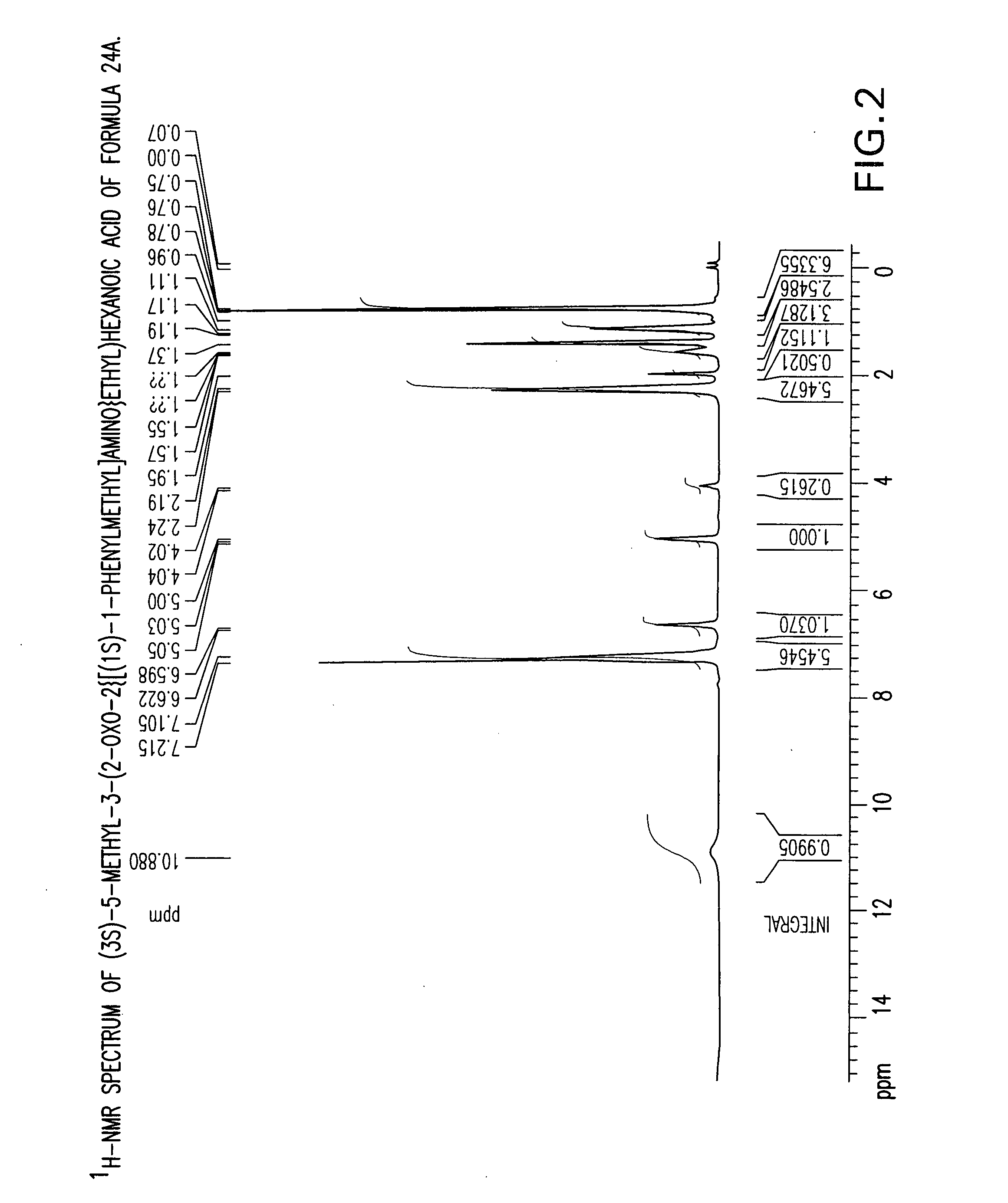
Chiral 3-carbamoylmethyl-5-methyl hexanoic acids, key intermediates for the synthesis of (S)-Pregabalin
The invention encompasses the synthesis of (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid, (S)-Pregabalin, via the intermediate, (3R)-5-methyl-3-(2-oxo-2{[(1R)-1-phenylethyl]amino}ethyl)hexanoic acid.
Owner:TEVA PHARM USA INC
Diaminopropionic acid derivatives
A compound of formula 1a which is useful for treating reperfusion injury, and salts, prodrugs, and related compounds.
Owner:F HOFFMANN LA ROCHE INC
Separating agent for optical isomers and process for producing the same
InactiveUS6217769B1Ion-exchanger regenerationCarboxylic acid amides optical isomer preparationSolventPolysaccharide
The invention provides separating agents for optical isomers which have a high optical resolving power inherent in polysaccharide derivatives and high solvent resistance, and which can be produced through short process steps; a process for producing the same, and a method for separating optical isomers. The invention also provides separating agents for optical isomers, wherein the surface of a polysaccharide derivative supported on a carrier or the surface of a pulverized or granulated polysaccharide derivative is coated with a polymer, which are produced by supporting the polysaccharide derivative on the carrier and then coating the surface thereof with the polymer to thereby immobilize the polysaccharide derivative on the substrate, or by grinding or spheroidizing the polysaccharide derivative and then coating the surface thereof with a polymer.
