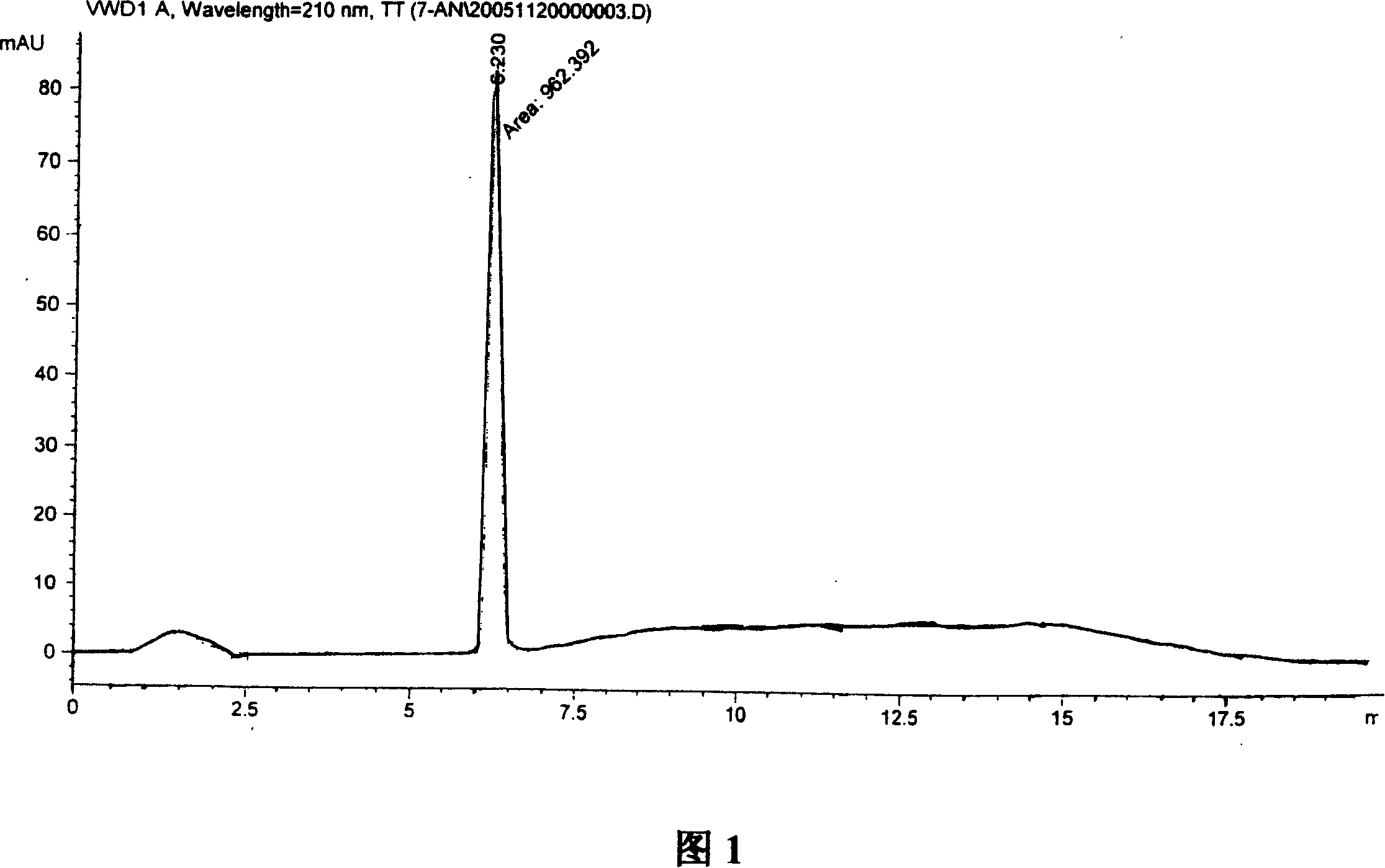
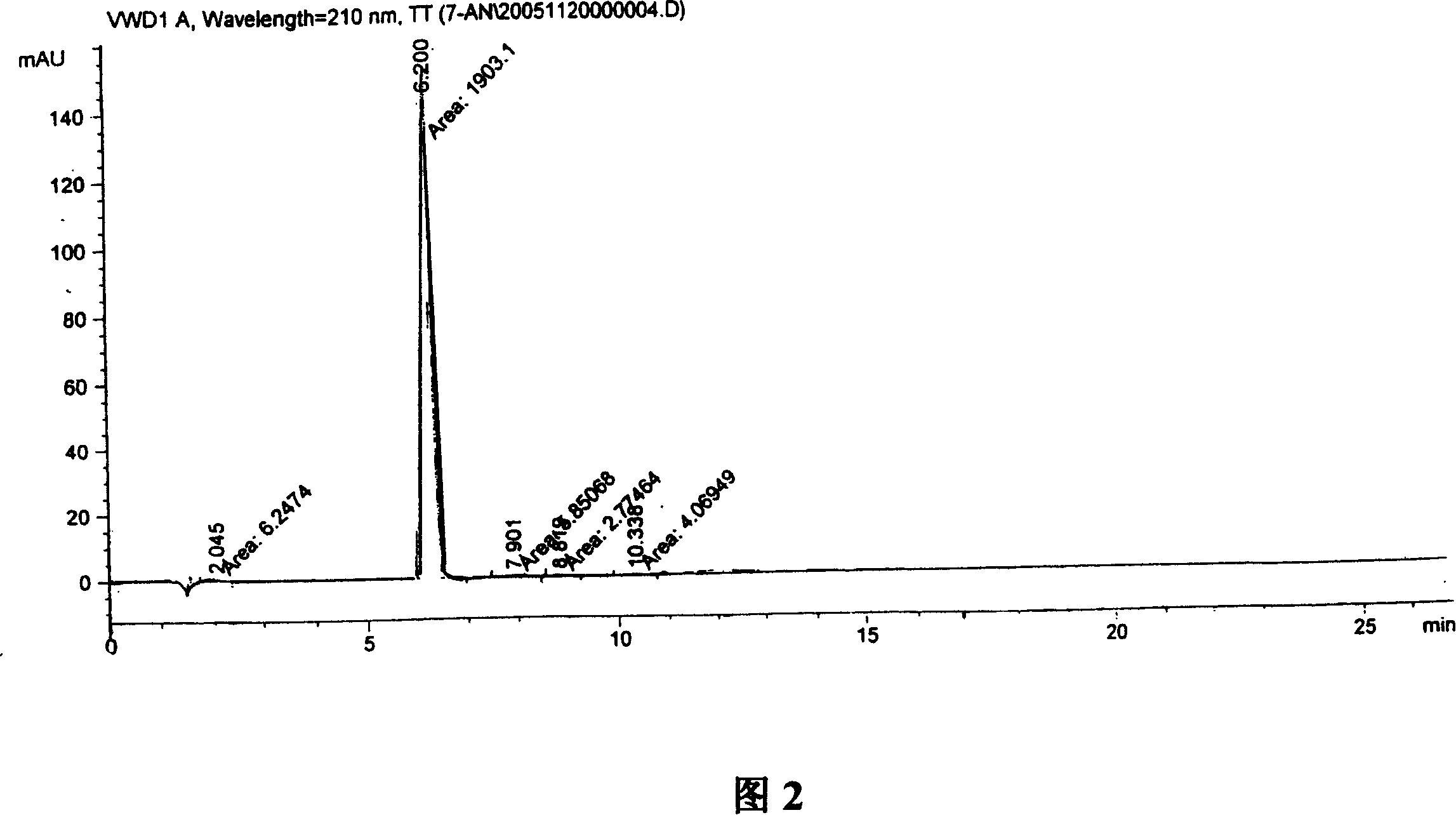
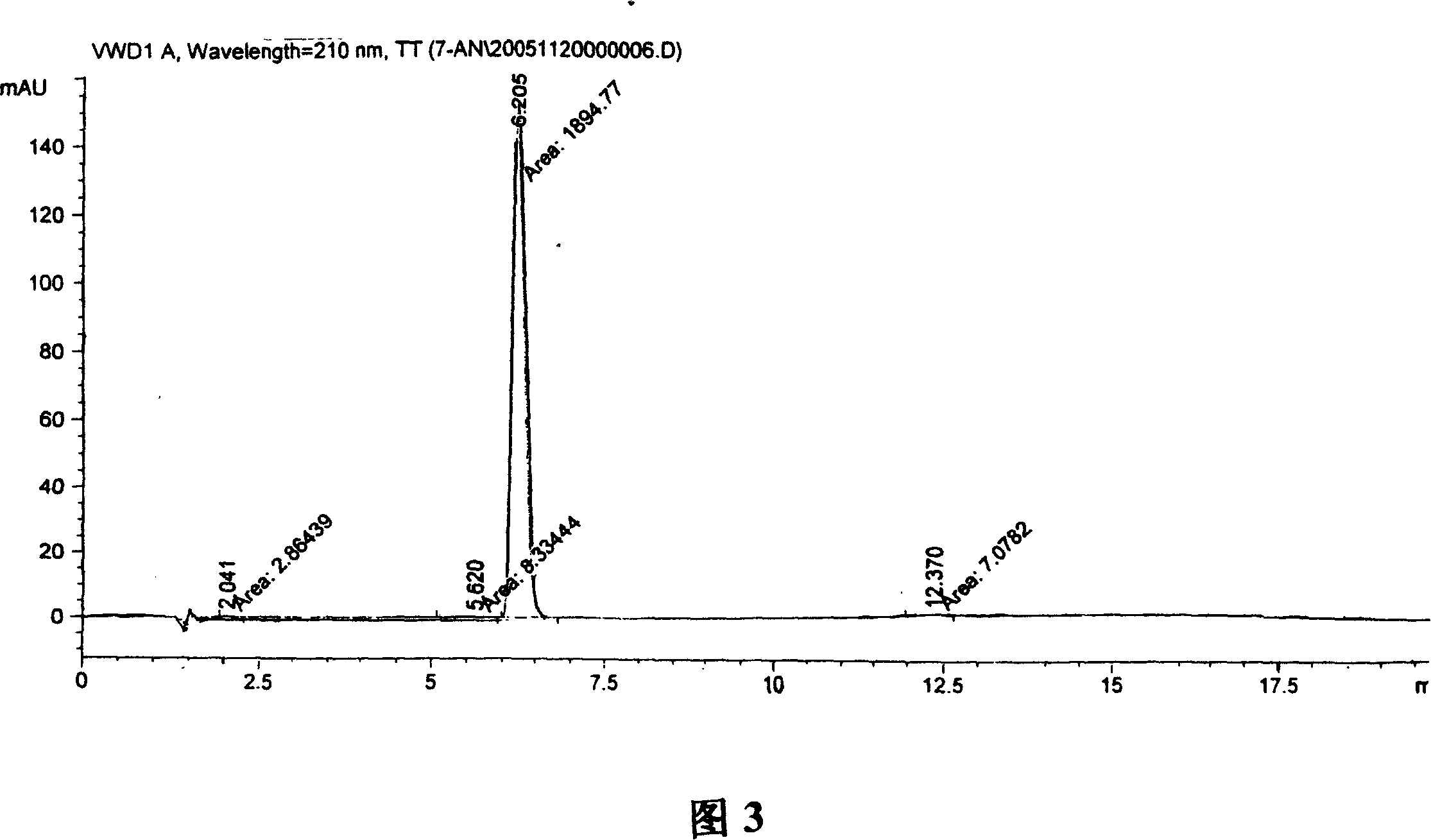
Synthesis, split and racemization of chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
A technology of aminobutyramide and chiral drugs, which is applied in the field of preparation of chiral drug intermediates, can solve the problem that CN1583721A does not carry out recycling, safety, three waste treatment and environmental protection, and L-2-aminobutyric acid is expensive, etc. problems, to achieve the effect of low equipment requirements, improved yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1.1: Preparation of 2-aminobutyric acid
[0040] In a 5000ml flask, 1500ml of 28% ammonia water was added, and 500g of 2-bromobutyric acid was added dropwise at room temperature. After the addition was completed, it was heated to 40°C and reacted for 12 hours. It was concentrated to dryness under reduced pressure, and the residual solid was refined with methanol to obtain 220 g of white crystal 2-aminobutyric acid with a yield of 71% and a content of 99.45%.
[0041] 1.2: Preparation of vortexed free base (±)-2-aminobutyramide
