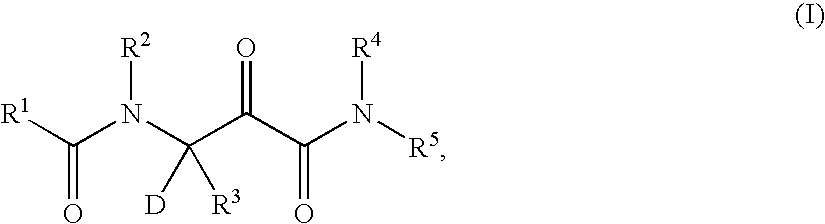
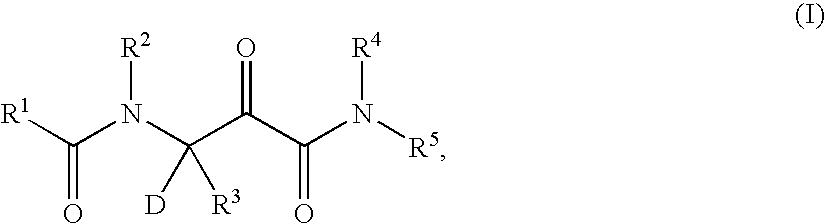
Deuterated hepatitis C protease inhibitors
a protease inhibitor and deuterated hepatitis c technology, applied in the field of deuterated hepatitis c protease inhibitors, can solve the problems of insufficient anti-hcv agents or treatments, inability to broadly effective treat the debilitating progression of chronic hcv, and significant side effects of interferons, so as to enhance the bioavailability of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N—((S)-1-(cyclopropylamino)-1,2-dioxo-3-deutero-hexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide
[0220]
Step a: Preparation of
[0221]
[0222] The deuterated sultam (i.e., compound vi shown in the scheme below) was prepared by known methods such as those described in Y. Elemes and U. Ragnarsson, J. of Chem. Soc., Perkin 1, 1996, 6, 537; W. Oppolzer, et.al., Helv. Chim. Acta., 1994, 25: 2363, by using the corresponding unsubstituted sultam and propyl iodide.
[0223] 17.32 g of compound vi (45.8 mmol) and 229 mL of THF were then charged into a 500 mL round-bottomed-flask with a magnetic stir bar and N2 inlet. The resulting solution was cooled to −78° C. and n-BuLi (31.5 mL of a 1.6 M solution in hexane, 50.3 mmol) was added with a syringe pump over an hour. The resulting yellow solution was aged for 30 minutes before a solution of HPMA (56 mL) and n-PrI (13.4 mL, 137 mmo...
example 2
Preparation of (2S,3S)-3-amino-3-deutero-N-cyclopropyl-2-hydroxyhexanamide hydrochloride
[0239]
[0240] The scheme shown above illustrate the total synthesis of the title compound. Each step is described in detail as follows.
Step 1: Preparation of 3-deutero-(E)-hex-2-en-1-ol
[0241] To a three-neck 250 mL round bottom flask equipped with mechanical stirrer and reflux condenser was charged 2-hexyn-1-ol (10 g, 0.1 mole) and THF (100 mL, 10 vol). The resulting mixture was cooled to 0±5° C. and then Red-A1 (65% in Toluene, 32 mL, 1.6 eq) was added slowly under a nitrogen atmosphere between 0° C. and 20° C. The resulting mixture was allowed to be warmed up to 25° C. and stirred for 5 hours. The reaction mixture was cooled down to −5±5° C. and D2O (8.2 g, 4 eq.) was added drop wise between 0° C. and 15° C. To the resulting mixture was charged IPAC (50 mL, 5 vol) and saturated NH4Cl solution (50 mL, 5 vol.). After stirring the mixture for 10 min, the white solid formed was filtered out. The ...
example 3
Assay for Measuring Epimerization Rate
[0258] The deuterated compounds of this invention undergo slow epimerization as follows:
[0259] The epimerization rate was measured according to the following assay. Specifically, 100 μL medium (buffer, rat plasma, dog plasma, or human plasma) was added into a 96-well deep plate. To the plasma was then added 10 μL acetonitrile solution containing a test compound (1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N—((S)-1-(cyclopropylamino)-1,2-dioxo-3-deutero-hexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide (at 1 uM or 10 uM) and 1200 μL ethyl acetate into the 96 deep-well plate (2 mL) by using a TomTec liquid handling workstation (Hamden, Conn., USA). The plate was then covered tightly and shaken with a vortex for 20 minutes before it was centrifuged at 3000 rpm for 10 minutes. After centrifuge, 900 μL of the supernatant was transferred to a new V-shape 96 deep-well plate using TomTec, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com