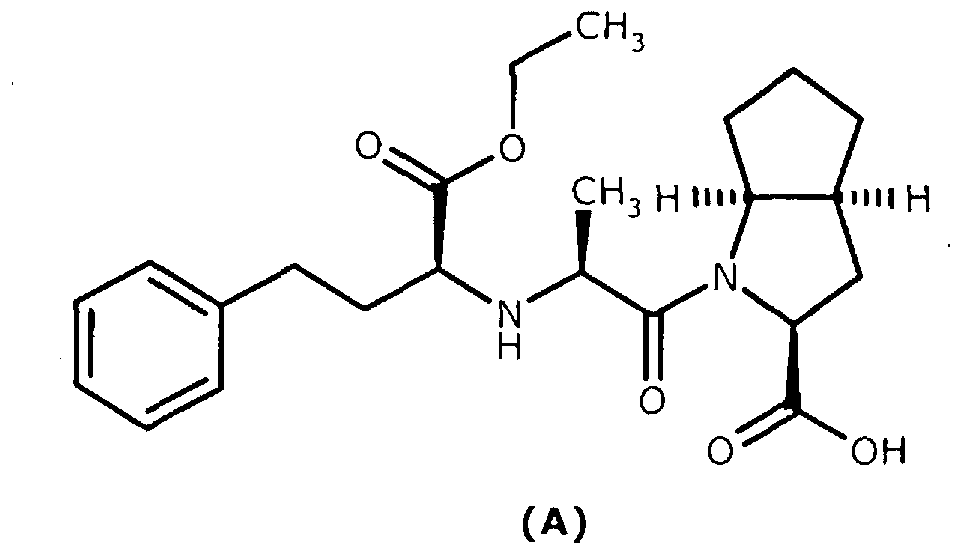
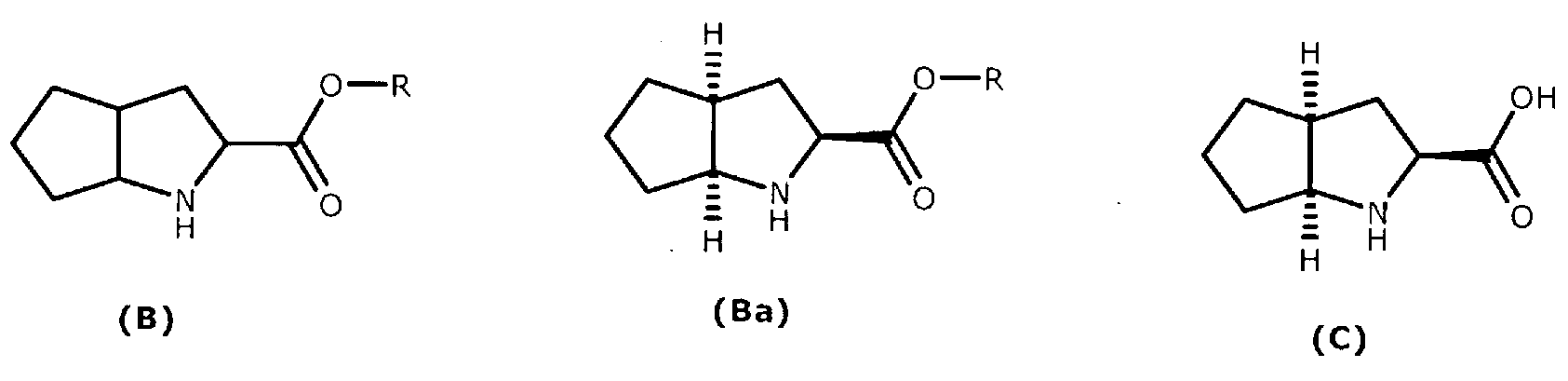
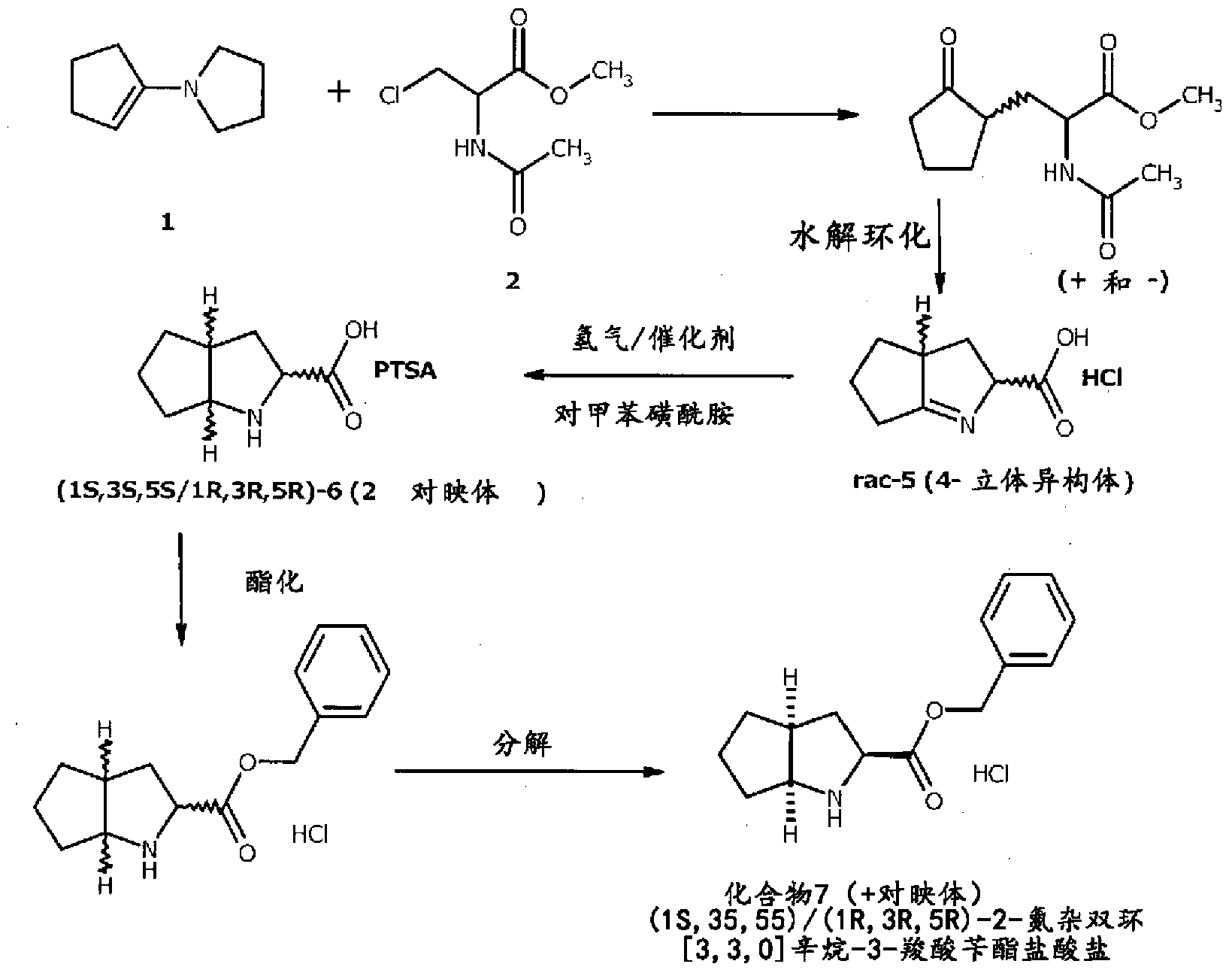
A method for preparing ramipril
A molecular formula, optical technology, used in the preparation of intermediates, angiotensin-converting enzyme (ACE) inhibitors, and the synthesis of stereoisomers, can solve the problem of unsuitable industrial production, long efforts, and difficulties in obtaining optical intermediates And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of (2S)-acetylamino-3-(2-oxocyclopentyl)-propionic acid (I)
[0048] 2-Acetamido-3-(2-oxocyclopentyl)-propionic acid methyl ester (90 g) was dissolved in water (350 ml) and stirring was continued until the solution was clear. The pH of the above solution was adjusted to 6.8 with freshly prepared sodium carbonate solution. Immobilized Protex 6L (0.75 g) was added to the solution with stirring. At 24-26° C., adjust the pH value of the reaction material to 6.2 to 6.8 with sodium carbonate, and react for 20 hours. The pH value of the above reaction solution was adjusted to 6 with freshly prepared 10% acetic acid solution. The catalyst was isolated by filtration of the reaction mass and the filtrate was extracted with dichloromethane (MDC) (3 x 250ml). After separation of the two phases, the pH of the aqueous layer was adjusted to 3 with activated amber 15 resin (30 g). The resin was activated with concentrated hydrochloric acid. The reaction material was f...