Owner:DAICEL CHEM IND LTD
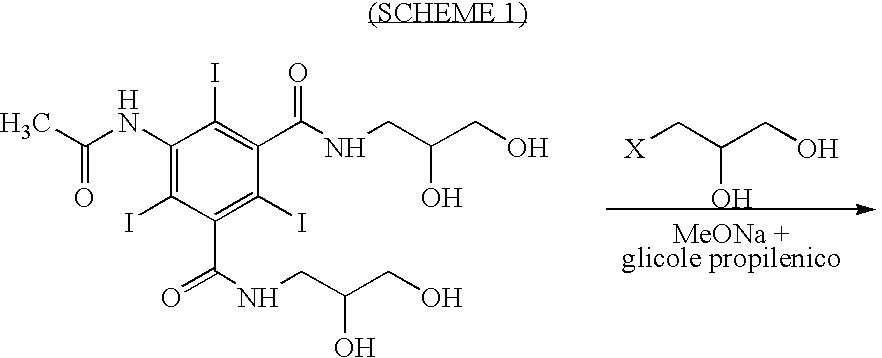
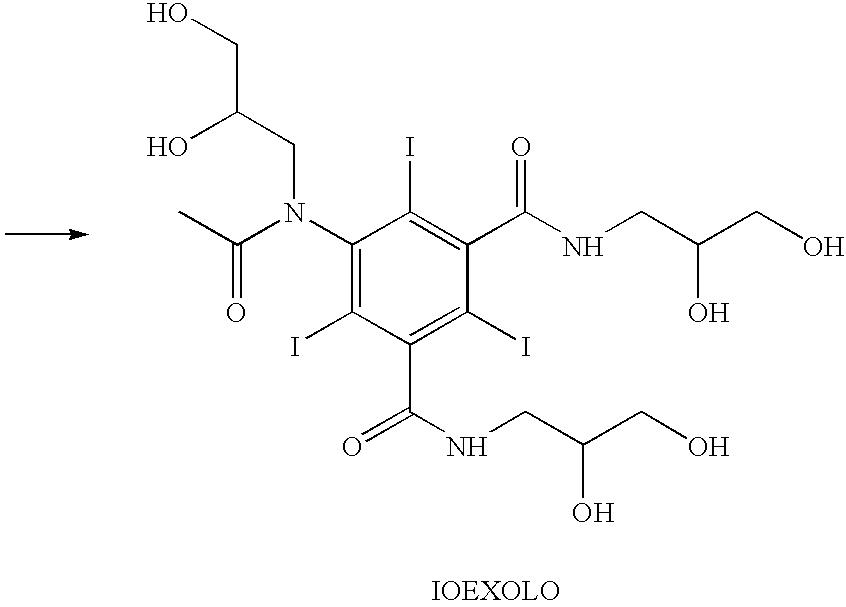
Process for the production of high purity iohexol
InactiveUS6897339B2Reduce impurityEliminate effectiveOrganic compound preparationCarboxylic acid amides optical isomer preparationPropanolPurification methods
Owner:CHEMI SPA
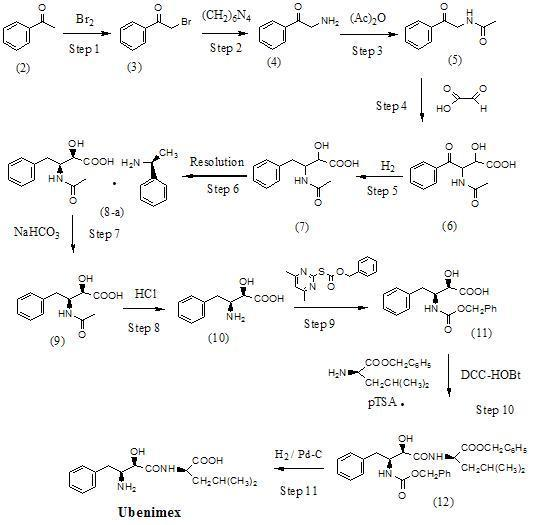
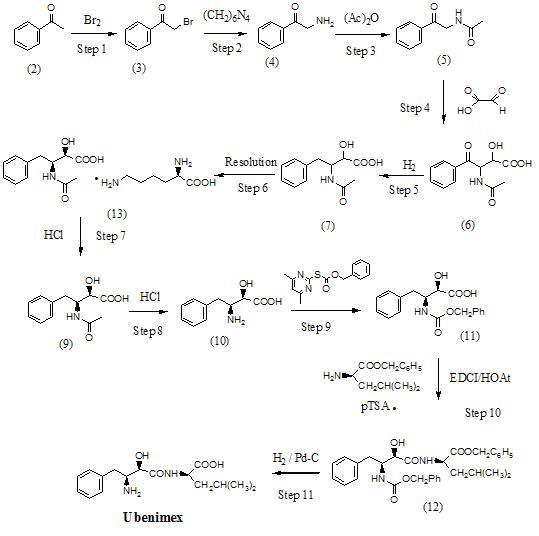
Preparation method for ubenimex
ActiveCN101891647AOrganic compound preparationCarboxylic acid amides optical isomer preparationArginine3-amino-2-hydroxy-4-phenylbutyric acid
The invention belongs to the field of antineoplastic agent preparation and provides a preparation method for ubenimex. The preparation method comprises the following steps of: preparing high-purity key intermediate (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid by taking L-lysine, L-arginine or L-histidine as a resolving reagent; and preserving the chirality of C-5 in forming a peptide chain by taking EDCI / HOAt as a condensing agent. Due to the adoption of the method, the problem that the (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid and the (2S,3R)-3-acetamino-2-hydroxy-4-phenyl butyric acid cannot be completely separated by the conventional resolving agent and the racemization problem in the condensation of amide are effectively solved; and the purity of the ubenimex prepared by the method can reach over 99.5 percent.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
Method for synthesizing intermediate L-2-aminobutyrylamide hydrochloride of chiral drug levetiracetam
InactiveCN102020584AMild reaction conditionsLow costCarboxylic acid amides optical isomer preparationSynthesis methodsUnit operation
The invention belongs to the field of the preparation of chiral drug intermediates and particularly relates to a method for synthesizing a intermediate L-2-aminobutyrylamide hydrochloride of chiral drug levetiracetam. The method is characterized by taking 2-bromobutyric acid as a starting raw material and comprising the steps of: firstly, preparing a 2-bromobutyric amide intermediate, reacting the 2-bromobutyric amide intermediate to generate DL-2-aminobutyrylamide, and finally, splitting and salifying the DL-2-aminobutyrylamide to synthesize the L-2-aminobutyrylamide hydrochloride. Compared with the traditional synthesis method, the method has the advantages of mild reaction conditions, low cost, higher yield, greatly improved process safety, cheap and easily-obtained raw materials, simple unit operations and low requirements on equipment and is suitable for industrial large-scale production, amidation reaction is carried out at normal temperature and normal pressure, and the reaction is easy to control.
