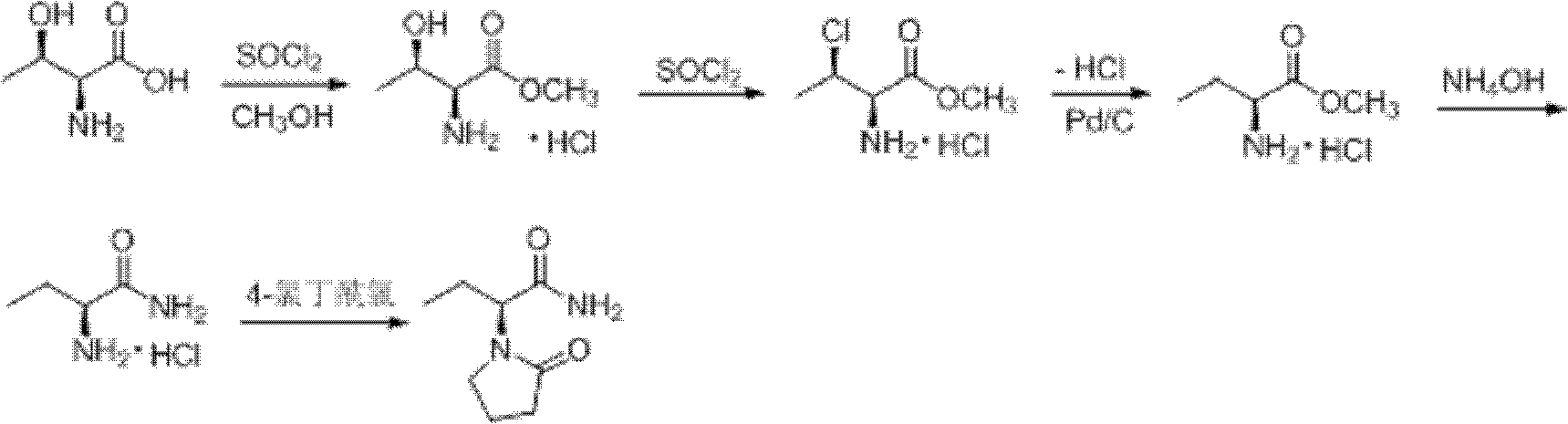Preparation method of L-2-aminobutanamide hydrochloride
A technology of aminobutyramide and DL-2-, applied in the preparation of carboxylic acid amide optical isomers, organic chemistry, etc., can solve the problems of product suction filtration and unstable quality, the impact of splitting process, production restrictions, etc., to achieve The effect of low equipment requirements, simple and safe unit operation, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
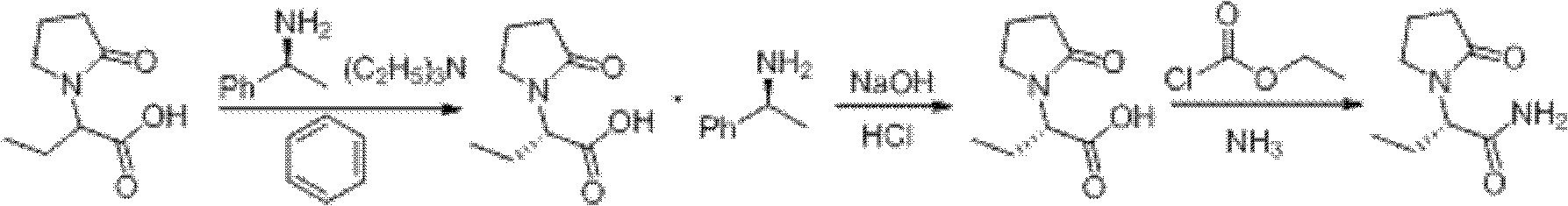
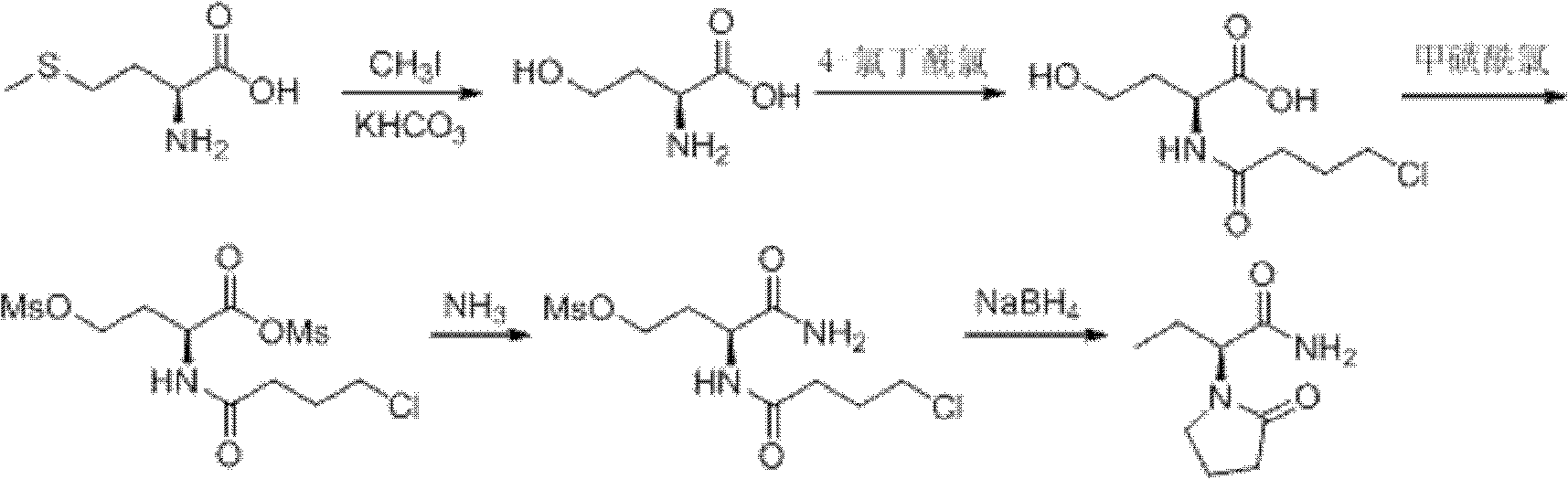
Image
Examples
Embodiment 1
[0039] 1.1 Purification:
[0040] In a 1000ml flask, dissolve 100g of crude DL-2-aminobutyramide in 500ml of water, add 106g of benzaldehyde, the solid slowly precipitates, continue to stir at room temperature for 5 hours, filter, wash with a small amount of water, and dry the solid under vacuum at 30°C. 158 g of vortexed Schiff base was obtained with a yield of 85.0%.
[0041] As a preferred version of the purification step, the aromatic aldehyde can also be salicylaldehyde, p-tolualdehyde, p-ethylbenzaldehyde, 3,4-dimethylbenzaldehyde and 2,4,6-trimethylbenzaldehyde One or several, wherein, the mol ratio of DL-2-aminobutyramide crude product to aromatic aldehyde can be 1: 0.8~2, preferably 1:1~1.5; Mixed DL-2-aminobutanamide crude product and The weight ratio of water may be 1:1-100, preferably 1:5-20.
[0042] 1.2 Split:
[0043] In a 1000ml flask, add 150g of Schiff's base and 500ml of absolute ethanol, stir to dissolve the solid, add 30g of L-tartaric acid / 150ml of abs...
Embodiment 2
[0052] 2.1 Purification:
[0053] In a 1000ml flask, dissolve 100g of DL-2-aminobutanamide crude product in 650ml of water, add 106g of benzaldehyde, the solid slowly precipitates out, continue to stir at room temperature for 5 hours, filter, wash with a small amount of water, and dry the solid under vacuum at 30°C to obtain 162 g of vortexed Schiff base, yield 87.1%.