[0042] In a 2000ml flask, add 88g (0.854mol) of 2-aminobutyric acid and 600ml of methanol, cool to below 0°C, add 142.6g (1.2mol) of thionyl chloride dropwise, after the addition is complete, reflux for 3 hours, and concentrate under reduced pressure Remove methanol, add 400ml of ammonia water, extract with 400ml of dichloromethane, dry over anhydrous magnesium sulfate, filter, concentrate under reduced pressure to remove solvent, add 660ml of ...
Embodiment 2
[0049] 2.1: Preparation of 2-aminobutyric acid
[0050] In a 5000ml flask, 2400ml of 28% ammonia water was added, and 800g of 2-bromobutyric acid was added dropwise at room temperature. After the addition was completed, it was heated to 40°C and reacted for 12 hours. It was concentrated to dryness under reduced pressure, and the residual solid was refined with methanol to obtain 340 g of white crystal 2-aminobutyric acid with a yield of 68.9% and a content of 99.3%.
[0051] 2.2: Preparation of vortexed free base (±)-2-aminobutyramide
[0052] In a 5000ml flask, add 325g 2-aminobutyric acid and 2200ml methanol, cool to below 0°C, add 527g (1.2mol) thionyl chloride dropwise, after the dropwise addition, reflux for 3 hours, concentrate under reduced pressure to remove methanol, add 1480ml Ammonia, extracted with 1500ml of dichloromethane, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to remove the solvent, added 2440ml of anhydrous methan...
Embodiment 3
[0059] 3.1: Preparation of 2-aminobutyric acid
[0060] In a 5000ml flask, 2400ml of 28% ammonia water was added, and 800g of 2-bromobutyric acid was added dropwise at room temperature. After the addition was completed, it was heated to 40°C and reacted for 12 hours. It was concentrated to dryness under reduced pressure, and the residual solid was refined with methanol to obtain 355 g of white crystal 2-aminobutyric acid, with a yield of 71.9% and a content of 99.0%.
[0061] 3.2: Preparation of vortexed free base (±)-2-aminobutyramide
[0062] In a 5000ml flask, add 350g 2-aminobutyric acid, 2300ml methanol, cool to below 0°C, add 549g thionyl chloride dropwise, after the dropwise addition, reflux for 3 hours, concentrate under reduced pressure to remove methanol, add 1540ml strong ammonia water, and use Extract with 1540ml of dichloromethane, dry with anhydrous magnesium sulfate, filter, concentrate under reduced pressure to remove solvent, add 2540ml of anhydrous methanol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com