Owner:浙江沙星医药化工有限公司
Novel asymmetric synthesis of (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid
The invention encompasses processes for the synthesis of (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid, (S)-Pregabalin, and intermediates of (S)-Pregabalin.
Owner:TEVA PHARM USA INC
Processes and intermediates for preparing steric compounds
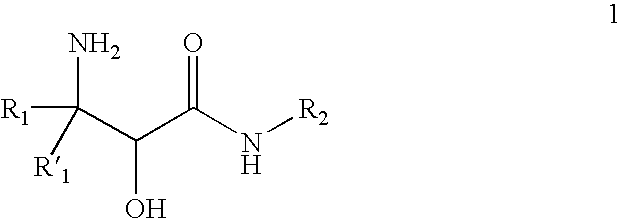
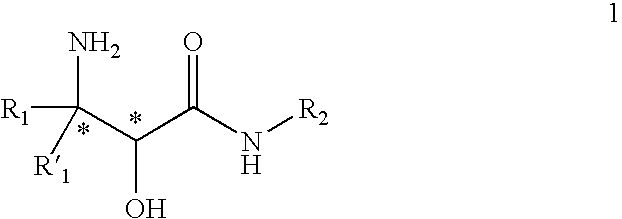
InactiveUS20070244334A1Organic compound preparationOrganic chemistry methodsBeta hydroxy acidStereochemistry
This invention relates to processes and intermediates for the preparation of an alpha-amino beta-hydroxy acid of Formula 1 wherein the variables R1, R′1 and R2 are defined herein and the compound of Formula 1 has an enantiomeric excess (ee) of 55% or greater.
Owner:VERTEX PHARMA INC
Formoterol tartrate polymorph
A method of preparation of a highly pure salt of R,R-formoterol L-tartrate is disclosed. The process provides the most thermodynamically stable polymorph by recrystallization of a novel polymorph.
Owner:SUNOVION PHARMA INC
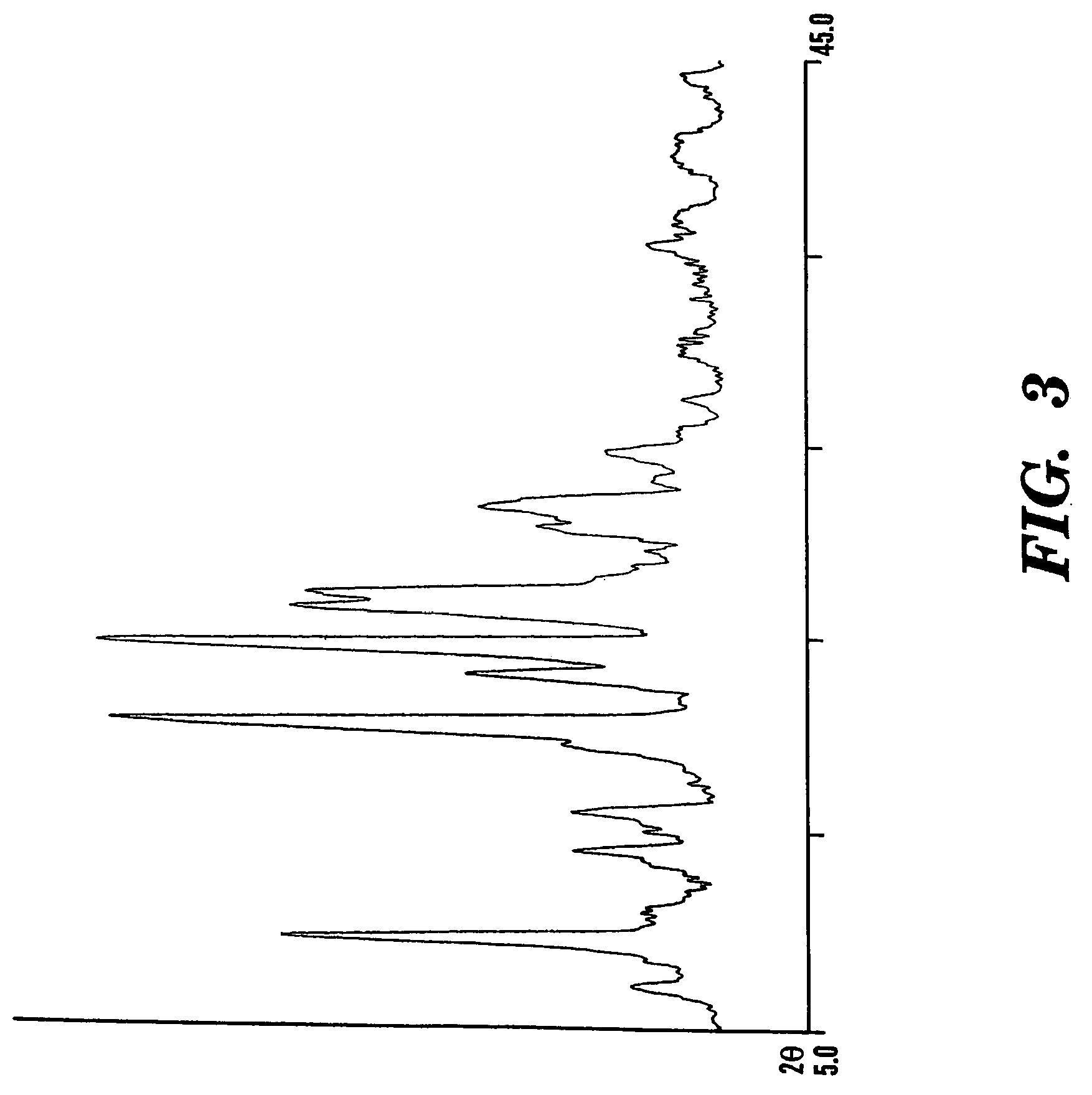
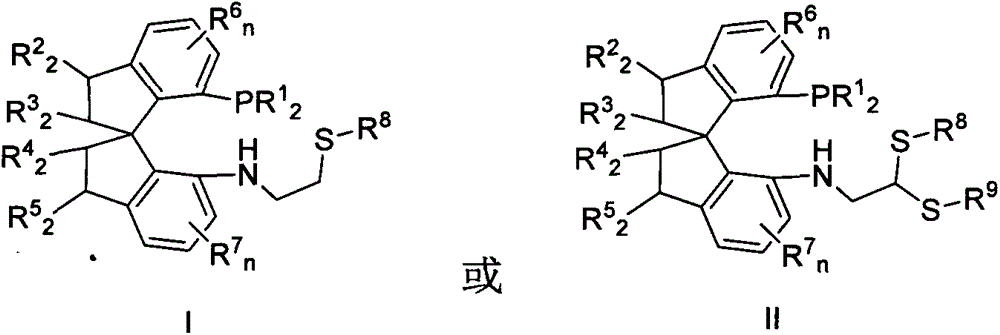
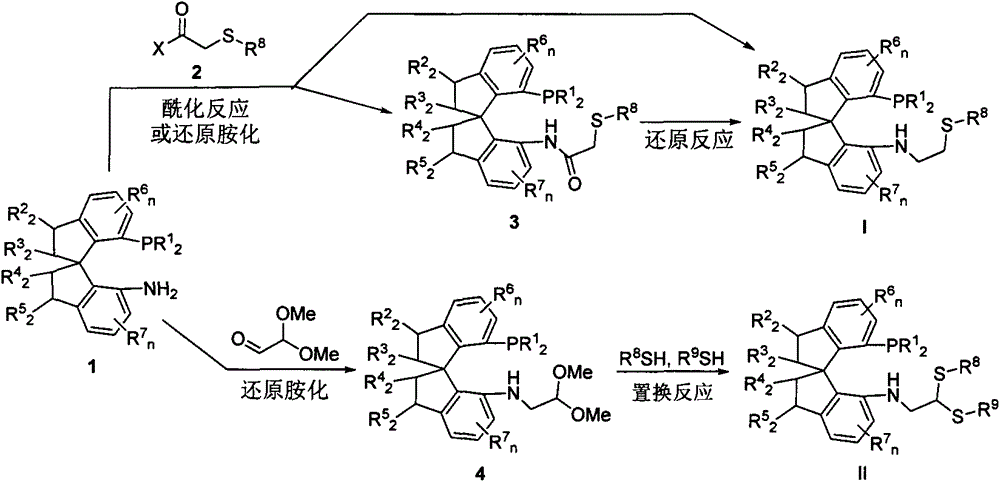
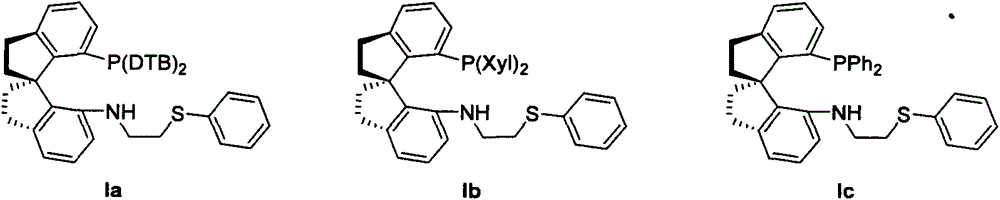
