Method for preparing (S)-2-aminobutanamide hydrochloride
A technology of aminobutyramide and hydrochloride, which is applied in the preparation of carboxylic acid amide, optical isomers of carboxylic acid amide, and preparation of organic compounds, etc. It can solve air pollution, high risk of production operation, and S-2 aminobutyramide High acid prices and other issues, to achieve the effects of no air pollution, less risk in production operations, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The present invention will be described in detail below in conjunction with specific embodiments.
[0019] 1. Ammonification:
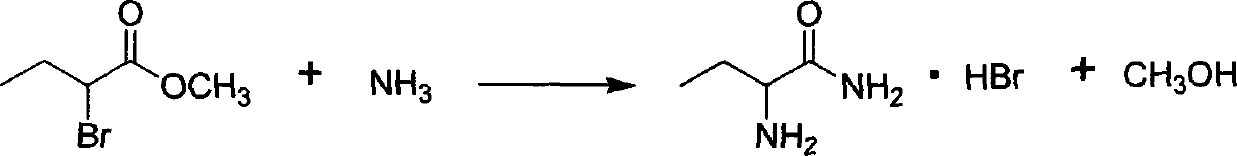
[0020] Reaction equation:
[0021] 5000 kilograms of ammoniacal liquor are added in the ammoniation still from the ammoniacal liquor tank, add 2-bromobutyrate methyl 800kg when opening the ammoniation still jacket freezing brine and cooling to-5 DEG C. -5°C--5°C temperature controlled reaction for 2 hours, the system was naturally heated to 45°C, sampled for analysis, high-pressure liquid phase analysis 2-bromobutyrate methyl ester reaction was completed and the reaction was terminated.
[0022] Add the ammoniated solution into the still, open the jacket steam to raise the temperature to 50°C, discharge the ammonia gas into the ammonia water storage tank (open the jacket cooling water in the ammonia water storage tank to cool down), after the ammonia discharge is completed, raise the temperature to 80°C, and distill and recover the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com