[0054] 2.2 Split:
[0055] In a 1000ml flask, add 150g of Schiff's base and 500ml of anhydrous methanol, stir to dissolve the solid, add 30g of L-tartaric acid / 150ml of anhydrous methanol solution at 20-25°C, react at 20-25°C for 6 hours, filter, and use A small amount of anhydrous methanol was washed, and the solid was vacuum-dried at 60° C. to constant weight to obtain 58.8 g of L-2-aminobutyramide tartrate as a solid, with a yield of 41.5%.
[0056] 2.3 Free into salt:
[0057] In a 500ml flask, add 50g of L-2-aminobutyramide tartrate, 250ml of absolute ethanol, cool to 0-5°C, inject ammonia gas to satura...
Embodiment 3
[0061] 3.1 Purification:
[0062] In a 1000ml flask, dissolve 100g of DL-2-aminobutanamide crude product in 650ml of water, add 122g of benzaldehyde, the solid slowly precipitates out, continue to stir at room temperature for 5 hours, filter, wash with a small amount of water, and dry the solid under vacuum at 30°C to obtain 187 g of vortexed Schiff base, yield 93.0%.
[0063] 3.2 Split:
[0064] In a 1000ml flask, add 180g of Schiff's base and 500ml of absolute ethanol, stir to dissolve the solid, add 33g of L-tartaric acid / 150ml of absolute ethanol solution at 20-25°C, react at 20-25°C for 6 hours, filter, and use After washing with a small amount of absolute ethanol, the solid was vacuum-dried at 70° C. to constant weight to obtain 70.0 g of L-2-aminobutyramide tartrate solid, with a yield of 45.0%.
[0065] 3.3 Free salt formation:
[0066] In a 500ml flask, add 60g of L-2-aminobutyramide tartrate, 300ml of absolute ethanol, cool to 0-5°C, feed ammonia to saturation, co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com