Chiral spiro phosphine-nitrogen-sulfur tridentate ligand and preparation method and application thereof
ActiveCN104892672AHigh catalytic activityHigh enantioselectivityCarbamic acid derivatives preparationIndium organic compoundsIridiumSulfur
The invention relates to a chiral spiro phosphine-nitrogen-sulfur tridentate ligand and a preparation method and application thereof. The chiral spiro phosphine-nitrogen-sulfur tridentate ligand is a compound with a formula I or formula II, or a racemate or optical isomer thereof, or a catalytically-acceptable salt thereof, and has the main structure characteristic of having a chiral spiro indan skeleton and a sulfoether group. The chiral spiro phosphine-nitrogen-sulfur tridentate ligand can be synthesized from chiral starting materials of 7-diaryl / alkyl phosphino-7'-amino-1,1'-spiro indan compound with a spiro skeleton. The chiral spiro phosphine-nitrogen-sulfur tridentate ligand and transition metal salt form a complex, which can be used in catalysis of an asymmetric catalytic hydrogenation reaction of a carbonyl compound. Especially, the iridium complex shows high catalytic activity (catalyst amount of 0.0002% mol) and enantioselectivity (up to 99.9%ee) in asymmetric hydrogenation reaction of beta-alkyl-beta-keto ester, and has practical value.
