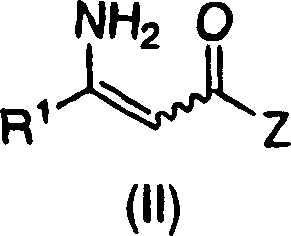
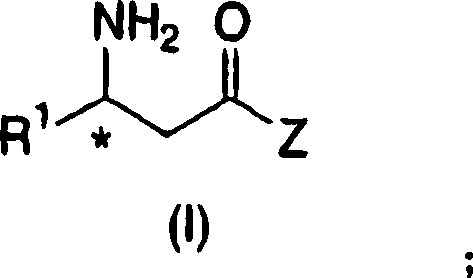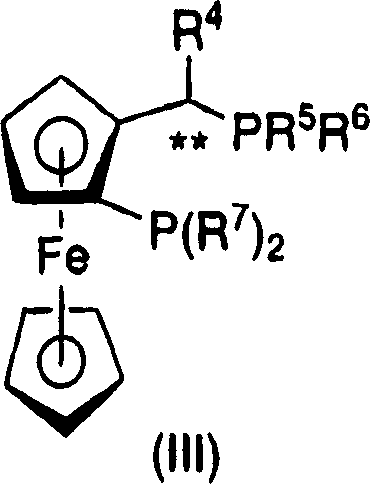Process for the preparation of enantiomerically enriched beta amino acid derivatives
An enantiomer, alkyl technology, applied in the field of β-amino acid derivatives, can solve problems such as synthesis difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0230]
[0231] (2R)-4-oxo-4-[3-trifluoromethyl-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazine-7(8H)- base]-1- (2,4,5-trifluorophenyl)butane-2-amine (2-5)
[0232] Preparation of 3-(trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo[4,3-a]pyrazine hydrochloride (1-4)
[0233] plan 1
[0234]
[0235] Step A: Preparation of bishydrazide (1-1)
[0236] Hydrazine (20.1 g, 35 wt% in water, 0.22 mol) was mixed with 310 mL of acetonitrile. 31.5 g of ethyl trifluoroacetate (0.22 mol) were added within 60 minutes. The internal temperature increased from 14°C to 25°C. The resulting solution was aged at 22-25°C for 60 minutes. The solution was cooled to 7°C. At a temperature below 16°C, 17.9 g of a 50 wt% aqueous NaOH solution (0.22 mol) and 25.3 g of chloroacetyl chloride (0.22 mol) were added simultaneously within 130 minutes. When the reaction was complete, the mixture was vacuum distilled at 27-30°C under a vacuum of 26-27 Hg to remove water and ethanol. During th...
Embodiment 2
[0287]
[0288] (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazine-7(8H )-base]-1- (2,4,5-trifluorophenyl)butane-2-amine (2-5)
[0289]
[0290] Bis(norbornadiene) rhodium(I) tetrafluoroborate {[Rh(nbd) 2 ] BF 4} (41.55mg, 0.1mmol), ligand D (69.73mg, 0.1mmol) and enaminoamide 2-4 (45 g, 111.1 mmol) into the flask. To this mixture was added 37.5 mL of a solvent mixture of methanol (very dry and degassed) and 112.5 mL of 2,2,2-trifluoroethanol (distilled and degassed). The slurry was then transferred to a stainless steel autoclave under nitrogen and sealed. The autoclave was then heated to 50°C and pressurized to 500 psig with hydrogen. Analysis of a sample taken after 17 hours using HPLC confirmed that the reaction was complete giving 94% assay yield and 94% ee.
Embodiment 3-5
[0292]
[0293] Table 1 a
[0294] Example
Ligand
metal precursor
%Yield b
%ee c
structure
3
4
5
A
B
C
[Rh(cod) 2 ] BF 4
[Rh(cod)Cl] 2
[Rh(cod)Cl] 2
77
58
15
88
76
78
R
R
S
[0295] a: Reaction conditions: 5 mol% metal precursor, 5 mol% ligand, 90 psig H in TFE 2 , 50°C, 18 hours;
[0296] b: Tested by HPLC; c: AS-RH chiral column eluted with 20% acetonitrile / water as mobile phase, tested by chiral HPLC
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com