Embodiment 2
[0050] Preparation of (2S)-2,3,3a,4,5,6-hexahydro-cyclopentopyrrole-2-carboxylic acid (IV) hydrochloride
[0051] The product obtained in Example 1 (15 g) was dissolved in water (30 ml), and concentrated hydrochloric acid (12 ml) was added. The mixture was heated to reflux and maintained at reflux temperature for 3 hours. MDC (3×45ml) was added to the reaction solution and extracted thoroughly. The water layer was vacuum distilled below 45°C to obtain the hydrochloride (11 g ) (purity: 99.1%; 97.3%ee).
Embodiment 3
[0053] Preparation of the hydrochloride salt of (2S,3aS,6aS)-octahydrocyclopentapyrrole-2-carboxylic acid (C)
[0054] While stirring, the hydrochloride (10 g) of (2S)-2,3,3a,4,5,6-hexahydro-cyclopentapyrrole-2-carboxylic acid (IV) obtained in Example 2 was added to acetic acid (60ml). The reaction mixture was heated to 40-45°C. Add 5% palladium-carbon catalyst (0.8g), and hydrogenate it at a hydrogen pressure of 5-6Kg / cm2 at a temperature of 55-60°C. After ensuring completion of the reaction, the reaction mass was filtered to remove the catalyst and concentrated. Acetone (20ml) was added and the reaction mixture was stirred at 30-35°C for 30 minutes. The mixture was cooled to 0-5 °C. The reaction mass was filtered and dried. The obtained solid was (2S,3aS,6aS)-octahydrocyclopentapyrrole-2-carboxylic acid hydrochloride (6 g) (purity: 99.2%; 97.5%ee).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com