Owner:ZHEJIANG RAYBOW PHARM CO LTD
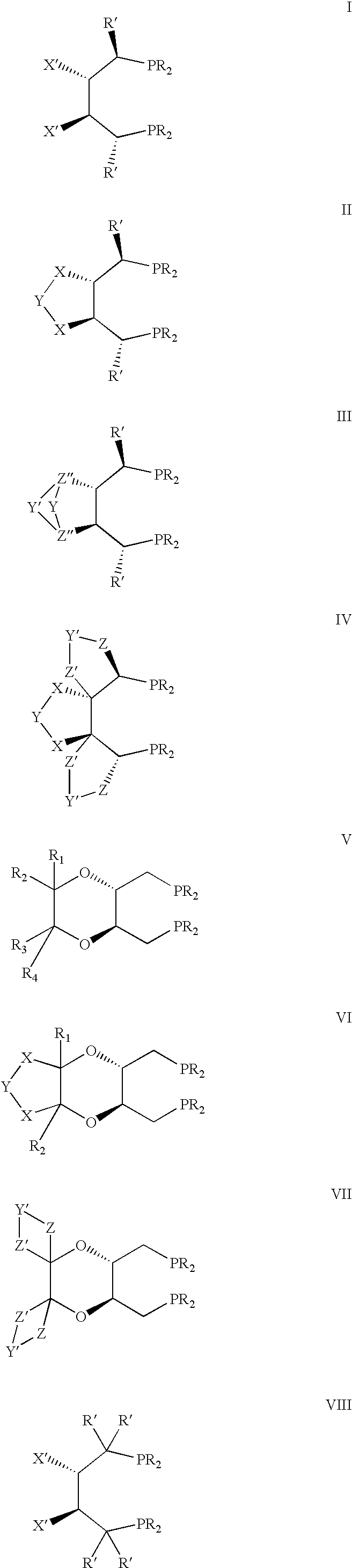
Chiral ligands, transition-metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6525210B1Carboxylic acid amides optical isomer preparationPreparation by carbon monoxide reactionIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include phospholanes, P,N ligands, N,N ligands, biphenols, and chelating phosphines. The ferrocene-based irridium (R,R)-f-binaphane complex reduces imines to the corresponding amines with 95-99.6% enantioselectivity and reduces beta-substituted-alpha-arylenamides with 95% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation of imines, asymmetric hydride transfer reactions, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
D-calcium pantothenate synthesis method
InactiveCN1765877AReduce usageSolve environmental problemsCarboxylic acid amides optical isomer preparationSynthesis methodsDiethyl oxalate
The invention discloses a synthesis method for D-calcium pantothenate, which comprises: synthesizing keto lactone pantothenate with diethyl oxalate, isobutanal and formaldehyde, reducing said product, using carbamidine carbonate to resolution out D-pantothenic carbonate and L- pantothenic carbonate; preparing the product with D-pantoic lactone, beta-alanine both from D-pantothenic carbonate and calcium metal. This invention avoids the application of hypertoxic sodium cyanide, stabilizes product quality, and increases material utilization ratio.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Process for the preparation of enantiomerically enriched beta amino acid derivatives
InactiveCN1972898AOrganic compound preparationCarboxylic acid amides optical isomer preparationMetalloleMedicinal chemistry
The present invention relates to a process for the efficient preparation of enantiomerically enriched beta amino acid derivatives wherein the amino group is unprotected. The product chiral beta amino acid derivatives are useful in the asymmetric synthesis of biologically active molecules. The process comprises an enantioselective hydrogenation of an amine-unprotected prochiral beta-amino acrylic acid or derivative thereof in the presence of a rhodium metal precursor complexed with a chiral mono- or bisphosphine ligand.
Owner:MERCK & CO INC +1
Chiral phosphines, transition metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6576772B1High enantioselectivityEnantioselectivity decreaseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include (R,S,S,R)-DIOP*. The ruthenium complex reduces enamide to the corresponding amine with up to 99% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation, hydride transfer, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, hydrocarboxylation, isomerization, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
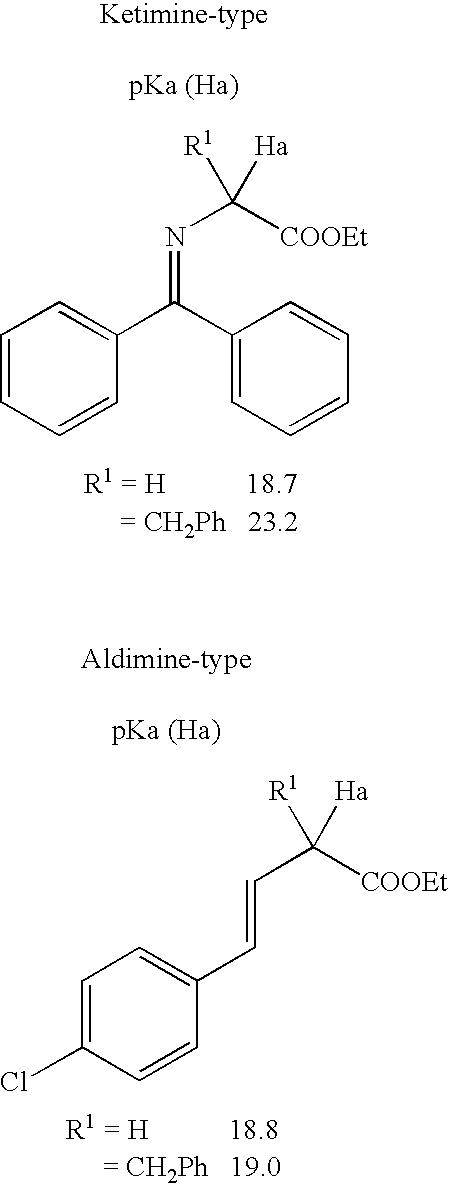
Process for production of mono-substituted alkylated compound using aldimine or derivative thereof
InactiveUS20090054679A1Less expensiveLess expensivelyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlkyl transferQuaternary ammonium cation
The present invention provides a method for producing asymmetrical mono-substituted alkylated compounds of α-amino acids that are represented by a specific formula, using an aldimine-type Schiff base. In the method of the present invention, the process of alkylating an aldimine-type Schiff base in a medium in the presence of an optically-active quaternary ammonium salt phase-transfer catalyst and an inorganic base is initiated, and subsequently the reaction is quenched at a time earlier than a time for completion of the stoichiometric reaction of the alkylation reaction, so that a mono-substituted alkylated product with high optical purity can be obtained.
Owner:KISHIDA CHEM
Method for preparing (S)-2-aminobutanamide hydrochloride
InactiveCN102898324ANo pollution in the processProduction operation risk is smallOrganic compound preparationCarboxylic acid amides optical isomer preparationHydrogen chlorideTartrate
The invention discloses a method for preparing (S)-2-aminobutanamide hydrochloride. The method comprises the following steps of: A1, ammoniating; A2, splitting; and A3, salifying: starting a salifying reaction kettle jacket for refrigerating a salt solution, adding hydrogen chloride isopropanol, cooling to 5 DEG C below zero, adding (S)-2-aminobutanamide tartrate obtained by splitting in batches, heating and refluxing for 2 hours, cooling, and centrifuging to obtain (S)-2-aminobutanamide hydrochloride, wherein the molar ratio of 2-aminobutanamide hydrochloride to hydrogen chloride isopropanol is 1:1.5. The method has the advantages of no use of virulent hydrocyanic acid, low production operation risk, prevention of air pollution, high product purity and low production cost.
Owner:FUXIN LONGRUI CHEM CO LTD
Formation of tetra-substituted enamides and stereoselective reduction thereof
InactiveUS7629470B2Carboxylic acid amides optical isomer preparationPreparation by cyanide reactionCouplingTetra
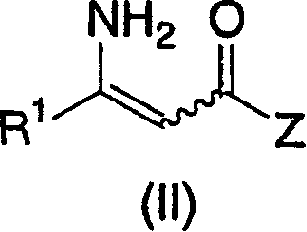
The present invention is directed to a practical process for the preparation of an enamide (II) by palladium catalyzed coupling of a primary amide (IV) with a compound of structural formula (III), as shown below: As well as to crystalline forms of a compound produced by this process, in particular, an anhydrous crystal form, Form B, and crystalline solvates falling into three patterns, Type 1, Type 2, and Type 3, and crystalline intermediate compounds produced in the process. Still further, the present invention relates to the stereoselective reduction of the tetrasubstituted enamide (II) to the corresponding amide (I).
Owner:MERCK SHARP & DOHME CORP
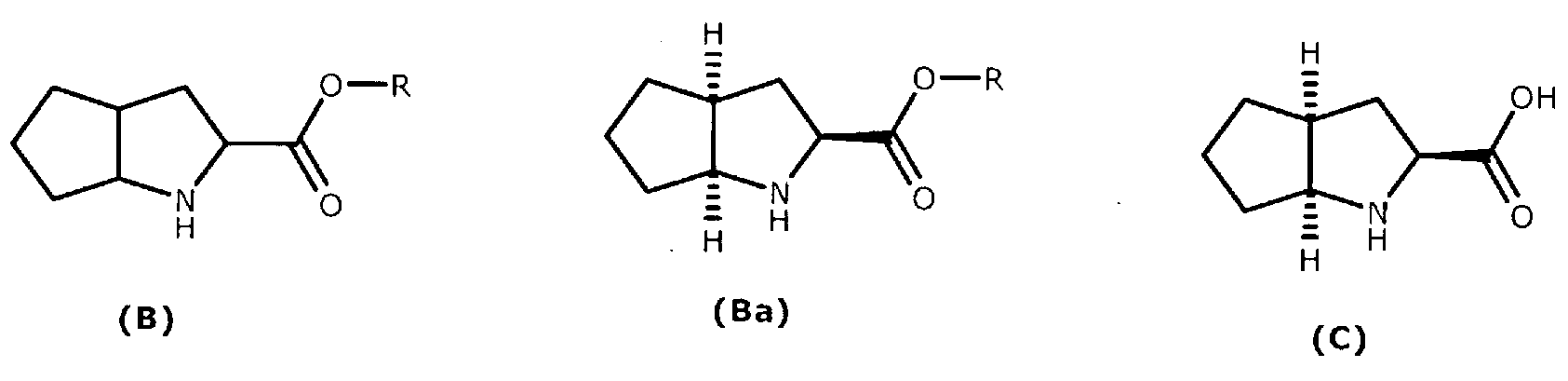
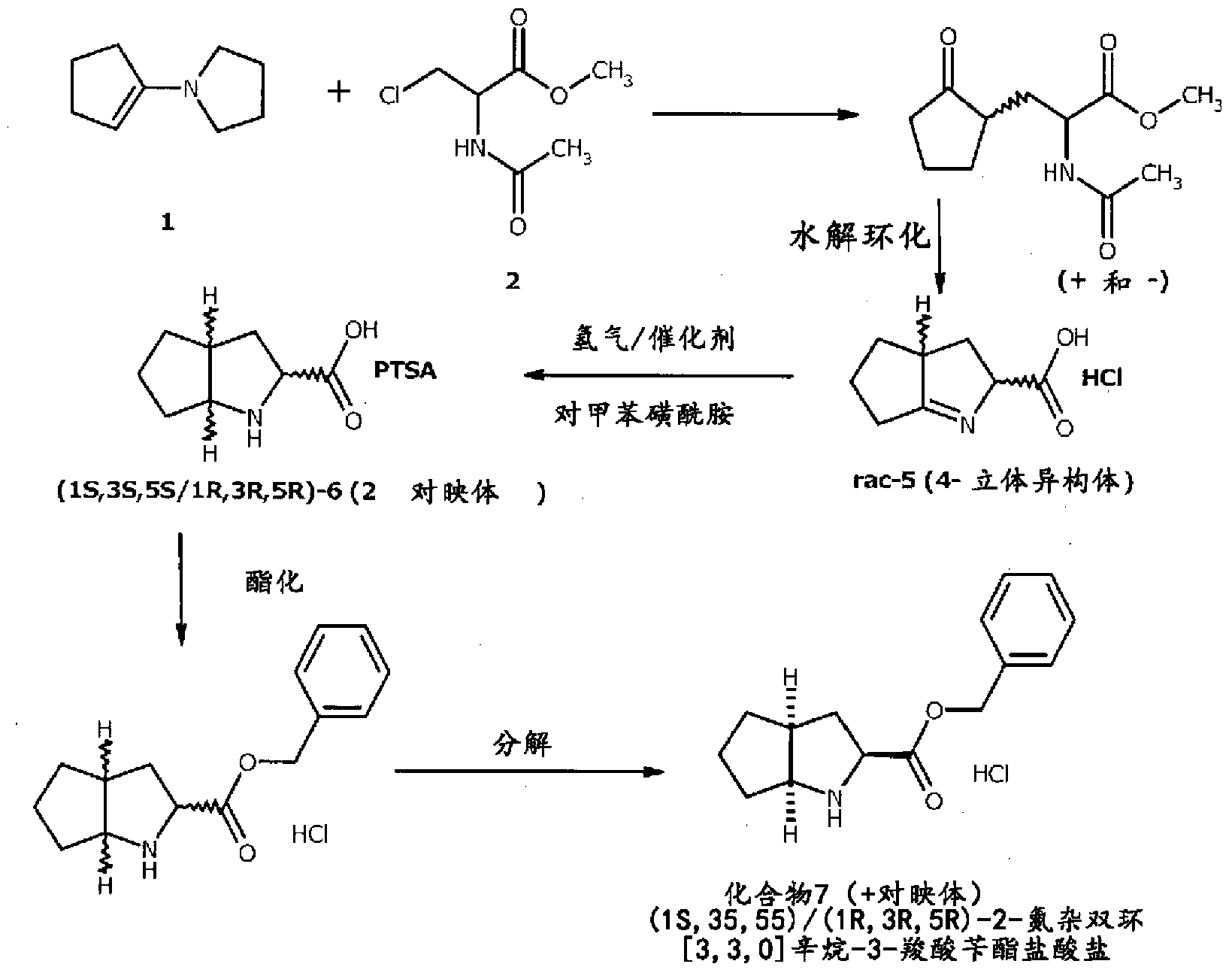
A method for preparing ramipril
ActiveCN103282350AAvoid wastingIncrease productionCarboxylic acid amides optical isomer preparationPropanoic acidSerine endopeptidase
Enantio-specific synthesis of optically pure (2S)-acetylamino-3-(2-oxo-cyclopentyl)- propionic acid (I) comprising converting enantiomeric mixture of (1 -4C alkyl)-2- acetylamino-3-(2-oxocyclopentyl) propionoate (II) (+ and -) under the influence of an Alkaline serine endopeptidase is disclosed. The invention further describes use of optically pure (2S)-acetylamino-3-(2-oxocyclopentyl)-propionic acid (I) formed by the process of present invention, in the preparation of Ramipril.
Owner:AARTI HEALTHCARE LTD
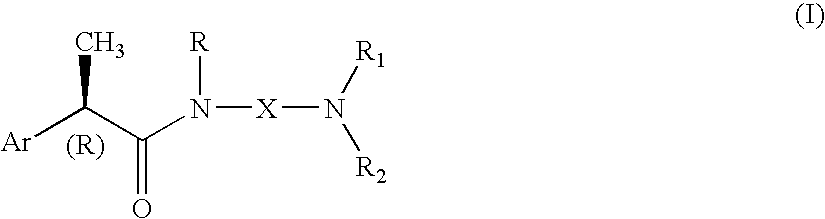
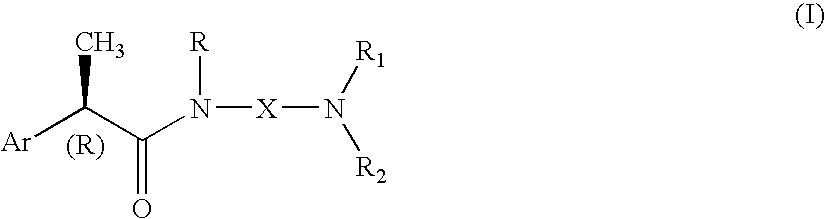
Omega aminoalkylamides of R-2 aryl propionic acids as inhibitors of the chemotaxis of polymorphonucleate and mononucleate cells
InactiveUS20050080067A1Suppression problemBiocidePeptide/protein ingredientsPropanoic acidEnantiomer
(R)-2-Arylpropionamide compounds of formula (I) are described. The process for their preparation and pharmaceutical preparations thereof are also described. The 2-Arylpropionamides of the invention are useful in the prevention and treatment of tissue damage due to the exacerbate recruitment of polymorphonuclear leukocytes (leukocytes PMN) and of monocytes at the inflammatory sites. In particular, the invention relates to the R enantiomers of omega-aminoalkylamides of 2-aryl propionic acids, of formula (I), for use in the inhibition of the chemotaxis of neutrophils and monocytes induced by the C5a fraction of the complement and by other chemotactic proteins whose biological activity is associated with activation of a 7-TD receptor. Selected compounds of formula (I) are dual inhibitors of both the C5a-induced chemotaxis of neutrophils and monocytes and the IL-8-induced chemotaxis of PMN leukocytes. The compounds of the invention are used in the treatment of psoriasis, ulcerative cholitis, glomerular nephritis, acute respiratory insufficiency, idiopathic fibrosis, rheumatoid arthritis and in the prevention and the treatment of injury caused by ischemia and reperfusion.
Owner:DOMPE FARM SPA
Asymmetric catalysis based on chiral phospholanes and hydroxyl phospholanes
InactiveUS20030040629A1Easy to handleAdd supportCarboxylic acid esters preparationCarboxylic acid amides optical isomer preparationNatural productCombinatorial chemistry
Chiral phosphine ligands derived from chiral natural products including D-mannitol and tartaric acid. The ligands contain one or more 5-membered phospholane rings with multiple chiral centers, and provide high stereoselectivity in asymmetric reactions.
Owner:PENN STATE RES FOUND
Preparation of capsicine
InactiveCN101298424ALarge amount of sampleSimple production processCarboxylic acid amides optical isomer preparationPlant ingredientsAlkaline waterBenzene
The invention discloses a method for preparing capsicine by adopting styrene macroporous resin. Compared with the technique disclosed by the existing invention and literature report, the method has the advantages of not using a large quantity of organic solvents, needing no special devices, large absorption amount of the absorption materials and being easy to regenerate, etc.; the method relates to a novel technique which is environment friendly for separating and refining capsicine from pepper. The method takes proper cracked peppers as the raw material and adopts alkaline water to extract; an extraction liquid is filtered or centrifugally deposited; flow speed is controlled to add the extraction liquid to a pre-processed resin column and then proper water and hydrous ethanol or hydrous acetone is used for washing in sequence; a single organic solvent is used for eluting; then an eluting liquid is decompressed, condensed and deposited, the capsicine crystallization is separated out and then the capsicine is obtained by drying. By taking the capsicine as a comparison product, the capsicine content of the crystallization detected by HPLC is more than 90 percent.
Owner:QILU UNIV OF TECH
Preparation method of L-2-aminobutanamide hydrochloride
ActiveCN102584622ASimple and safe operationQuality improvementCarboxylic acid amides optical isomer preparationUnit operationHydrochloride
The invention provides a preparation method of L-2-aminobutanamide hydrochloride. The method comprises the following steps: based on crude DL-2-aminobutanamide as a starting raw material, reacting with aromatic aldehyde to form DL-Schiff-base, separating, dissociating, and salifying to obtain L-2-aminobutanamide hydrochloride, wherein the Schiff base can be directly separated by using L-tartaric acid, the chiral purity ee value of the product is no less than 99%, the quality is stable, and the separation mother liquor can be recycled through racemization. According to the invention, the raw materials used in each step of the method are cheap and available, the unit operations are simple and safe, the post-treatment is convenient, the requirements on the equipment are low and the method issuitable for industrialized production.
Owner:ABA CHEM NANTONG
Method for preparing D-asparagine and D-homoserine
InactiveCN101333175AReduce pollutionReduce manufacturing costOrganic compound preparationCarboxylic acid amides optical isomer preparationSolventReducing agent
A preparation method for D-asparagine and D-homoserine comprises the following steps: a, DL-aspartic acid methyl ester is taken as raw material, L-DBTA as resolving agent, water as solvent to carry through resolution reaction for 0.5-5 hours at 40-95 DEG C so as to get D-aspartic acid methyl ester-L-DBTA salt; b, the D-aspartic acid methyl ester-L-DBTA salt is stirred and hydrolyzed for 2-4 hours at room temperature in the presence of alkali to get D-aspartate-beta-methyl ester; c, the D-aspartate-beta-methyl ester is ammonolyzed in a solvent with ammonia gas, ammonia or liquid ammonia to get the D-asparagine; d, the D-aspartate-beta-methyl ester is deoxidized with reductant for 1-10 hours at 0-80 DEG C, neutralized with acid and then concentrated and processed through hydrogen or sodium cation exchange resin to get the D-homoserine. The invention provides a technology which uses cheap DL-aspartate to prepare D-asparagine and D-homoserine through esterification, resolving, ammonolysis, deoxidizing and other processes, thereby laying a solid foundation for the industrial production of two important D-type amino acids.
Owner:SOUTHEAST UNIV
Enantioselective process for cycloalkenyl ?-substituted alanines
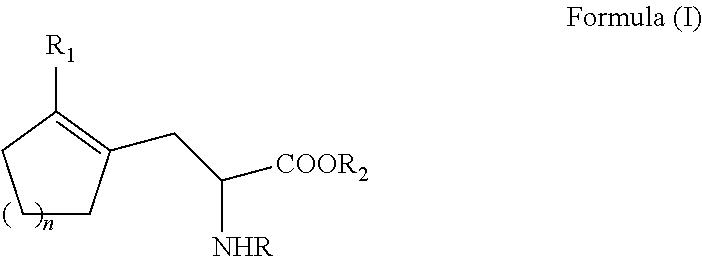
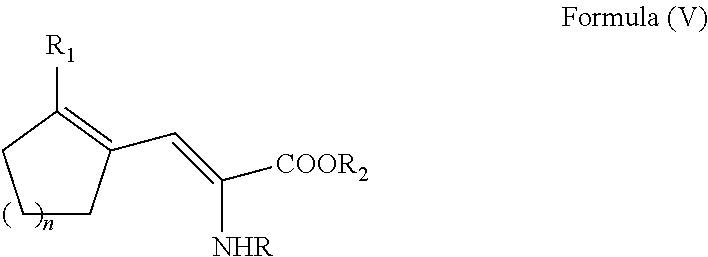
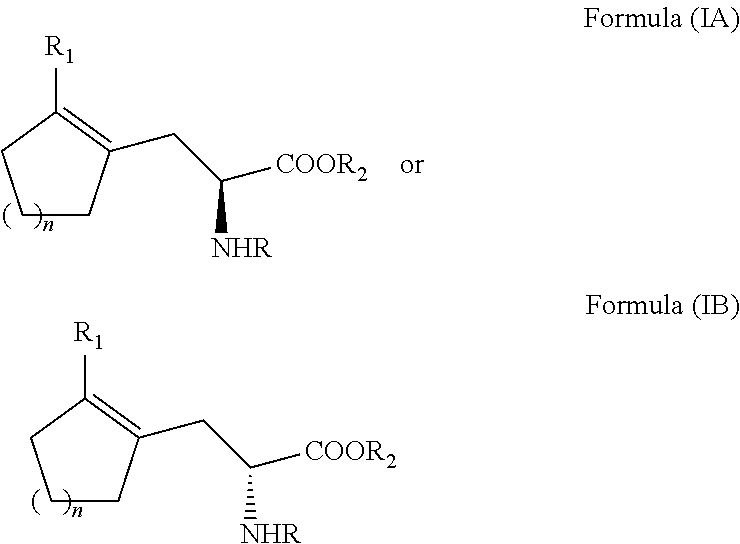
ActiveUS20110257408A1Easy to convertHigh yieldOrganic compound preparationCarboxylic acid amides optical isomer preparationCycloalkeneAsymmetric hydrogenation
A process for preparing an enantiomerically enriched cycloalkene-substituted alanine compound having the structure:by asymmetrically hydrogenating a dehydro amino acid compound having the structure:in a suitable reaction media in the presence of a catalyst having a transition metal moiety complexed to a chiral phosphine ligand to prepare enantiomerically enriched cycloalkene substituted alanine compounds having the structure of Formula (IA) or (IB), which are key intermediates for the ACE inhibitors ramipril and perindolpril:
Owner:CHIRAL QUEST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com